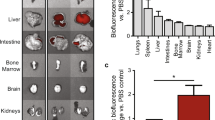
Abstract
Exosomes of human cardiosphere-derived cells (CDCs) are very promising for treating cardiovascular disorders. However, the current challenge is inconvenient delivery methods of exosomes for clinical application. The present study aims to explore the potential to enhance the therapeutic effect of exosome (EXO) from human CDCs to myocardial hypertrophy. A heart homing peptide (HHP) was displayed on the surface of exosomes derived from CDCs that were forced to express the HHP fused on the N-terminus of the lysosomal-associated membrane protein 2b (LAMP2b). The cardiomyocyte-targeting capability of exosomes were analyzed and their therapeutic effects were evaluated in a mouse model of myocardial hypertrophy induced by transverse aorta constriction (TAC). The molecular mechanisms of the therapeutic effects were dissected in angiotensin II-induced neonatal rat cardiomyocyte (NRCMs) hypertrophy model using a combination of biochemistry, immunohistochemistry and molecular biology techniques. We found that HHP-exosomes (HHP-EXO) accumulated more in mouse hearts after intravenous delivery and in cultured NRCMs than control exosomes (CON-EXO). Cardiac function of TAC mice was significantly improved with intravenous HHP-EXO administration. Left ventricular hypertrophy was reduced more by HHP-EXO than CON-EXO via inhibition of β-MHC, BNP, GP130, p-STAT3, p-ERK1/2, and p-AKT. Similar results were obtained in angiotensin II-induced hypertrophy of NRCMs, in which the beneficial effects of HHP-EXO were abolished by miRNA-148a inhibition. Our results indicate that HHP-EXO preferentially target the heart and improve the therapeutic effect of CDCs-exosomes on cardiac hypertrophy. The beneficial therapeutic effect is most likely attributed to miRNA-148a-mediated suppression of GP130, which in turn inhibits STAT3/ERK1/2/AKT signaling pathway, leading to improved cardiac function and remodeling.
Similar content being viewed by others
Background
Cardiac hypertrophy is initially a compensatory adaptive response to many cardiovascular diseases including chronic hypertension, aortic stenosis, mitral or aortic regurgitation, myocardial infarction, and genetic hypertrophic cardiomyopathy, among others [1]. Although current treatment regimens have significantly curtailed the progression of cardiac hypertrophy, unmet medical need remains in clinical practice. Therefore, novel therapeutics that can improve cardiac remodeling and heart functions are urgently needed.
Exosomes are nano-sized extracellular vesicles secreted from cells with ~ 30 to ~ 200 nm in diameter, and contain various signaling molecules that can be shuttled to recipient cells to modulate the pathophysiology of the latter [2]. Emerging evidence shows that exosome not only plays an important role in cell-cell communication in physiological processes, but also mediates the pathogenesis of many diseases and the therapeutic effect of cell therapy [3]. Cardiosphere-derived cells (CDCs) have been shown to reduce cardiomyocyte death, stimulate angiogenesis, suppress interstitial fibrosis, inhibit inflammation, and promote tissue regeneration after acute myocardial infarction [4, 5]. These therapeutic effects could be fully recapitulated by exosomes-derived from the CDCs, and abrogated by the inhibition of exosome secretion [6]. Moreover, retro-orbital injection of exosomes derived from human CDCs improved cardiac hypertrophy and dysfunction in an angiotensin II (Ang II)-induced cardiac hypertrophy mouse model [7]. Intra-myocardial injection of CDCs-exosomes in porcine acute myocardial infarction models significantly decreased cardiac remodeling and improved cardiac functions [8]. Although the therapeutic effect of CDCs-exosomes is very promising in the preclinical animal models, the translational potential is restricted. On the one hand, naturally occurring exosomes either lack or possess limited, tissue tropism, which tends to be trapped and quickly cleared by macrophages of the mononuclear phagocyte system in the liver, kidney, and lungs upon systemic delivery [1: Table S3 [46]. The PCR products were purified and cloned into pLVX-IRES-ZsGreen1 plasmid with XhoI and BamHI restriction enzymes, generating plasmid pLVX-IRES-ZsGreen1-HHP-hLAMP2b (HHP-pLVX). Using the same strategy, a FLAG Tag (DYKDDDDK) was fused in-frame with LAMP2b, generating plasmid pLVX-IRES-ZsGreen1-FLAG-hLAMP2b (FLAG-pLVX). The plasmids were sequenced to confirm their identities (Qingke Biotech Inc., Chengdu, China).
Preparation of human cardiosphere-derived cells and exosomes
Human atrial specimens were obtained from patients underwent heart surgery at **amen Cardiovascular Hospital, **amen University. Human cardiosphere-derived cells (CDCs) were isolated and cultured as described previously [47]. Briefly, atrial specimen was minced into small pieces of approximately 1–2 mm3, which were enzymatically digested and then plated to allow the cardiac explants to grow. After 5–7 days, cells surrounding the explants were harvested and seeded onto poly-D-lysine-coated dishes to allow cardiosphere formation. Two days later, cardiospheres were collected and plated on fibronectin-coated dishes to generate CDCs. The CDCs was expanded to at least passage 4 for downstream experiments.
CDCs of passage 4 were transfected with lentiviral hLAMP2b (LAMP), HHP and FLAG, respectively. The stable cell lines expressing LAMP (LAMP-CDCs), HHP (HHP-CDCs), and FLAG (FLAG-CDCs) were established by sorting green fluorescent protein (GFP) positive cells using flow cytometry. Stable cell lines, LAMP-CDCs and HHP-CDCs were expanded to passage 8 in Iscove's Modified Dulbecco's Media (IMDM) supplemented with 10% FBS. The cells were refreshed with serum-free IMDM and further incubated for 2 weeks. The conditioned medium (CdM) was harvested, filtrated through a 0.45 μm filter, and then stored at − 80 °C. Exosomes from HHP-CDCs and LAMP-CDCs were isolated from the CdM by gradient centrifugation as described previously [48]. Briefly, the CdM was thawed, then spun at 110,000 × g for 2 h at 4 °C. The pellet was dissolved into cold PBS, and spun again at 110,000 × g for 70 min at 4 °C. The pellet was then dissolved in cold PBS and stored at −80 °C for further analysis and applications.
Preparation of neonatal rat cardiomyocytes and treatment with Angiotensin II
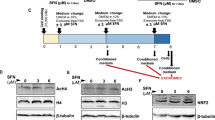
Neonatal rat cardiomyocytes (NRCMs) were isolated by collagenase and trypsin enzymatic dissociation from 1–2 days old newborn rat hearts as previously described [49]. NRCMs were seeded onto 6-well plates in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 20% fetal bovine serum (FBS) and penicillin/streptomycin. After 48 h grown at 37 °C with 5% CO2, NRCMs were subjected to 1 μM Ang II treatment in the presence of 50 μg/ml exosomes or controls for 48 h. H9C2 cardiomyocytes were seeded onto 6-well plates in DMEM supplemented with 10% FBS and penicillin/streptomycin. After 24 h grown at 37 °C with 5% CO2, H9C2 cells were pretreated with 10 μM SC144 for 1 h, and then exposed to 1 μM Ang II with or without 50 μg/ml HHP-EXO for 24 h.
Transverse aortic constriction model
Transverse aortic constriction (TAC) surgery was performed on 10-week-old C57BL/6 male mice [50]. Briefly, mice were anesthetized, intubated, and placed on a ventilator. Sternotomy was performed to expose the aorta, and a 6–0 propene suture was placed around the aorta and tightened around a blunt 27-gauge needle positioned between the right innominate artery and left common carotid artery of the aortic arch. The needle was then removed, and the chest was closed. At day 7 after the TAC, cardiac function was evaluated with echocardiography to confirm that the TAC procedure was successful. Animals with unsuccessful ligation (no change in peak flow velocity of aortic arch on the site of the constriction) were excluded from the study. The mice with successful TAC were randomly divided into 3 groups (n = 12), PBS control, CON-EXO, and HHP-EXO groups. Starting on day 8 post-TAC, 4 mg/kg exosomes were tail-vein injected on every third day for a total of 7 times.
Echocardiography and arterial blood pressure measurement
Cardiac function and gross morphology were assessed by echocardiography using Vevo 2100 (Visual Sonics, Canada) equipped with a 40-MHz MS550D probe and a high-frequency ultrasound system as described previously [49]. Echocardiograms were performed 3 days before the surgery (Control), and on day 7, 14, 21, 28 and 42 post-TAC. Arterial blood pressure was measured on day 42 using the carotid artery catheter method [51]. Briefly, mice were anesthetized by 2% isoflurane and body temperature was kept at 37 °C. The right carotid artery was isolated, clamped and intubated with a PE10 catheter pre-filled with heparin solution (0.1 IU/ml in saline). The catheter was connected to RM6240 multichannel physiological signal acquisition and processing system with a YPJ01 pressure transducer (Chengdu Instrument Factory, China). Aortic pressure was recorded for approximately 30–60 s.
Histological analysis
Hearts, lungs and kidneys were harvested after blood pressure measurement, rinsed in saline, and weighed. Lungs were dried in an oven at 60 °C for 5 days and re-weighed as dry weight. Hearts were embedded in optimal cutting temperature (OCT) medium. Frozen sections of 6.0 μm were fixed in 4% paraformaldehyde, and stained with H&E (Hematoxylin-eosinstaining) and WGA (Wheat Germ Agglutnin) WGA and Masson’s trichrome solutions, respectively [49]. Slides were observed and images were acquired using the tissue cytometry system TissueFAXS (TissueGnostics, Austria). Cardiac fibrosis of the LV was evaluated using ImageJ software.
Western blot
Western blot was performed as previously described [49]. Briefly, equal amount of protein lysates was resolved on 10% polyacrylamide gel and transferred to PVDF membranes. The membranes were incubated respectively with primary antibodies against GP130, phospho-p44/42 MAPK (ERK1/2) (Thr202/Tyr204), phospho-AKT (Ser473), p44/42 MAPK (ERK1/2), AKT, β-MHC, BNP, phospho-STAT3 (p-Tyr705), STAT3, and GAPDH (Additional file 1: Table S2), for overnight at 4 °C. The membranes were washed and incubated with their corresponding species-specific horseradish peroxidase (HRP) conjugated secondary antibodies. The immunoreactive bands were developed with enhanced chemiluminescence (ECL) and images were acquired and quantitated on BioRad ChemiDoc MP imaging system.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 9 software. Unpaired two-tailed Student’s t test was employed to determine the differences between two groups or one-way ANOVA followed by Tukey’s post hoc test was used for comparison among multiple groups. Date were presented as mean ± SEM, and P < 0.05 was considered statistically significant.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- HHP:
-
Heart homing peptide
- CDCs:
-
Cardiosphere-derived cells
- TAC:
-
Transverse aorta constriction
- Ang II:
-
Angiotensin II
- CRIP2:
-
Cysteine-rich protein 2
- NRCMs:
-
Neonatal rat cardiomyocytes
- LVEF:
-
Left ventricular ejection fraction
- LVFS:
-
Left ventricular fractional shortening
- LVV,s:
-
Left ventricular end-systolic volume
- LVAW,d:
-
Diastolic left ventricular anterior wall thickness
- LVAW,s:
-
Systolic left ventricular anterior wall thickness
- LVH:
-
Left ventricular hypertrophy
- MBP:
-
Mean blood pressure
- NT-proBNP:
-
N-terminal pro B-type natriuretic peptide
- AMI:
-
Acute myocardial infarction
- GP130:
-
Glycoprotein 130
References
Shimizu I, Minamino T. Physiological and pathological cardiac hypertrophy. J Mol Cell Cardiol. 2016;97:245–62.
Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem. 2019;88:487–514.
Nikfarjam S, Rezaie J, Zolbanin NM, Jafari R. Mesenchymal stem cell derived-exosomes: a modern approach in translational medicine. J Transl Med. 2020;18:449.
Kreke M, Smith RR, Marban L, Marban E. Cardiospheres and cardiosphere-derived cells as therapeutic agents following myocardial infarction. Expert Rev Cardiovasc Ther. 2012;10:1185–94.
Cheng K, Malliaras K, Li TS, Sun B, Houde C, Galang G, Smith J, Matsushita N, Marban E. Magnetic enhancement of cell retention, engraftment, and functional benefit after intracoronary delivery of cardiac-derived stem cells in a rat model of ischemia/reperfusion. Cell Transplant. 2012;21:1121–35.
Ibrahim AGE, Cheng K, Marban E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Reports. 2014;2:606–19.
Cambier L, Giani JF, Liu W, Ijichi T, Echavez AK, Valle J, Marban E. Angiotensin II-induced end-organ damage in mice is attenuated by human exosomes and by an exosomal Y RNA fragment. Hypertension. 2018;72:370–80.
Gallet R, Dawkins J, Valle J, Simsolo E, de Couto G, Middleton R, Tseliou E, Luthringer D, Kreke M, Smith RR, et al. Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur Heart J. 2017;38:201–11.
Wan Z, Zhao L, Lu F, Gao X, Dong Y, Zhao Y, Wei M, Yang G, **ng C, Liu L. Mononuclear phagocyte system blockade improves therapeutic exosome delivery to the myocardium. Theranostics. 2020;10:218–30.
Chen P, Wang L, Fan X, Ning X, Yu B, Ou C, Chen M. Targeted delivery of extracellular vesicles in heart injury. Theranostics. 2021;11:2263–77.
Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA, Lee JJ, Kalluri R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546:498–503.
Dang XTT, Kavishka JM, Zhang DX, Pirisinu M, Le MTN. Extracellular vesicles as an efficient and versatile system for drug delivery. Cells. 2020;9:2191.
Yu Y, Li W, Mao L, Peng W, Long D, Li D, Zhou R, Dang X. Genetically engineered exosomes display RVG peptide and selectively enrich a neprilysin variant: a potential formulation for the treatment of Alzheimer’s disease. J Drug Target. 2021;29(10):1128–38.
**tong D, **aorong Z. Targeted therapeutic delivery using engineered exosomes and its applications in cardiovascular diseases. Gene. 2016;575:377–84.
Zhang L, Hoffman JA, Ruoslahti E. Molecular profiling of heart endothelial cells. Circulation. 2005;112:1601–11.
Vandergriff A, Huang K, Shen D, Hu S, Hensley MT, Caranasos TG, Qian L, Cheng K. Targeting regenerative exosomes to myocardial infarction using cardiac homing peptide. Theranostics. 2018;8:1869–78.
Mentkowski KI, Lang JK. Exosomes engineered to express a cardiomyocyte binding peptide demonstrate improved cardiac retention in vivo. Sci Rep. 2019;9:10041.
Smyth T, Kullberg M, Malik N, Smith-Jones P, Graner MW, Anchordoquy TJ. Biodistribution and delivery efficiency of unmodified tumor-derived exosomes. J Control Release. 2015;199:145–55.
Wiklander OP, Nordin JZ, O’Loughlin A, Gustafsson Y, Corso G, Mager I, Vader P, Lee Y, Sork H, Seow Y, et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles. 2015;4:26316.
Blanch P, Armario P, Oliveras A, Fernandez-Llama P, Vazquez S, Pareja J, Alvarez E, Calero F, Sierra C, de la Sierra A. Association of either left ventricular hypertrophy or diastolic dysfunction with 24-hour central and peripheral blood pressure. Am J Hypertens. 2018;31:1293–9.
Yan W, Dong ZC, Wang JJ, Zhang YL, Wang HX, Zhang B, Li HH. Deficiency of the immunoproteasome LMP10 subunit attenuates angiotensin II-induced cardiac hypertrophic remodeling via autophagic degradation of gp130 and IGF1R. Front Physiol. 2020;11:625.
Aminzadeh MA, Rogers RG, Fournier M, Tobin RE, Guan X, Childers MK, Andres AM, Taylor DJ, Ibrahim A, Ding X, et al. Exosome-mediated benefits of cell therapy in mouse and human models of duchenne muscular dystrophy. Stem Cell Reports. 2018;10:942–55.
Raso A, Dirkx E, Philippen LE, Fernandez-Celis A, De Majo F, Sampaio-Pinto V, Sansonetti M, Juni R, El Azzouzi H, Calore M, et al. Therapeutic delivery of miR-148a suppresses ventricular dilation in heart failure. Mol Ther. 2019;27:584–99.
Barile L, Lionetti V, Cervio E, Matteucci M, Gherghiceanu M, Popescu LM, Torre T, Siclari F, Moccetti T, Vassalli G. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovasc Res. 2014;103:530–41.
Eguchi S, Takefuji M, Sakaguchi T, Ishihama S, Mori Y, Tsuda T, Takikawa T, Yoshida T, Ohashi K, Shimizu Y, et al. Cardiomyocytes capture stem cell-derived, anti-apoptotic microRNA-214 via clathrin-mediated endocytosis in acute myocardial infarction. J Biol Chem. 2019;294:11665–74.
Namazi H, Mohit E, Namazi I, Rajabi S, Samadian A, Hajizadeh-Saffar E, Aghdami N, Baharvand H. Exosomes secreted by hypoxic cardiosphere-derived cells enhance tube formation and increase pro-angiogenic miRNA. J Cell Biochem. 2018;119:4150–60.
Volpe JJ. Commentary - exosomes: realization of the great therapeutic potential of stem cells. J Neonatal Perinatal Med. 2020;13:287–91.
Marban E, Cingolani E. Heart to heart: cardiospheres for myocardial regeneration. Heart Rhythm. 2012;9:1727–31.
Johnston PV, Sasano T, Mills K, Evers R, Lee ST, Smith RR, Lardo AC, Lai S, Steenbergen C, Gerstenblith G, et al. Engraftment, differentiation, and functional benefits of autologous cardiosphere-derived cells in porcine ischemic cardiomyopathy. Circulation. 2009;120:1075–83.
Lee ST, White AJ, Matsushita S, Malliaras K, Steenbergen C, Zhang Y, Li TS, Terrovitis J, Yee K, Simsir S, et al. Intramyocardial injection of autologous cardiospheres or cardiosphere-derived cells preserves function and minimizes adverse ventricular remodeling in pigs with heart failure post-myocardial infarction. J Am Coll Cardiol. 2011;57:455–65.
Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, Czer LS, Marban L, Mendizabal A, Johnston PV, et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379:895–904.
Antimisiaris SG, Mourtas S, Marazioti A. Exosomes and exosome-inspired vesicles for targeted drug delivery. Pharmaceutics. 2018;10(4):218.
Takahashi Y, Nishikawa M, Shinotsuka H, Matsui Y, Ohara S, Imai T, Takakura Y. Visualization and in vivo tracking of the exosomes of murine melanoma B16-BL6 cells in mice after intravenous injection. J Biotechnol. 2013;165:77–84.
Kooijmans SAA, Fliervoet LAL, van der Meel R, Fens MHAM, Heijnen HFG, van Bergen EnHenegouwen PMP, Vader P, Schiffelers RM. PEGylated and targeted extracellular vesicles display enhanced cell specificity and circulation time. J Control Release. 2016;224:77–85.
Emanueli C, Shearn AI, Angelini GD, Sahoo S. Exosomes and exosomal miRNAs in cardiovascular protection and repair. Vascul Pharmacol. 2015;71:24–30.
de Couto G, Gallet R, Cambier L, Jaghatspanyan E, Makkar N, Dawkins JF, Berman BP, Marban E. Exosomal MicroRNA transfer into macrophages mediates cellular postconditioning. Circulation. 2017;136(2):200–14.
Hirai K, Ousaka D, Fukushima Y, Kondo M, Eitoku T, Shigemitsu Y, Hara M, Baba K, Iwasaki T, Kasahara S, et al. Cardiosphere-derived exosomal microRNAs for myocardial repair in pediatric dilated cardiomyopathy. Sci Transl Med. 2020;12(573):eabb3336.
Silver JS, Hunter CA. gp130 at the nexus of inflammation, autoimmunity, and cancer. J Leukoc Biol. 2010;88:1145–56.
Tsutamoto T, Hisanaga T, Wada A, Maeda K, Ohnishi M, Fukai D, Mabuchi N, Sawaki M, Kinoshita M. Interleukin-6 spillover in the peripheral circulation increases with the severity of heart failure, and the high plasma level of interleukin-6 is an important prognostic predictor in patients with congestive heart failure. J Am Coll Cardiol. 1998;31:391–8.
Hirano T, Nakajima K, Hibi M. Signaling mechanisms through gp130: a model of the cytokine system. Cytokine Growth Factor Rev. 1997;8:241–52.
Fischer P, Hilfiker-Kleiner D. Role of gp130-mediated signalling pathways in the heart and its impact on potential therapeutic aspects. Br J Pharmacol. 2008;153(Suppl 1):S414-427.
Mir SA, Chatterjee A, Mitra A, Pathak K, Mahata SK, Sarkar S. Inhibition of signal transducer and activator of transcription 3 (STAT3) attenuates interleukin-6 (IL-6)-induced collagen synthesis and resultant hypertrophy in rat heart. J Biol Chem. 2012;287:2666–77.
Ye S, Luo W, Khan ZA, Wu G, Xuan L, Shan P, Lin K, Chen T, Wang J, Hu X, et al. Celastrol attenuates angiotensin II-induced cardiac remodeling by targeting STAT3. Circ Res. 2020;126:1007–23.
Akers WS, Cross A, Speth R, Dwoskin LP, Cassis LA. Renin-angiotensin system and sympathetic nervous system in cardiac pressure-overload hypertrophy. Am J Physiol Heart Circ Physiol. 2000;279:H2797-2806.
Zhou Z, Peters AM, Wang S, Janda A, Chen J, Zhou P, Arthur E, Kwartler CS, Milewicz DM. Reversal of aortic enlargement induced by increased biomechanical forces requires AT1R inhibition in conjunction with AT2R activation. Arterioscler Thromb Vasc Biol. 2019;39:459–66.
El-Andaloussi S, Lee Y, Lakhal-Littleton S, Li J, Seow Y, Gardiner C, Alvarez-Erviti L, Sargent IL, Wood MJ. Exosome-mediated delivery of siRNA in vitro and in vivo. Nat Protoc. 2012;7:2112–26.
Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham MR, Marban E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908.
Mao L, Li X, Gong S, Yuan H, Jiang Y, Huang W, Sun X, Dang X. Serum exosomes contain ECRG4 mRNA that suppresses tumor growth via inhibition of genes involved in inflammation, cell proliferation, and angiogenesis. Cancer Gene Ther. 2018;25:248–59.
Wu WY, Cui YK, Hong YX, Li YD, Wu Y, Li G, Li GR, Wang Y. Doxorubicin cardiomyopathy is ameliorated by acacetin via Sirt1-mediated activation of AMPK/Nrf2 signal molecules. J Cell Mol Med. 2020;24:12141–53.
Tavakoli R, Nemska S, Jamshidi P, Gassmann M, Frossard N. Technique of minimally invasive transverse aortic constriction in mice for induction of left ventricular hypertrophy. J Vis Exp. 2017;(127):e56231.
Zhao X, Ho D, Gao S, Hong C, Vatner DE, Vatner SF. Arterial pressure monitoring in mice. Curr Protoc Mouse Biol. 2011;1:105–22.
Acknowledgements
The authors would like to thank Ms. Tang-Ting Chen, Institute of Cardiovascular Research, Southwest Medical University, for her guidance on the transverse aortic constriction mouse model.
Funding
The research has been supported, in part, by grants from Central Government for Local Development of Science and Technology of Sichuan Province (2020ZYD047 to LM), Collaborative Innovation Center for Prevention and Treatment of Cardiovascular Disease of Sichuan Province (xtcx2019-15 to LM), Luzhou-Southwest Medical University joint scientific funding (2018LZXNYD-ZK25 to LM), Special Supporting grant for Young Scientists of Southwest Medical University (2021–2023 to LM), and the National Nature Science Foundation of China (81700268 to LM, U1605226 to YW).
Author information
Authors and Affiliations
Contributions
Y. W. and L. M. conceived the research; L. M. performed the study, and wrote the first draft of the manuscript; Y-D. L. performed the echocardiography; R-L. C. performed the examination of arterial blood pressure; X-X. Z., F. S., C. W., Y. H., and Y-X. H. participated in cell culture and animal experiments; G. L. guided the data analysis; X. D. edited and finalized the final draft of the manuscript; and L. M., G. L., G-R. L., and Y. W. reviewed and proofread the manuscript. All authors read and approved the final draft of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures involving human specimens were in accordance with the ethical standards of **amen University and with the 1964 Helsinki declaration. An informed consent was obtained from each participant prior to the relevant surgery. All animal study protocols were approved by the ethical committee of **amen University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Schematic illustration of displaying a homing peptide/FLAG on the surface of exosomes. The coding sequence of HHP/FLAG (red bar) was fused in-frame to the LAMP2b cDNA between the signal peptide (SP) and the N-terminus, which was then cloned into pLVX-IRES-ZsGreen1 expression plasmid. Forced expression of the plasmid in CDCs would display the HHP/FLAG (red oval) on the surface of exosomes. Figure S2. Schematic illustration of mice treatment schedule. The TAC mice were randomly divided into 3 groups, PBS control, CON-EXO, and HHP-EXO (n = 12 each). Exosomes (4 mg/kg) or PBS were tail-vein injected on day 8, 10, 12, 14, 16, 18, 20 post-TAC. Echocardiographic studies were performed 3 days (Control) prior to, and on day 7, 14, 21, 28 and 42 after, the TAC. The mean arterial blood pressure was evaluated, serum was collected, and the hearts were harvested on day 42 post-TAC. Figure S3. Cardiac hypertrophy after exosome treatment. A Coronal sections of the hearts among groups by HE staining. B Quantitation of heart weight/body weight (left panel) and heart weight/tibial length (right panel) ratios among groups. Data are presented as ‘Mean ± STDEV’, n = 9-12 animals, *P < 0.05 and **P < 0.01. Figure S4. HHP-EXO and SC144 perform similar effect of inhibiting GP130-STAT pathway. H9C2 cardiomyocytes were pretreated with SC144 (10μM) for 1h, and then exposure to Ang II (1 μM) with or without HHP-EXO (50μg/ml) for 24h, the expression of β-MHC, GP130, p-STAT3, STAT3, p-ERK1/2, ERK, p-AKT and AKT was detected by Western blotting. Table S1. Parameters of cardiac function and related serum kinases levels in TAC mice with different treatments. Table S2. Reagents and antibodies used in the present study. Table S3. Primers for cloning of LAMP2b fusion plasmids used in the present study.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mao, L., Li, YD., Chen, RL. et al. Heart-targeting exosomes from human cardiosphere-derived cells improve the therapeutic effect on cardiac hypertrophy. J Nanobiotechnol 20, 435 (2022). https://doi.org/10.1186/s12951-022-01630-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12951-022-01630-3