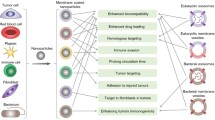
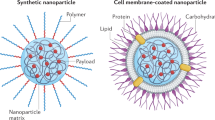
Abstract
Inorganic nanoparticles (INPs) have been paid great attention in the field of oncology in recent past years since they have enormous potential in drug delivery, gene delivery, photodynamic therapy (PDT), photothermal therapy (PTT), bio-imaging, driven motion, etc. To overcome the innate limitations of the conventional INPs, such as fast elimination by the immune system, low accumulation in tumor sites, and severe toxicity to the organism, great efforts have recently been made to modify naked INPs, facilitating their clinical application. Taking inspiration from nature, considerable researchers have exploited cell membrane-camouflaged INPs (CMCINPs) by coating various cell membranes onto INPs. CMCINPs naturally inherit the surface adhesive molecules, receptors, and functional proteins from the original cell membrane, making them versatile as the natural cells. In order to give a timely and representative review on this rapidly develo** research subject, we highlighted recent advances in CMCINPs with superior unique merits of various INPs and natural cell membranes for cancer therapy applications. The opportunity and obstacles of CMCINPs for clinical translation were also discussed. The review is expected to assist researchers in better eliciting the effect of CMCINPs for the management of tumors and may catalyze breakthroughs in this area.
Graphical Abstract

Similar content being viewed by others
Introduction
Cancer is the second leading cause of death and has been doing great harm to human health [1,2,3,4]. There are an estimated 19.3 million [95% uncertainty interval (UI): 19.0–19.6 million] new cases of cancer (18.1 million excluding non-melanoma skin cancer) and almost 10.0 million (95% UI: 9.7–10.2 million) deaths from cancer (9.9 million excluding non-melanoma skin cancer) worldwide in 2020 [5]. Conventional methods to treat cancer include surgery, chemotherapy, and radiotherapy. However, if tumors are non-resectable or metastasized, chemotherapy is the only therapeutic resolution to control the size and spread of cancer. As one of the most common clinical strategies, chemotherapy still has unsatisfactory performance due to severe adverse effects and the low targeting ability of anti-cancer drugs [6, 7]. To deal with the difficulties above, targeting drug delivery systems (TDDS) based on nanoparticles (NPs), including organic NPs (ONPs) and inorganic NPs (INPs), have been widely developed [8,9,10,11,12,13]. Taking advantage of their unique (bio)physicochemical characteristics, such as small size (100–200 nm) and optimized surface potential (negative or neutral), NPs can enhance the accumulation of anti-cancer drugs in cancers and reduce their side effects [14]. Compared with ONPs, INPs possess unique optical, electric, and magnetic characteristics, making them have a promising application prospect in tumor diagnosis and treatment [15,16,17,18,19,80]. MSC homing is the process by which MSC migrates to the targeted tissue and colonizes under the action of various biotic factors. As for cancer, persistent wound healing is a definite process during its development. The over-expressed adhesion molecules, chemokines, and growth factors, which are the integral components of tumor stroma, induce MSC to migrate to tumor sites actively [32]. Meanwhile, these specific signaling molecules rich in the tumor microenvironment can bind to the corresponding MSC membrane surface proteins, all these factors contributing to MSC tumor-homing behavior. Therefore, the MSC membrane is an ideal potential carrier for establishing a drug delivery system that promises to minimize adverse off-target effects for cancer treatment.
In 2016, He et al. combined the MSC membrane with dual photosensitizers-loaded mesoporous silica through a mechanical extrusion method (Fig. 7a) [81]. CLSM images of the UCNPs@mSiO2 and MSC membrane-coated UCNPs@mSiO2 (SUCNPs@mSiO2) in different circumstances in vitro are shown in Fig. 7b. It demonstrates that when incubating the UCNPs@mSiO2 and SUCNPs@mSiO2 with cancer cells, the SUCNPs@mSiO2 showed higher fluorescence intensity, which indicated that the MSC membrane is reliable for tumor affinity. Fluorescence intensity is quantified by the flow cytometry in Fig. 7c–e. For in vivo tumor targeting evaluation of SUCNPs@mSiO2, He et al. injected Cy7-SUCNPs@mSiO2 into the tail vein of mice and used Cy7-UCNPs@mSiO2 with the same amount as a control. As shown in Fig. 7f, the fluorescence intensity of Cy7-SUCNPs@mSiO2 at the tumor site was more than the control group, which indicated the tumor homing effect of SUCNPs@mSiO2 endowed by the MSC membrane. Collectively, MSC membrane camouflaging is a reasonable pathway to improve the efficacy of anti-cancer therapy in vivo. This precocious work verified that this STM-camouflaged UCNPs-based nanoplatform had a promising ability of deep-tissue PDT for cancer treatment. Collectively, MSC membrane camouflaging is a reasonable pathway to improve the efficacy of tumor affinity in vitro and tumor homing in vivo. As illustrated in Fig. 7g, compared with the tumors in the control groups, tumors in the PS-loaded SUCNPs@mSiO2-injected mice were remarkably inhibited. This pioneering work verified that this STM-camouflaged UCNPs-based nanoplatform had a promising ability of deep-tissue PDT for cancer treatment. MSCs in this work has advantages of easy cultivation and amplification in vitro, low immunogenicity, and low ethical controversy. More investigations can be carried out by exploiting the merits of other stem cell membranes to endow MSC-INPs with more biological functions.
a The fabrication process of SUCNPs@mSiO2 and their mechanism in photodynamic therapy. b Enhanced in vitro cancer cell accumulation of SUCNPs@mSiO2. CLSM images demonstrate the tumor cell binding of UCNPs@mSiO2 and SUCNPs@mSiO2. All scale bars are 200 nm. c Quantitative analysis of HeLa cells by flow cytometry. d Percentages of HeLa cells with increased fluorescence in (c). e Quantification of the mean fluorescence intensity of HeLa cells in (c). f In vivo fluorescence images of mice at 1, 2, 4, 8, 12, and 24 h after injection of Cy7-SUCNPs@mSiO2. The red circles indicate the tumor sites. g Photographs of mice show the tumor size change after various treatments at different time points. Reprinted with permission from Refs [81]
In 2017, Chang et al. designed a biomimetic nanoplatform of MSC membrane-camouflaged superparamagnetic iron oxide NPs (STM-SPIO), which achieved self-assembling through sonication. (Fig. 8a) [82]. After being co-incubated with DI H2O and cell culture medium for 10 min, 4 h, and 24 h, respectively, the particle size of the biomimetic nanoplatform did not change significantly, which signified the adequate colloidal stability for further therapeutic use. (Fig. 8e) Macrophages were incubated with various concentrations (10, 20, and 30 μg/mL) of SPIO (Top) and STM-SPIO (Bottom). STM-SPIO showed lower macrophages uptake than the bare SPIO (Fig. 8b c), which implied better biocompatibility and a longer circulation time of STM-SPIO. As shown in Fig. 8d, when exposed to an alternating magnetic field (AMF) in vitro, the 150 μg/mL Magnetic+ membrane-coated group achieved the highest temperature, representing the most effective PTT efficacy. The prostate cancer cells were killed rapidly via the magnetocaloric effect (Fig. 8f). In conclusion, the multifunctional nanoplatform holds immense prospects for extensive biomedical applications for active targeting drug delivery systems, magnetic resonance imaging, and magnetic hyperthermia therapy in the future. However, this work only verified the efficient anti-cancer function of this biomimetic nanoplatform in vitro. It is also interesting to identify their biosafety and explore other biomedical applications, including tissue repair, antibacterial, and so on.
a Schematic representation of STM-SPIO preparation procedure. b Macrophage uptake of SPIO and STM-SPIO of three different concentrations. c Quantitative measurement of intracellular Fe level in the macrophages by using inductively coupled plasma mass spectrometry (ICP-MS). d Effect of alternating magnetic field (AMF) treatment on prostate cancer cells solutions. e The particle size of MSC membrane-camouflaged superparamagnetic iron oxide NPs incubated with water, 25% and 50% FBS-containing DMEM at 30 min, 4 h, and 24 h, respectively. f Viability assay of prostate cancer cells. Reprinted with permission from Refs [82]
However, the source of MSCs is scarce, greatly limiting the development of MSCM-INPs. Meanwhile, it costs a great deal of money and medical resources to acquire MSCM, which also restricts the clinical applications of MSCM-INPs compared with other types of CMCINPs.
White blood cell membrane-camouflaged inorganic nanoparticles (WBCM-INPs)
White blood cell (WBC) exists widely in blood and lymph with the ability to migrate actively, which mainly exerts immune defense and regulation functions in vivo [100]. According to its granularity and shape difference, WBC is divided into five major types, including monocytes/macrophages, neutrophils, eosinophils, basophils, and lymph cells [101]. WBC expresses abundant signal and adhesion molecules on its surface, which makes the WBCM-INPs partly preserve the functions of the WBC. Therefore, WBCM-INPs have a wide prospect of application in TDDS [83, 84].
Long-term chronic inflammation is a significant symbol of the malignant tissue, and therefore macrophages and neutrophils can target and get enriched in tumor sites [83]. These characteristics of WBC provide creative ideas to design a carrier for tumor targeting therapy. MSNs are always widely used as a drug delivery platform due to their porous structure and large specific surface area, but the bare MSNs cannot target the specific tumor site. Xuan et al. coated the macrophage membrane (MPCM) on DOX-loaded MSNs and proved the MPCM-INPs carrying drugs could target and get enriched in the tumor site to kill the tumor tissue after injection (Fig. 9a) [85]. The results showed that the MPCM-camouflaged MSNs could cause higher toxicity to 4T1 breast cancer cells in vitro than free DOX and DOX-loaded MSNs. Figure 9b showed the reduced tumor volume after being treated with MPCM-camouflaged NPs in mice tumor-bearing models over time (3, 10, and 15 d). Due to the excellent targeting ability of the MPCM, the MPCM-camouflaged MSNs had a higher accumulation in the tumor than bare MSNs, and a lower accumulation in organs such as liver and spleen, which indicated that MPCM-camouflaged MSNs also had the ability of immune escape (Fig. 9c). This work suggested that the inflammatory environment can serve as a targeting area to increase the concentration of CMCINPs at tumor sites, which takes advantage of the interaction between WBCM and the unique tumor microenvironment. However, the specific mechanism of interaction needs further study. As an example, the specific molecules on WBCM that can interact with the tumor microenvironment should be confirmed. Meanwhile, it should be noted that the interaction is also affected by the type and the stage of tumors.
a The preparation process of the MPCM-camouflaged MSNCs and application for subsequent in vivo cancer therapy. b The tumor growth in mice treated with DOX@MPCM-camouflaged MSNCs. c The amount distribution of NPs in tumors and different organs. d The brief synthesis process of MPCM-AuNS. e The relative signal intensity of AuNS, MPCM-AuNS, and PBS after intravenous injection. f The images of mice bearing 4T1 tumor injected AuNS and MPCM-AuNS under the fluorescence time-lapse. Reprinted with permission from Refs [85, 86]
In addition to the ability of targeting, immune escape and prolonged circulation time are also significant. Since the gold NPs can convert light to thermal energy, inducing local temperature increase in tumor sites, they can be used for cancer PTT [40]. However, the half-life of circulation is short due to the intense clearance by the immune system. Xuan et al. reported the macrophage cell membrane-camouflaged Au nanoshells (MPCM-AuNSs) through a top-down assembly method to prolong the blood circulation time (Fig. 9d) [86]. First, Xuan et al. coated the mesoporous silica onto the gold core, and then the composite particles were camouflaged with a macrophage membrane. Meanwhile, they loaded dye on the silica to achieve the PTT and imaging integration simultaneously. As shown in Fig. 9e, through the detection of the relative signal intensity, they found that the MPCM-AuNSs had a longer circulation time than bare AuNSs. And in the in vivo experiments, lower clearance of NPs achieved only when AuNSs were camouflaged with MPCM (Fig. 9f). The longer circulation time expressed better effects of killing tumors and fewer adverse effects. Then, they treated the nude mice bearing the 4T1 tumor in different therapeutic conditions, and the tumor volume was much smaller in the mice injected with the MPCM-AuNSs than in other control groups. In summary, MPCM-camouflaged AuNSs retain the natural properties of MPCM, which improved the PTT efficacy modulated by AuNSs and other metal INPs. It is worth mentioning that the WBCs in this work utilized natural macrophages. However, to the best of our knowledge, the natural macrophages can be polarized into M1 or M2 types under specific stimulation. Since different types of macrophages have various influences on the process of tumor progression, more research could focus on camouflaging INPs with various types of macrophages to investigate their anti-cancer and biological functions.
Nanoswimmer can translate various types of energy to mechanical movement, and it is often used for biosensing, drug delivery, and PTT [87]. As a kind of nanoswimmers, liquid metal has excellent biocompatibility, and the movement of liquid metal can be regulated through the alternating frequency and voltage of the ultrasonic field, and therefore this nanoswimmer is given the ability of autonomous motion [88, 89]. However, when the bare gallium nanoswimmer (GNS) enters the circulatory system, they will be affected by the “biofouling effect” vulnerably, which will enhance the effects of immune clearance and viscous resistance [90]. It was reported that Wang et al. synthesized the leukocyte membrane-camouflaged gallium nanoswimmers (LMGNSs) (Fig. 10a) [91]. It showed a fascinating capability of the anti-biofouling after camouflaging with the WBC membrane. As shown in Fig. 10b, c, after incubation with Rhodamine-BSA, the fluorescence signal of GNSs was higher than LMGNSs. It proved that the LMGNSs were not susceptible to protein adsorption. To evaluate the motion behavior of the nanoswimmer, Wang et al. tested the movement distance of GNSs and LMGNSs (Fig. 10d, e). The LMGNSs moved with a longer distance than GNSs in serum and blood, representing a better anti-biofouling effect. And through the coefficient detection of the velocity and diffusion (Fig. 10f, g), the LMGNSs showed a better locomotivity in the biological media. Meanwhile, this nanoswimmer certainly exhibited the ability of photothermal effect, making it possible for PTT. This biomimetic nanoplatform integrated the abilities of active driven motion, imaging, cancer cell targeting, drug delivery, antibio-fouling, and PTT altogether, making this nanoswimmer a next-generation theranostics platform. The leukocyte membrane here can act as a protective measure to prevent the directionally moving nanoswimmer from being affected by the biofouling effect, thus prolonging the motion cycle of the nanoswimmer. With the rise of remote-controlled INPs for cancer diagnosis and therapy, this WBCM-camouflaging technology can be applied extensively to prolong the duration of action of INPs. However, intricate motion of INPs, such as rotation, bounce, might be affected by the coated WBCM.
a Schematic illustration of the fabrication process of LMGNSs. b The CLSM images of GNSs after co-cultured with Rhodamine-labeled BSA for 24 h. c The CLSM images of LMGNSs after co-cultured with Rhodamine-labeled BSA for 24 h. d The movement distance of GNSs in the blood and serum. e The movement distance of LMGNSs in the blood and serum. f The velocity of GNSs and LMGNSs in PBS, serum, and blood media. g The mean-squared displacement (MSD) and diffusion coefficient of the GNSs and LMGNSs in the different solutions. Reprinted with permission from Refs [91]
Construction of IMS and the procedure of CTC enrichment.Reprinted with permission from Refs [96]
In terms of the detection of cancer cells, WBCM-INPs exhibit a creative application due to the combination of targeting and immune escape. Circulating tumor cells (CTCs) is considered a crucial step in forming metastases, which determines that screening and detecting CTCs is important for early cancer diagnosis, treatment monitoring, and prognostic evaluation [92, 93]. However, the amount of CTCs in the blood is very low among the surrounding background cells, which makes it hard to collect them [94]. **ong et al. developed biomimetic immuno-magnetosomes (IMSs) to enrich CTCs. (Fig. 11) The leukocyte membrane was modified with azide (N3), paving the way for subsequent Ab decoration by chemical modification, which endows the leukocyte membrane with efficient CTCs recognition. Then the MNCs were camouflaged by the pre-engineered leukocyte membrane to form IMSs. IMSs would be repelled when encountered a leukocyte in the bloodstream because of its homology [95]. Therefore, unspecific leukocyte absorption would be suppressed to a large extent. Surprisingly, the results showed that about 90% of the CTCs could be captured from the peripheral blood in a short period with an undetectable leukocyte background, which firmly verified IMSs are superior candidates for CTCs recognition and managing cancer therapy. This WBCM-camouflaging technology significantly increases the ability of INPs to detect specific circulatory morbid substances in terms of sensitivity and degree of purity, overcoming the dilemma of false positive and false negative results caused by background interference.
WBCM-INPs are no doubt an emerging and effective cancer therapy nanoplatform. The INPs inherit the function of the WBC stably, like inflammation sites aggregation and immune escape, which can overcome the limitations of the INPs when applying for tumor therapy. Moreover, compared to the maturely established RBCM-INPs that have been studied a lot, the WBCM-INPs are more functional because the WBC plays a relatively complex role in the human body and has complicated surface molecules. And the WBCM-INPs also inherit the transendothelial migration ability from WBC. Despite the advantages above, the source of the WBC is still a challenge, and the immune rejection should be reckoned with when choosing cell lines from donors. The application of patients' autologous WBC needs to consider the sufficient amount of WBC. It is worth mentioning that if choosing to use the tumor cell line as the source of the cell membrane, it is a necessity to abandon the carcinogenicity of its nucleic acid.
Platelet membrane-camouflaged inorganic nanoparticles (PLTM-INPs)
Platelets are a kind of akaryotes released from the cytosol of megakaryocytes in bone marrow hematopoietic tissue and have a direct effect on thrombosis. Uncontrolled platelet activation can lead to some chronic inflammations, including atherosclerotic thrombosis and even inflammation in cancer [97]. Besides, plenty of research showed that platelets play an essential role in the pathogenesis and progression of malignant tumors. Recently, a significant cross-communication of tumor cells and platelets was found [98]. Cancer cells can “educate” platelets by affecting their RNA profiles, making a difference in the number of circulating platelets, and changing the state of platelet activation. Meanwhile, “educated” platelets can produce excess activators, including cyclic uptake and platelet-specific biomolecules, which are released from platelets upon activation and promote the development of malignant tumors. The process of primary tumor-induced platelet production, aggregation, and activation contribute to the prethrombotic state in the blood. Platelet activation is critical for tumor growth and metastatic outbreaks. Moreover, some crucial components in the tumor microenvironment, such as vascular endothelial growth factors, platelet-derived factors, and transforming growth factor β, can promote the development of malignant tumors [99]. CTC can contact, activate, and be promoted to proliferate by platelets. Compared with RBCM, platelet membrane (PLTM) encapsulation has the advantage of targeting inflammation and tumor sites. Researchers have utilized this characteristic to transport drugs and INPs to tumor sites [100]. There are two mechanisms of targeted therapy with platelet encapsulated NPs-passive targeting and active targeting [101]. Passive targeting is based on the surface ligands of platelet membrane-camouflaged NPs (PNPs) that have autologous antigens derived from PLTM, such as CD47, which helps PNPs escape the immune system elimination and deliver more drugs to the inflammation sites. In terms of active targeting, a variety of receptors on the surface of PNPs can also interact directly with specific components of malignant tissues. For example, the cell adhesion molecule P-selectin glycoprotein ligand-1 (PSGL-1) can specifically recognize the CD44 that is overexpressed on the surface of the tumor cell membrane. All these interactions endow PNPs with the ability to target tumors actively.
Compared with active targeting, passive targeting is more widely used. Pei et al. improved the precise delivery of drugs with the participation of PLTM. They prepared IR780 (a typical NIR fluorescent dye) PLGA and DOX for IR780@PLGA/DOX NPs by single emulsification to treat breast cancer [102]. NPs that contained drugs and photothermal agents wrapped with natural PLTM could achieve no recognition and little clearance by the immune system. As shown in Fig. 12a, b, the photothermal fluorescence imaging in vivo indicated that the PLTM-INPs could circulate in the blood for a more extended period and accumulate more at the tumor sites compared with the bare core INPs, which released more antitumor drugs to achieve better PTT results. Fluorescence imaging of tumors in vivo could be obviously observed from 12 to 120 h after injection. It is worth mentioning that the tumors on mice models treated with PLTM-INPs thoroughly vanished without recurrence during 18 d observation period [102]. This study provided a new idea to design photothermal agents and drug delivery systems by loading them into PLTM vesicles.
IR780 embedded within the nanoparticles is used as the photothermal agent, which could also be applied in vivo fluorescence imaging of 4T1 tumor-bearing mice at 12, 24, 48, 72, 96, and 120 h after intravenous injection of (a) bare core INPs and (b) PLTM-INPs lied in NIR fluorescence imaging. c Comparison of representative in vivo IR of mice bearing MCF-7 tumor injected with different components after laser irradiation for 5 min. d magnetic resonance imaging contrast after all kinds of nanoparticles injected with the same amount. Red arrows indicated the sites of MCF-7 tumors in mice. e After PTT treatment with different kinds of nanoparticles, the highest tumor temperature in treated mice increased. White circles indicate the tumor sites. Reprinted with permission from Refs [32, 103]
To take advantage of the long circulation and cancer-targeting ability of PLTM, Liu et al. developed PLTM-camouflaged Fe3O4 magnetic NPs (PLTM-MNs) that inherited tumor targeting molecules from PLTM and the photothermal conversion and magnetic reactions of the Fe3O4 NPs [103]. In a mouse model, PLTM-MNs had similar blood retention to RBCM-MNs within 48 h. More importantly, PLTM-MNs uptake was lower in the liver and spleen rich in RES and macrophages compared with MNs and RBCM-MNs, while the amount of internalization was higher in tumor sites. Quantitative measurements of mice tissues by inductively coupled plasma atomic emission spectrometers showed PLTM-MNs had a lower uptake and better tumor-targeting ability. The results showed that PLTM could be used to enhance PTT in cancer therapy. The tumor-bearing mice injected with PLTM-MNs and exposing laser irradiation regardless of external magnetic field (MF) exhibited the highest tumor temperature increase from 34.4 to 56.1 ℃ (Fig. 12c). Moreover, the T2-weighted MR images confirmed that PLTM-MNs possessed better tumor accumulation capability than other groups, which indicated that it was able to run MRI examination to achieve personalized diagnosis and treatment of tumors (Fig. 12d) [32].
Liu et al. did experiments with gold nanoclusters wrapped with PLTM, which took advantage of specific adhesion to injured vessels and tumor tissues of PLTM [77]. The results showed that the PLTM@AuNRs system circulatory performance of the 48 h was more stable and lasting than the exposed AuNRs in vivo, while the major organ microphysiological systems accumulated less. Notably, compared with the absence of laser irradiation, the tumor site accumulated more AuNRs after laser irradiation, which may be explained as that the PLTM@AuNRs had self-healing ability of the tumor injury site by active targeting. Therefore, after each treatment, the maximum tumor temperature of PLTM@AuNRs treated mice increased continuously, and positive feedback appeared through this self-reinforcing characteristic of PLTM@AuNRs, resulting in an enhanced PTT effect, which was proved by the representative in vivo IR thermal images before and after each treatment (Fig. 12e). This work provided a new angle on the design of biomimetic PLTM-INPs for personalized diagnosis and therapy of various diseases with injured vessels. However, controlling temperature increase to avoid burning normal tissues is a tricky problem since INPs that have a photothermal property can also accumulate apart from tissues with injured vessels.
Apart from encapsulating small-molecule anti-cancer INPs, such as DOX and Fe3O4 NPs, PLTM-INPs were also utilized for delivering gene to achieve gene therapy for cancer, such as using small interfering RNA (siRNA) to silence tumor-relevant genes. In one design, Zhuang et al. loaded the synthesized siRNA into zeolite imidazole ester skeleton-8 (zif-8) of the porous metal–organic skeleton (MOF) INPs and then coated PLTM onto this inorganic nanoplatform to form P-MOF-siRNA (Fig. 13a) [104]. The P-MOF-siRNA showed the lowest immunogenicity, and a binding test showed the highest affinity to tumor cells than the RBC membrane-camouflaged NPs (R-MOF-siRNA). The nude mice treated with P-MOF-siRNA exhibited a more robust tumor growth inhibition rate and a higher survival rate than those treated with bare INPs (Fig. 13b–d). Overall, Zhuang et al. successfully construct a biomimetic nanoplatform for effective siRNA delivery. In consideration of long-term toxicity use in human patients, the nanoplatform utilizing cell membrane-camouflaged MOF as delivery vehicles could help expand the field of nucleic acid-based therapies, such as immune modulation and gene therapy, since any kind of RNA molecule can be easily loaded into the porous MOF. It is also worth mentioning that multiple anti-cancer nanomedicines could be encapsulated into the same MOF to achieve multifunctions.
a Platelet membrane–coated siRNA-loaded MOFs (P-MOF-siRNA) for gene silencing. b Growth kinetics of SK-BR-3 tumors implanted subcutaneously into nu/nu mice and treated intravenously with P-MOF-siRNA or R-MOF-siRNA every 3 days for a total of four administrations (n = 5; mean ± SEM). c Survival of the mice over time (n = 5). d Body weight of the mice in (a) over time (n = 5; mean ± SD). Reprinted with permission from Refs [104]
PLTM-INPs can not only increase tumor sites accumulation with the ability of CTC-targeting, but also reduce systemic toxicity. With rich sources, low cost, simple extraction, and low immunogenicity, PLTM-INPs have the potential to be one of the best candidates for the biomimetic system for cancer therapy.
Hybrid cell membrane-camouflaged inorganic nanoparticles (HCM-INPs)
After the previous discussion, cell membrane-camouflaged INPs possess various unique characteristics, but in some circumstances, the single-cell membrane-camouflaged INPs are not enough to meet ideal demands. For instance, the RBCM cannot target the tumor for lack of associated tumor adhesive molecules [100]. Compared with the single-cell membrane-camouflaged INPs, the HCM-INPs concentrate multiple functions from different source cells on one platform. It is a relatively new method that gives INPs specialized functions. It can inherit the targeting ability of CCM, as well as the immune evasion ability of RBC. New functions can be developed through the combination of different types of cell membranes. And on account of different tumors, HMC-INPs can enable the implementation of personalized therapies. Since the first synthesis of HCM-INPs by Zhang’s group, there have been many HCM-INPs platforms getting designed for different aspects [104,105,106].
Wang et al. proposed CuS NPs that were coated by fusing RBCM with B16-F10 CCM (CuS@[RBC-B16]NPs) [70]. CuS exhibits excellent drug loading efficiency and the ability of photothermal conversion, which has the potential to become a multifunctional platform. It showed highly specific self-recognition and visible longer circulatory time after being camouflaged by the RBC-B16 membrane (Fig. 14a). To characterize the HCM, a Förster resonance energy transfer (FRET) pair dyes, DiD and DiI were used for indicating the fusion of the two kinds of cell membranes. With the addition of the RBCM, the fluorescence intensity changed accordingly. The two dyes become scattered with the fusion of the RBCM (Fig. 14b, c). As shown in Fig. 14d, the gp100 (Characteristic molecules of B16-F10 membrane) and CD47 (Characteristic molecules of RBCM) could be found in the [RBC-B16] membrane and CuS@[RBC-B16] NPs groups making use of the Western Blot analysis. CuS@[RBC-B16] NPs showed superior efficiency and persistence in photothermal conversion (Fig. 14e). As shown in Fig. 14f, g, after being incubated with DiI-dyed CuS@[RBC-B16] NPs, the B16 showed higher fluorescence intensity than other cells in flow cytometric profiles. This study gave a new insight to design personalized anticancer nanoplatform by combining RBCMs with homotypic CCMs to coat the surface of the INPs. This work gives an inspiring idea and method of hybridizing different types pf cell membranes to achieve combination of multiple biological functions, and a series of strategies were shown to characterize HCM-INPs, providing guidance for the following research on HCM-INPs. Since RBCM is easy and cheap to require, more attempts can carry out to hyrid RBCM with various natural cell membranes to endow CMCINPs with a variety of biological functions.
a The process of membrane fusion and the hybrid membrane was coated on the CuS NPs and the brief principle of tumor treatment. b FRET pair dyes of DiD and DiI, or single DiD or DiI, were employed to label the B16-F10 cell membrane. c The FRET with the addition of RBCM. d Western blot protein analysis of CD47 and gp120 in different systems. e Infrared thermal imaging of water and CuS@[RBC-B16]. Flow cytometric profiles (f and g) Mean fluorescence intensity of the four cell lines B16-F10, HT1080, NHDF, A549 upon 4 h incubation with DiI dyed CuS@[RBC-B16] NPs. Reprinted with permission from Refs [70]
The Fe3O4 NPs are frequently used and clinically acceptable to conduct the PTT [107]. However, the odd is that the Fe3O4 NPs accumulate little in the tumor due to the immune clearance and lack of the ability to target. Moreover, the first injection of INPs will cause a quick clearance of the following injection, and this circumstance is called the accelerated blood clearance phenomenon (ABC phenomenon). To deal with these defects, Bu et al. developed hybrid cancer stem cell-platelet membrane-camouflaged Fe3O4 NPs ([CSC-P]MNs) as shown in Fig. 15a to solve the ABC phenomenon during the treatment of the head and neck squamous cell carcinoma [108]. The detection of the Fe can reflect the circulation time of Fe3O4 NPs. As shown in Fig. 15b, d, the circulation time of [CSC-P]MNs was longer after the second injection compared with other groups, and in Fig. 15c, e, the accumulation of [CSC-MNs] in the liver and spleen changed little, which indicated that the ABC phenomenon was mitigated. As shown in Fig. 15f, g, through the cellular uptake experiments, the CAL27 cancer cells emerged with a higher uptake rate for [CSC-P]MNs compared with RAW246.7 macrophage-like cells, reflecting that [CSC-P]MNs had a good tumor-targeting ability. In conclusion, the [CSC-P]MNs were reliable HCM-camouflaged nanoplatforms for immune evasion, magnetic resonance imaging, tumor targeting, and PTT. The combination of platelet and cancer stem cell membrane can overcome multiple defects of INPs at the same time, such as the ABC phenomenon, ensuring INPs play a durable and efficient function. However, determining the optimal mass ratio of different cell membranes to realize the most efficient functions of HCM-INPs is a time-consuming process. Meanwhile, the reconstruction of HCM might change the distribution of the surface cell membrane proteins, which might influence its functions.
a The synthesis process of [CSC-P]MNs and the principle of treatment. b The clearance after the first injection. c The accumulation amount of different NPs in different organs after the first injection. d The clearance rate of different NPs after the second injection. e The accumulation in different organs after the second injection. f The Fe content in RAW264.7 macrophage-like cells in cellular uptake experiment. g The Fe content in CAL27 cancer cells in cellular uptake experiment. Reprinted with permission from Refs [108]
Conclusion and perspective
This review highlights the recent advances in CMCINPs for cancer treatment and several strategies to prepare the CMCINPs, which are summarized in Table 2. Compared with the bare inorganic cores, the CMCINPs overcame many challenges, including fast immune clearance, limited targeting ability, toxicity to the human body, and essentially retaining the features of INPs, which could realize the personalized and efficient tumor treatment with fewer side effects. After coating with the different cell membranes, the INPs can also inherit various functions of the cell membranes. The RBCM-INPs can prolong blood circulation time, reduce RES uptake ratio, and escape immune clearance. The MSCM-INPs can target the tumor and damaged tissue. The CCM-INPs have been widely used for homogeneous tumor targeting. The WBCM-INPs have been proven to have the ability of inflammation sites targeting, specific tumor-targeting, detection of cancer cells, and transendothelial migration. The PLTM-INPs have been reported to apply in drug delivery, detoxification, and CTC-targeting. The HCM-INPs can fuse different cell membranes’ functions to construct a versatile therapeutic platform.
To the best of our knowledge, there are a series of clinical trials based on cell-derived vesicles are underway, such as a Phase I clinical trial from 2011 investigating the ability of plant exosomes to deliver curcumin to colon tumors and normal colon tissue (NCT01294072), and a Phase II clinical trials from 2013 recruiting 30 malignant ascites or pleural effusion patients to evaluate the safety and effectiveness of tumor cell-derived MVs (NCT01854866) [110]. However, none of the clinical trials are about CMCINPs applicated in cancer therapy. Moreover, there are little survey based-on cell membrane-derived vesicles conducted to promote clinical translations for medical applications. Up to now, drug candidates concentrated on cell membrane vesicles barely derived from red blood cells and macrophages, which is applicated for MRSA pneumonia (No. CTI-005), sepsis (No. CTI-111), coronavirus (No. CTI-118), cytokine release syndrome (No. CTI-156), and inflammatory bowel disease (No. CTI-168), which utilizes the surface porous structure acting as a nanosponge to absorb the toxins, bacteria, cytokines, virus, and so on. All these clinical trials have not been come into Phase I study. Though the cell membrane-camouflaged technique has got some developments, there are still some problems unsolved that obstruct the clinical translation of the CMCINPs, and issues primarily focus on cell source, biosafety, mechanisms of action at biointerfaces, the preparation of the CMCINPs, and the optimization of the production process.
The source of the cells is one of the most difficulties which confine the works in the laboratory. For some cell types, it is hard to acquire sufficient cell membrane vesicles from the patient and thus restrict the yield’s development. Moreover, when using the cells from donors, the blood type and the immune rejection are necessary to be considered. There has been scant report about the in vivo reaction between CMCINPs and the human system, such as hemolytic reactions, using the RBCM-INPs with different types of ABO antigen or other CMCINPs with various human leukocyte antigens (HLA). The use of the cancer cell line is also a considerable choice, but the potential carcinogenicity of the nucleic acids might occur since they are not removed thoroughly.
Additionally, the challenges also include biosafety, for which it is hard to make sure all of the cell membrane vesicles are utilized to coat onto INPs, and the uncoated INPs will induce an intense immune reaction so that the high output membrane coating technology is urgently needed. More clinical research, like pharmacokinetics and post-marketing surveillance, need to be conducted with bare inorganic cores.
Most of the current understanding of the mechanism of action of CMCINPs tends to concentrate on the interactions of the surface molecules of the cell membrane, especially the cluster of differentiation or the shadowing of INPs surfaces. Nevertheless, the detailed characterization of the molecules confines the research of the concrete mechanism in plenty of research. Furthermore, whether the interaction between INPs and cell membranes can change the conformation of the molecules or not has not been demonstrated in most research. Moreover, although there are many coating methods, the mechanism of some methods and the concrete parameter control are not clear, which restricts the standardized production. With the development of cell biology, different molecular mechanism and other surface molecules such as carbohydrates should be discussed.
The standardized production process is necessary to achieve large-scale production and clinical translation. The fabrication process of CMCINPs is relatively complex and expensive, and every step should be performed in an aseptic environment to ensure the quality of the products. Meanwhile, the short storage time of CMCINPs makes it challenging to meet the needs of clinical applications. The cell source mentioned above is also one of the challenges. All these factors limit the clinical translation of CMCINPs.
As an emerging technique, the great potential of CMCNIPs in medical care will undoubtedly promote researchers to explore more in the future, and it will be a boon to human health. Even though there are still many difficulties ahead, from the current work progress, we can promise that the CMCINPs have great potential in therapy and diagnosis for cancers, potentially bringing a significant change in current cancer treatment modalities.
Availability of data and materials
Not applicable.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(2018):394–424.
Zhao Y, Li X, Zhang H, Yan M, Jia M, Zhou Q. A transcriptome sequencing study on genome-wide gene expression differences of lung cancer cells modulated by fucoidan. Front Bioeng Biotechnol. 2022;10:844924.
Helmink BA, Khan MAW, Hermann A, Gopalakrishnan V, Wargo JA. The microbiome, cancer, and cancer therapy. Nat Med. 2019;25:377–88.
Li Y, Zheng X, Chu Q. Bio-based nanomaterials for cancer therapy. Nano Today. 2021;38:101134.
Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A, Bray F. Cancer statistics for the year 2020: an overview. Int J Cancer Int J Canc. 2021;149:778–89.
Chen W, Zheng R, Zhang S, Zhao P, Zeng H, Zou X, He J. Annual report on status of cancer in China, 2010. Chin J Cancer Res. 2014;26:48–58.
Mitragotri S, Pa B, Langer R. Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nat Rev Drug Discov. 2014;13:655–72.
Qi H, Yang J, Yu J, Yang L, Shan P, Zhu S, Wang Y, Li P, Wang K, Zhou Q. Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes. Nanotechnol Rev. 2022;11:1511–24.
Chen W, Zheng R, Zeng H, Zhang S, He J. Annual report on status of cancer in China. Chinese J Cancer Res. 2011;27(2015):2–12.
Yu Z, Li Q, Wang J, Yu Y, Wang Y, Zhou Q, Li P. Reactive oxygen species-related nanoparticle toxicity in the biomedical field. Nanoscale Res Lett. 2020;15:115.
Guo F, Yuan C, Huang H, Deng X, Bian Z, Wang D, Dou K, Mei L, Zhou Q. Regulation of T cell responses by nano-hydroxyapatite to mediate the osteogenesis. Front Bioeng Biotechnol. 2022;10:884291.
Yan M, Zhang Y, Wu Z, Li Y, Dou K, Wang B, Wang Y, Zhou Q. Recent progress in advanced biomaterials for long-acting reversible contraception. J Nanobiotechnol. 2022;20:138.
Xu S, Zhou Q, Jiang Z, Wang Y, Yang K, Qiu X, Ji Q. The effect of doxycycline-containing chitosan/carboxymethyl chitosan nanoparticles on NLRP3 inflammasome in periodontal disease. Carbohydr Polym. 2020;237:116163.
Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33:941–51.
Zheng W, Zhou Q, Yuan C. Nanoparticles for oral cancer diagnosis and therapy. Bioinorg Chem Appl. 2021;2021:9977131.
Ji Y, Han Z, Ding H, Xu X, Wang D, Zhu Y, An F, Tang S, Zhang H, Deng J, Zhou Q. Enhanced eradication of bacterial/fungi biofilms by glucose oxidase-modified magnetic nanoparticles as a potential treatment for persistent endodontic infections. ACS Appl Mater Interfaces. 2021;13:17289–99.
Liu X-F, Zhang J, Liu J-J, Zhou Q-H, Liu Z, Hu P-Y, Yuan Z, Ramakrishna S, Yang D-P, Long Y-Z. Bifunctional CuS composite nanofibers via in situ electrospinning for outdoor rapid hemostasis and simultaneous ablating superbug. Chem Eng J. 2020;401: 126096.
Liu X, Ji Y, Du Y, **g X, Zhao Y, Dou K, Yu L, Chu L, Zhou Q, Sun M. A “green” all-organic heterostructure functionalized by self-assembled fullerene small molecule with enhanced photocatalytic activity. Appl Surf Sci. 2022;585: 152738.
Wang Z, Wang X, Wang Y, Zhu Y, Liu X, Zhou Q. NanoZnO-modified titanium implants for enhanced anti-bacterial activity, osteogenesis and corrosion resistance. J Nanobiotechnol. 2021;19:353.
**ong J, Wu M, Chen J, Liu Y, Chen Y, Fan G, Liu Y, Cheng J, Wang Z, Wang S, Liu Y, Zhang W. Cancer-erythrocyte hybrid membrane-camouflaged magnetic nanoparticles with enhanced photothermal-immunotherapy for ovarian cancer. ACS Nano. 2021;15:19756–70.
Jaque D, Richard C, Viana B, Soga K, Liu X, GarcíaSolé J. Inorganic nanoparticles for optical bioimaging. Adv Opt Photonics. 2016;8:1–103.
Alexis F, Pridgen E, Molnar LK, Farokhzad OC. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol Pharm. 2008;5:505–15.
Gref R, Lück M, Quellec P, Marchand M, Dellacherie E, Harnisch S, Blunk T, Müller RH. “Stealth” corona-core nanoparticles surface modified by polyethylene glycol (PEG): influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption. Colloids Surf B Biointerfaces. 2000;18:301–13.
Chapman AP. PEGylated antibodies and antibody fragments for improved therapy: a review. Adv Drug Deliv Rev. 2002;54:531–45.
Lubich C, Allacher P, de la Rosa M, Bauer A, Prenninger T, Horling FM, Siekmann J, Oldenburg J, Scheiflinger F, Reipert BM. The mystery of antibodies against polyethylene glycol (PEG)-What do we Know. Pharm Res. 2016;33:2239–49.
Gao W, Zhang L. Coating nanoparticles with cell membranes for targeted drug delivery. J Drug Target. 2015;23:619–26.
Luk BT, Zhang L. Cell membrane-camouflaged nanoparticles for drug delivery. J Control Release. 2015;220:600–7.
Maeda H. The enhanced permeability and retention (EPR) effect in tumor vascularture: the key role of tumor-selective macromolecular drug targeting. Advan Enzyme Regul. 2001;41:1898–2207.
Fang RH, Jiang Y, Fang JC, Zhang L. Cell membrane-derived nanomaterials for biomedical applications. Biomaterials. 2017;128:69–83.
M. Iero, V. Valenti R FAU - Huber, P. Huber V FAU - Filipazzi, G. Filipazzi P FAU - Parmiani, S. Parmiani G FAU - Fais, L. Fais S FAU - Rivoltini, L. Rivoltini, Tumour-released exosomes and their implications in cancer immunity. Cell Death Differ. 15 (2008) 80–88.
Azmi AS, Bao B, Sarkar FH. Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review. Cancer Metastasis Rev. 2013;32:623–42.
Xu C-H, Ye P-J, Zhou Y-C, He D-X, Wei H, Yu C-Y. Cell membrane-camouflaged nanoparticles as drug carriers for cancer therapy. Acta Biomater. 2020;105:1–14.
Zhai Y, Su J, Ran W, Zhang P, Yin Q, Zhang Z, Yu H, Li Y. Preparation and application of cell membrane-camouflaged nanoparticles for cancer therapy. Theranostics. 2017;7:2575–92.
Parodi A, Quattrocchi N, van de Ven AL, Chiappini C, Evangelopoulos M, Martinez JO, Brown BS, Khaled SZ, Yazdi IK, Enzo MV, Isenhart L, Ferrari M, Tasciotti E. Synthetic nanoparticles functionalized with biomimetic leukocyte membranes possess cell-like functions. Nat Nanotechnol. 2013;8:61–8.
Arriaga LR, López-Montero I, Monroy F, Orts-Gil G, Farago B, Hellweg T. Stiffening effect of cholesterol on disordered lipid phases: a combined neutron spin echo + dynamic light scattering analysis of the bending elasticity of large unilamellar vesicles. Biophys J. 2009;96:3629–37.
An X, Salomao M, Guo X, Gratzer W, Mohandas N. Tropomyosin modulates erythrocyte membrane stability. Blood. 2007;109:1284–8.
Guo P, Huang J, Zhao Y, Martin CR, Zare RN, Moses MA. Nanomaterial preparation by extrusion through nanoporous membranes. Small. 2018;14:e1703493.
Chugh V, Vijaya Krishna K, Pandit A. Cell membrane-coated mimics: a methodological approach for fabrication, characterization for therapeutic applications, and challenges for clinical translation. ACS Nano. 2021;15:17080–123.
Chen HY, Deng J, Wang Y, Wu CQ, Li X, Dai HW. Hybrid cell membrane-coated nanoparticles: a multifunctional biomimetic platform for cancer diagnosis and therapy. Acta Biomater. 2020;112:1–13.
Zou S, Wang B, Wang C, Wang Q, Zhang L. Cell membrane-coated nanoparticles: research advances. Nanomedicine. 2020;15:625–41.
Hu C-MJ, Zhang L, Aryal S, Cheung C, Fang RH, Zhang L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc Natl Acad Sci USA. 2011;108:10980–5.
Godfrin Y, Horand R F FAU - Franco, E. Franco R FAU - Dufour, E. Dufour E FAU - Kosenko, B.E. Kosenko E FAU - Bax, A. Bax BE FAU - Banz, O.A. Banz A FAU - Skorokhod, J.M. Skorokhod OA FAU - Lanao, V. Lanao JM FAU - Vitvitsky, E. Vitvitsky V FAU - Sinauridze, V. Sinauridze E FAU - Bourgeaux, K.C. Bourgeaux V FAU - Gunter, G. KC, International seminar on the red blood cells as vehicles for drugs. Expert Opin Biol Ther. 12 (2012) 127–133.
Gutiérrez Millán C, Colino Gandarillas CI, Sayalero Marinero ML, Lanao JM. Cell-based drug-delivery platforms. Ther Deliv. 2011;3:25–41.
Hu C-MJ, Fang RH, Zhang L. Erythrocyte-inspired delivery systems. Adv Healthc Mater. 2012;1:537–47.
Hao R, **ng R, Xu Z, Hou Y, Gao S, Sun S. Synthesis, functionalization, and biomedical applications of multifunctional magnetic nanoparticles. NanoBiotechnology. 2010;22:2729–42.
Shen S, Wang S, Zheng R, Zhu X, Jiang X, Fu D, Yang W. Magnetic nanoparticle clusters for photothermal therapy with near-infrared irradiation. Biomaterials. 2015;39:67–64.
Rao L, Xu J-H, Cai B, Liu H, Li M, Jia Y, **ao L, Guo S-S, Liu W, Zhao X-Z. Synthetic nanoparticles camouflaged with biomimetic erythrocyte membranes for reduced reticuloendothelial system uptake. Nanotechnology. 2016;27:85106.
Rao L, Cai B, Bu L-L, Liao Q-Q, Guo S-S, Zhao X-Z, Dong W-F, Liu W. Microfluidic electroporation-facilitated synthesis of erythrocyte membrane-coated magnetic nanoparticles for enhanced imaging-guided cancer therapy. ACS Nano. 2017;11:3496–505.
Yang M, Peng B, Zhuang Q, Li J, Liu H, Cheng K, Ming Y. Facile preparation of multifunctional upconversion nanoprobes for multimodal imaging and dual-targeted photothermal therapy Angew. Chemie-International Ed. 2011;50:7385–90.
Ke PC, Lin S, Parak WJ, Davis TP, Caruso F. A Decade of the Protein Corona. ACS Nano. 2017;11:11773–6.
Ding H, Lv D Y FAU–Ni J, Ni D FAU–Wang Z. Wang J FAU–Tian, W. Tian Z FAU–Wei, G. Wei W FAU–Ma, G. Ma, Erythrocyte membrane-coated NIR-triggered biomimetic nanovectors with programmed delivery for photodynamic therapy of cancer, Nanoscale. 7 (2015) 9806–9815.
Martínez-Carmona M, Ho QP, Morand J, García A, Ortega E, Erthal LCS, RuizHernandez E, Santana MD, Ruiz J, ValletRegí M, Gunko YK. Amino-functionalized mesoporous silica nanoparticle-encapsulated octahedral organoruthenium complex as an efficient platform for combatting cancer. Inorg Chem. 2020;59:10275–84.
Kulyk K, Azizova L, Cunningham JM, Mikhalovska L, Borysenko M, Mikhalovsky S. Nanosized copper(ii) oxide/silica for catalytic generation of nitric oxide from S-nitrosothiols. J Mater Chem B. 2020;8:4267–77.
Takahashi T, Yamada Y, Kataoka K, Nagasaki Y. Preparation of a novel PEG–clay hybrid as a DDS material: Dispersion stability and sustained release profiles. J Control Release. 2005;107:408–16.
Can NO, Arli G, Lafci Y. A novel RP-HPLC method for simultaneous determination of potassium sorbate and sodium benzoate in soft drinks using C 18-bonded monolithic silica column. J Sep Sci. 2011;34:2214–22.
Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev. 2013;65:36–48.
Rosenholm JM, Mamaeva V, Sahlgren C, Lindén M. Nanoparticles in targeted cancer therapy: mesoporous silica nanoparticles entering preclinical development stage. Nanomedicine. 2011;7:111–20.
Zhao Y, Luo M Z FAU–Li Q, Li M FAU–Qu X. Qu Q FAU–Ma, S-H. Ma X FAU–Yu, Y. Yu SH FAU–Zhao, Y. Zhao. A preloaded amorphous calcium carbonate/doxorubicin@silica nanoreactor for pH-responsive delivery of an anticancer drug., Angew Chem Int Ed Engl. 2015; 54: 919–922.
Su J, Sun H, Meng Q, Zhang P, Yin Q, Li Y. Enhanced blood suspensibility and laser-activated tumor-specific drug release of theranostic mesoporous silica nanoparticles by functionalizing with erythrocyte membranes. Theranostics. 2017;7:523–37.
Fang RH, Hu C-MJ, Chen KNH, Luk BT, Carpenter CW, Gao W, Li S, Zhang D-E, Lu W, Zhang L. Lipid-insertion enables targeting functionalization of erythrocyte membrane-cloaked nanoparticles. Nanoscale. 2013;5:8884–8.
Maeda H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv Drug Deliv Rev. 2015;91:3–6.
Dash P, Piras AM, Dash M. Cell membrane coated nanocarriers - an efficient biomimetic platform for targeted therapy. J Control Release. 2020;327:546–70.
Zhu J-Y, Zheng D-W, Zhang M-K, Yu W-Y, Qiu W-X, Hu J-J, Feng J, Zhang X-Z. Preferential cancer cell self-recognition and tumor self-targeting by coating nanoparticles with homotypic cancer cell membranes. Nano Lett. 2016;16:5895–901.
Sun H, Su J, Meng Q, Yin Q, Chen L, Gu W, Zhang Z, Yu H, Zhang P, Wang S, Li Y. Cancer cell membrane-coated gold nanocages with hyperthermia-triggered drug release and homotypic target inhibit growth and metastasis of breast cancer. Adv Funct Mater. 2017;27:1604300.
Sun Q, Wu J, ** L, Hong L, Wang F, Mao Z, Wu M. 0000–0001–7990–2856, Cancer cell membrane-coated gold nanorods for photothermal therapy and radiotherapy on oral squamous cancer. J Mater Chem B. 2020;8:7253–63.
Yu Z, Zhou P, Pan W, Li N, Tang B. A biomimetic nanoreactor for synergistic chemiexcited photodynamic therapy and starvation therapy against tumor metastasis. Nat Commun. 2018;9:5044.
Fang RH, Hu C-MJ, Luk BT, Gao W, Copp JA, Tai Y, O’Connor DE, Zhang L. Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery. Nano Lett. 2014;14:2181–8.
Kroll AV, Fang RH, Jiang Y, Zhou J, Wei X, Yu CL, Gao J, Luk BT, Dehaini D, Gao W, Zhang L. Nanoparticulate delivery of cancer cell membrane elicits multiantigenic antitumor immunity. Adv Mater. 2017;29:1703969.
Rao L, Bu L-L, Cai B, Xu J-H, Li A, Zhang W-F, Sun Z-J, Guo S-S, Liu W, Wang T-H, Zhao X-Z. Cancer cell membrane-coated upconversion nanoprobes for highly specific tumor imaging. Adv Mater. 2016;28:3460–6.
Wang D, Dong H, Li M, Cao Y, Yang F, Zhang K, Dai W, Wang C, Zhang X. Erythrocyte-cancer hybrid membrane camouflaged hollow copper sulfide nanoparticles for prolonged circulation life and homotypic-targeting photothermal/chemotherapy of melanoma. ACS Nano. 2018;12:5241–52.
Li S-Y, Cheng H, **e B-R, Qiu W-X, Zeng J-Y, Li C-X, Wan S-S, Zhang L, Liu W-L, Zhang X-Z. Cancer cell membrane camouflaged cascade bioreactor for cancer targeted starvation and photodynamic therapy. ACS Nano. 2017;11:7006–18.
Liu C-M, Chen G-B, Chen H-H, Zhang J-B, Li H-Z, Sheng M-X, Weng W-B, Guo S-M. Cancer cell membrane-cloaked mesoporous silica nanoparticles with a pH-sensitive gatekeeper for cancer treatment. Colloids Surfaces B Biointerfaces. 2019;175:477–86.
Nie D, Dai Z, Li J, Yang Y, ** Z, Wang J, Zhang W, Qian K, Guo S, Zhu C, Wang R, Li Y, Yu M, Zhang X, Shi X, Gan Y. Cancer-cell-membrane-coated nanoparticles with a yolk-shell structure augment cancer chemotherapy. Nano Lett. 2020;20:936–46.
Pan W, Ge Y, Yu Z, Zhou P, Cui B, Li N, Tang B. A cancer cell membrane-encapsulated MnO2 nanoreactor for combined photodynamic-starvation therapy. Chem Commun. 2019;55:5115–8.
Li J, Wang X, Zheng D, Lin X, Wei Z, Zhang D, Li Z, Zhang Y, Wu M, Liu X. Cancer cell membrane-coated magnetic nanoparticles for MR/NIR fluorescence dual-modal imaging and photodynamic therapy. Biomater Sci. 2018;6:1834–45.
Pittenger MF, Discher DE, Péault BM, Phinney DG, Hare JM, Caplan AI. Mesenchymal stem cell perspective: cell biology to clinical progress. Npj Regen Med. 2019;4:22.
Zhen X, Cheng P, Pu K. Recent advances in cell membrane-camouflaged nanoparticles for cancer phototherapy. Small. 2019;15:1–19.
Hass R, Kasper C, Böhm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011;9:12.
Lindvall O, Kokaia Z, Martinez-Serrano A. Stem cell therapy for human neurodegenerative disorders–how to make it work. Nat Med. 2004;10:S42–50.
Leibacher J, Henschler R. Biodistribution, migration and homing of systemically applied mesenchymal stem/stromal cells. Stem Cell Res Ther. 2016;7:7.
Gao C, Lin Z, Wu Z, Lin X, He Q. Stem-Cell-Membrane camouflaging on near-infrared photoactivated upconversion nanoarchitectures for in vivo remote-controlled photodynamic therapy. ACS Appl Mater Interfaces. 2016;8:34252–60.
Lai PY, Huang RY, Lin SY, Lin YH, Chang CW. Biomimetic stem cell membrane-camouflaged iron oxide nanoparticles for theranostic applications. RSC Adv. 2015;5:98222–30.
Murata M. Inflammation and cancer. Environ Health Prev Med. 2018;23:1–8.
Fang RH, Kroll AV, Gao W, Zhang L. Cell membrane coating nanotechnology. Adv Mater. 2018;30:1–34.
Xuan M, Shao J, Dai L, He Q, Li J. Macrophage cell membrane camouflaged mesoporous silica nanocapsules for in vivo cancer therapy. Adv Healthc Mater. 2015;4:1645–52.
Xuan M, Shao J, Dai L, Li J, He Q. Macrophage cell membrane Camouflaged Au nanoshells for in vivo prolonged circulation life and enhanced cancer photothermal therapy. ACS Appl Mater Interfaces. 2016;8:9610–8.
Ariga K, Li J, Fei J, Ji Q, Hill JP. Nanoarchitectonics for dynamic functional materials from atomic-/molecular-level manipulation to macroscopic action. Adv Mater. 2016;28:1251–86.
Yan J, Malakooti MH, Lu Z, Wang Z, Kazem N, Pan C, Bockstaller MR, Majidi C, Matyjaszewski K. Solution processable liquid metal nanodroplets by surface-initiated atom transfer radical polymerization. Nat Nanotechnol. 2019;14:684–90.
Dickey MD. Stretchable and soft electronics using liquid metals. Adv Mater. 2017;29:1606425.
Gao C, Lin Z, Lin X, He Q. Cell Membrane-camouflaged colloid motors for biomedical applications. Adv Ther. 2018;1:1–15.
Wang D, Gao C, Zhou C, Lin Z, He Q. Leukocyte membrane-coated liquid metal nanoswimmers for actively targeted delivery and synergistic chemophotothermal therapy. Research. 2020;2020:1–10.
Marx V. Targeted proteomics. Nat Methods. 2013;10:19–22.
Alix-Panabières C, Pantel K. Challenges in circulating tumour cell research. Nat Rev Cancer. 2014;14:623–31.
Fierer JO, Veggiani G, Howarth M. SpyLigase peptide–peptide ligation polymerizes affibodies to enhance magnetic cancer cell capture. Proc Natl Acad Sci. 2014;111:E1176–81.
Rodriguez PL, Harada T, Christian DA, Pantano DA, Tsai RK, Discher DE. Minimal “Self” peptides that inhibit phagocytic clearance and enhance delivery of nanoparticles. Science. 2013;339:971–5.
**ong K, Wei W, ** Y, Wang S, Zhao D, Wang S, Gao X, Qiao C, Yue H, Ma G, **e HY. Biomimetic immuno-magnetosomes for high-performance enrichment of circulating tumor cells. Adv Mater. 2016;28:7929–35.
Dovizio M, Ballerini P, Fullone R, Tacconelli S, Contursi A, Patrignani P. Multifaceted functions of platelets in cancer: from tumorigenesis to liquid biopsy tool and drug delivery system. Int J Mol Sci. 2020;21:9585.
Plantureux L, Mège D, Crescence L, Dignat-George F, Dubois C, Panicot-Dubois L. Impacts of cancer on platelet production, activation and education and mechanisms of cancer-associated thrombosis. Cancers. 2018;10:441.
Wang S, Li Z, Xu R. Human Cancer and Platelet Interaction, a Potential Therapeutic Target. Int J Mol Sci. 2018;19:1246.
Spanjers JM, Städler B. Cell membrane coated particles. Adv Biosyst. 2020;4:1246.
Wang S, Duan Y, Zhang Q, Komarla A, Gong H, Gao W, Zhang L. Drug targeting via platelet membrane-coated nanoparticles. Small Struct. 2020;1:2000018.
Pei W, Huang B, Chen S, Wang L, Xu Y, Niu C. Platelet-mimicking drug delivery nanoparticles for enhanced chemo-photothermal therapy of breast cancer. Int J Nanomedicine. 2020;15:10151–67.
Rao L, Bu L-L, Meng Q-F, Cai B, Deng W-W, Li A, Li K, Guo S-S, Zhang W-F, Liu W, Sun Z-J, Zhao X-Z. Antitumor Platelet-Mimicking Magnetic Nanoparticles. Adv Funct Mater. 2017;27:1604774.
Zhuang J, Gong H, Zhou J, Zhang Q, Gao W, Fang RH, Zhang L. Targeted gene silencing in vivo by platelet membrane–coated metal-organic framework nanoparticles. Sci Adv. 2022;6:eaaz6108.
Gong C, Yu X, You B, Wu Y, Wang R, Han L, Wang Y, Gao S, Yuan Y. Macrophage-cancer hybrid membrane-coated nanoparticles for targeting lung metastasis in breast cancer therapy. NanoBiotechnology. 2020;18:1–7.
Dehaini D, Wei X, Fang RH, Masson S, Angsantikul P, Luk BT, Zhang Y, Ying M, Jiang Y, Kroll AV, Gao W. Erythrocyte-platelet hybrid membrane coating for enhanced nanoparticle functionalization. Adv Mater. 2017;29:1606209.
Chakravarty R, Valdovinos F HF FAU - Chen, CM Chen F FAU - Lewis, PA, Lewis CM FAU - Ellison, H Ellison PA FAU - Luo, ME Luo H FAU - Meyerand, RJ. Meyerand ME FAU - Nickles, W. Nickles RJ FAU - Cai, W. Cai, Intrinsically germanium-69-labeled iron oxide nanoparticles: synthesis and in-vivo dual-modality PET/MR imaging., (n.d.).
Bu LL, Rao L, Yu GT, Chen L, Deng WW, Liu JF, Wu H, Meng QF, Guo SS, Zhao XZ, Zhang WF, Chen G, Gu Z, Liu W, Sun ZJ. Cancer stem cell-platelet hybrid membrane-coated magnetic nanoparticles for enhanced photothermal therapy of head and neck squamous cell carcinoma. Adv Funct Mater. 2019;29:1–11.
Piao J-G, Wang L, Gao F, You Y-Z, **ong Y, Yang L. Erythrocyte membrane is an alternative coating to polyethylene glycol for prolonging the circulation lifetime of gold nanocages for photothermal therapy. ACS Nano. 2014;8:10414–25.
Chen L, Hong W, Ren W, Xu T, Qian Z, He Z. Recent progress in targeted delivery vectors based on biomimetic nanoparticles. Signal Transduct Target Ther. 2021;6:225.
Acknowledgements
Not applicable.
Funding
The authors are very grateful for the financial support by National Natural Science Foundation of China (Grant No. 31900957, 52103170), Shandong Provincial Natural Science Foundation (Grant No. ZR2019QC007, ZR2019BC020), Innovation and technology program for the excellent youth scholars of higher education of Shandong province (Grant No. 2019KJE015), Traditional Chinese Medicine Science and Technology Project of Shandong province (Grant No. 2021Q069), and National College Students Innovation and Entrepreneurship Training Program of China (S202011065041).
Author information
Authors and Affiliations
Contributions
WS, FJ, TZ, and FL wrote different sections of the original draft; LL, KD, QZ, and ZH edited the manuscript; YR, JX, and visualized the figures. QZ and QZ contributed to the conceptualization, revised the original draft, provided funding and supervision. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors have approved the final draft of this manuscript for submission and have given consent for the publication of identifiable details.
Competing interests
The authors declare no competing financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Song, W., Jia, P., Zhang, T. et al. Cell membrane-camouflaged inorganic nanoparticles for cancer therapy. J Nanobiotechnol 20, 289 (2022). https://doi.org/10.1186/s12951-022-01475-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12951-022-01475-w