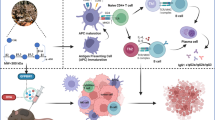
Abstract
Background
Poly(D, L-lactic-co-glycolic acid) (PLGA) nanoparticles have potential applications as a vaccine adjuvant and delivery system due to its unique advantages as biodegradability and biocompatibility.
Experimental
We fabricated cationic solid lipid nanoparticles using PLGA and dimethyl-dioctadecyl-ammonium bromide (DDAB), followed by loading of model antigen OVA (antigen ovalbumin, OVA257-264) to form an OVA@DDAB/PLGA nano-vaccine. And we investigated the intracellular signaling pathway in dendritic cells in vitro and antigen transport pathway and immune response in vivo mediated by an OVA@DDAB/PLGA nano-vaccine.
Results
In vitro experiments revealed that the antigen uptake of BMDCs after nanovaccine incubation was two times higher than pure OVA or OVA@Al at 12 h. The BMDCs were well activated by p38 MAPK signaling pathway. Furthermore, the nano-vaccine induced antigen escape from lysosome into cytoplasm with 10 times increased cross-presentation activity than those of OVA or OVA@Al. Regarding the transport of antigen into draining lymph nodes (LNs), the nano-vaccine could rapidly transfer antigen to LNs by passive lymphatic drainage and active DC transport. The antigen+ cells in inguinal/popliteal LNs for the nano-vaccine were increased over two folds comparing to OVA@Al and OVA at 12 h. Moreover, the antigen of nano-vaccine stayed in LNs for over 7 days, germinal center formation over two folds higher than those of OVA@Al and OVA. After immunization, the nano-vaccine induced a much higher ratio of IgG2c/IgG1 than OVA@Al. It also effectively activated CD4+ T, CD8+ T and B cells for immune memory with a strong cellular response.
Conclusion
These results indicated that DDAB/PLGA NP was a potent platform to improve vaccine immunogenicity by p38 signaling pathway in BMDCs, enhancing transport of antigens to LNs, and higher immunity response.
Graphical Abstract

Similar content being viewed by others
Introduction
Vaccination plays a crucial role in controlling the dissemination of virus and reducing morbidity and mortality [1]. Subunit vaccines of recombinant proteins and epitope peptides are emerging in interest as a safer alternative to traditional vaccines [2,3,4]. However, the ability of these vaccines to elicit a long-lasting and potent immune response is limited because their drawbacks as comparatively lower molecular weights and weaker immunogenicity [5]. The need for adjuvants is urgent and increasing. Al, the most common adjuvant licensed for human use, has been widely employed for about 90 years [6, 7]. Its application is limited by disadvantages such as side effects and anergy to cellular immunity, which drives the development of new delivery system and adjuvants for these subunit vaccines [8, 9].
Biodegradable nanoparticles are being investigated as a delivery vector for vaccines and adjuvant systems in recent years that can boost weak antigen effectiveness by enhancing antigen processing and/or as immune-potentiating adjuvants to induce or potentiate immune responses. These nanoparticles are made of biodegradable materials such as polysaccharides, proteins, fatty acids lipids and polymers [10,4b). Within 24 h after OVA injection, pure antigen showed a rapid decline at the injection site, which easily entered the lymphatic circulation and quickly spread to the spleen, kidney, liver, and other major organs within 4 h (Fig. 4b, c). As time progressed the antigen distribution in major organs gradually decreased or even cleared. In contrast the OVA@DDAB/PLGA Nv groups showed the excellent ability of efficient antigen delivery to draining LNs. The radioactive signals of proximal LNs as inguinal LNs and popliteal LNs remained strong at 48 h post-injection (Fig. 4b). As time progressed the antigen mainly distributed into the spleen with trace found in the liver, kidneys. What is notable is that even 14 days post-injection antigens were still present in these locations. It was found that the OVA@DDAB/PLGA Nv at the injection site could be transported faster than OVA@Al, but slower than OVA (Fig. 4c). The OVA@DDAB/PLGA Nv not only formed a short-term "antigen repository" for antigen delivery, but also enhanced antigen transportation to LNs. Both of these effects are favorable for inducing an immune response. The Fig. 4d shows the diagram of internal organs in mice.
The biodistribution results indicated that the OVA@DDAB/PLGA Nv could effectively deliver antigen to secondary lymphoid organs such as the spleen and draining lymph nodes. Therefore, we further quantitatively studied the dynamic antigen biodistribution for different formulations in vivo. After intramuscular injection with 89Zr-OVA, 89Zr-OVA@Al or 89Zr-OVA@DDAB/PLGA Nv, the primary and secondary lymphoid tissues of mice were isolated at 0.5, 3, 6, 12, 24, 48, and 72 h to monitor radioactivity for calculating the biodistribution value (Fig. 5a; %ID/g: antigen uptake rate per gram of tissue at different times). The results showed that pure antigen (OVA) could be rapidly transported from the injection site to spleen with antigen uptake peaking at 6 h post injection (Fig. 5b, c). 89Zr-OVA@Al showed similar rapid antigen delivery to the spleen and peaking 6 h post injection as well (Fig. 5c). In contrast 89Zr-OVA@DDAB/PLGA Nv exhibited delayed antigen delivery to spleen with uptake peaking at 12 h post injection. A notable phenomenon is that 89Zr-OVA@DDAB/PLGA Nv showed two antigen transport peaks at 3 h and 12 h in draining LNs while 89Zr-OVA and 89Zr-OVA@Al only had a single peak at 12 h (Fig. 5d–f). Furthermore, antigen transported to the popliteal and inguinal LNs via the 89Zr-OVA@DDAB/PLGA Nv were maintained there for more than 72 h, while the antigen signal from 89Zr-OVA and 89Zr-OVA@Al dropped down to a very low level after 12 h (Fig. 5d, e). 89Zr-OVA@DDAB/PLGA Nv not only enhanced antigen delivery to proximal LNs, but also greatly promoted antigen transport to distal LNs such as cervical LNs (Fig. 5f). According these results, we hypothesize that 89Zr-OVA@DDAB/PLGA Nv transported antigen through two pathways: the first was direct transport into draining lymph nodes and the second was indirect transport by immune cells uptake such as DCs.
Dynamic antigen biodistribution of different vaccine formulations in mice. a Experimental design to evaluate the dynamic antigen biodistribution. b–f 89Zr-OVA biodistribution in injection site (b), spleen (c) Popliteal LNs (d), inguinal LNs (e), and cervical LNs (f). The primary organs and secondary lymphoid tissues of mice were isolated at 0.5, 3, 6, 12, 24, 48, and 72 h, and then radioactivity was quantified to calculate %ID/g value (% ID/g: antigen uptake rate per gram of tissue at different times). g immunohistochemical methods quantified the antigen in draining LNs (the brown areas represent the antigen OVA), and stained sections were measured by the automatic multispectral imaging system (PerkinElmer Vectra II), bar = 200 μm. Data are expressed as Mean ± STD (n = 6)
OVA@DDAB/PLGA Nv Induced germinal centers formation in draining LNs and proliferative response of splenocyte
Antigen amounts in popliteal and inguinal LNs measured by flow cytometry also confirmed that OVA@DDAB/PLGA Nv promoted much more two folds antigen transport to lymph nodes as popliteal and inguinal LNs at 12 h than OVA and OVA@Al (Additional file 1: Fig. S5; P < 0.001). Immunohistochemical images suggested that the OVA@DDAB/PLGA Nv showed much higher ability in transferring antigen to LNs than OVA and OVA@Al (Fig. 5g and Additional file 1: Fig. S6). Moreover, antigens presented with the OVA@DDAB/PLGA Nv persisted in the LNs even seven days post immunization. These results implied that OVA@DDAB/PLGA Nv promoted both antigen migration into draining LNs at the early stage and subsequent continuous antigen stimulation to immune cells in LNs.
Maturation and activation of DCs is a prerequisite of antigen presentation, and directly affects the interaction with T cells [24, 28, 29]. The expression of co-stimulatory molecules CD86 on DCs was determined by flow cytometry. OVA@DDAB/PLGA Nv increased the expression of CD86 molecules on DCs in draining LNs (Additional file 1: Fig. S7a; NVs vs pure antigen: p < 0.0001). The expression of MHC I and MHC II on DCs from secondary LNs were also increased, which inducing by OVA@DDAB/PLGA Nv (Additional file 1: Fig. S6b, c). These results indicated that OVA@DDAB/PLGA Nv significantly promoted antigen cross-presentation. Expression of MHC II in the OVA@Al group gradually decreased after 24 h of immunization, while the MHC II in the OVA@DDAB/PLGA Nv group remained high (Additional file 1: Fig. S7c). These higher levels of MHC I and MHC II molecule expression on DCs suggested a stronger MHC-restricted antigen presenting pathway, which was favorable for T cell-mediated immunity. Therefore, these results indicated that the OVA@DDAB/PLGA Nv were superior to OVA@Al for inducing maturation and activation of DCs in secondary lymphocytes.
The OVA@DDAB/PLGA Nv showed great difference in antigen transportation from injection site to LNs and spleen compared to OVA and OVA@Al. Therefore, we further explored whether this difference would have a significant influence on subsequent immune activations. One of the most important functions of vaccines is to induce an effective specific and/or neutralizing antibodies against pathogens, which are mainly related to the level of follicular helper CD4+ T cells (Tfh) and formation of germinal centers represent B cells activation capacity. Flow cytometry was employed to detect the populations of follicular helper CD4+ T cells (CD4+ CXCR5hi PD-lhi) and germinal centers (GL-7hi B220+) in LNs. The OVA@DDAB/PLGA Nv significantly induced more cells populations of germinal centers in LNs over two-fold than OVA and OVA@Al (Fig. 6a and Additional file 1: Fig. S8b; for OVA and OVA@Al, P < 0.0001). And all three groups induced over 40% higher levels of follicular helper CD4+ T cells (Fig. 6b and Additional file 1: Fig. S8c). Immunohistochemical results showed that OVA@DDAB/PLGA Nv induced more germinal centers than the other two groups, notably 28 days post immunization (Fig. 6c and S9). Meanwhile, the proliferation and activation of lymphocytes in the spleen was important for immune system stimulation. The splenocyte from mice injected with OVA@DDAB/PLGA Nv showed much stronger proliferation ability than those with OVA (P < 0.0001) and OVA-Al (P < 0.01) (Fig. 6d-e). These results indicated that the OVA@DDAB/PLGA Nv could effectively promote germinal centers formation and splenocyte proliferation, which would be favorable for an effective immune response.
The OVA@DDAB/PLGA Nv induced the formation of germinal centers in draining LNs and proliferative response of splenocytes from immunized mice. a The number of germinal center (GL-7hi B220+ cells) in draining LNs. b The follicular helper CD4+ T cells (Tfh, CXCR5hi PD-1hi CD4+ T cells) in draining LNs. c Germinal centers in draining LNs determined by immunohistochemical staining; The circular brown signal is the germinal center B cell, and the dense circular positive area is the germinal center, which is circular or oval with a diameter of about 0.1 to 1.0 mm, and the bright area can be identified from the outside to the inside, bar = 1.0 mm. d OD450 nm value for immunized mice splenocytes stimulated with OVA in vitro were measured by CCK-8 kit; e Proliferation index of splenocytes. Data were expressed as means ± SEM (n = 6). (**p < 0.01; ***p < 0.001; ****p < 0.0001)
OVA@DDAB/PLGA Nv induced the splenocyte activation and cytokines secretion
As a major indicator of lymphocyte activation in the spleen, the antigen specific CD4+ T, CD8+ T and B cells were evaluated and analyzed by flow cytometry after mice splenocytes were re-stimulated with antigen in vitro. CD69 and CD19 usually serve as the activation marker of T cells and B cells, which was used to measure lymphocyte activation. The mice were immunized three times and the spleen cells were isolated 35 days after the first immunized (Fig. 7a). The immunized splenocytes restimulated with OVA in vitro were employed for measuring CD4+ T cells (CD4+ CD69+ cells), CD8+ T cells (CD8+ CD69+ cells) and B cells (CD19+ CD69+ cells) by flow cytometry. The results suggested that the OVA@DDAB/PLGA Nv could promote more T and B cells activation than OVA and OVA@Al (Fig. 7b–d and Additional file 1: Fig. S10b–d; for CD4+ T cells comparing with OVA, p < 0.01; for CD4+ T cells comparing with OVA@Al, p < 0.05; for CD8+ T cells comparing with OVA, p < 0.001; for CD8+ T cells comparing with OVA@Al, p < 0.01; for CD19+ B cells comparing with OVA, p < 0.0001; for CD19+ B cells comparing with OVA@Al, p < 0.01). These results indicated that the OVA@DDAB/PLGA Nv could induce a strong activation of effector immune cells (T cells and B cells), which was necessary for enhancing system immune response.
OVA@DDAB/PLGA Nv induced splenocyte activation and cytokines secretion. a The schematic treatment schedule of the vaccine immunization. b, c, d The activation of CD4+ T cells (b) CD8+ T cells (c) and B cells (d) from mice after being immunized with different vaccine formulations were measured by flow cytometry. e, f, g, h The secretion of Th1-type cytokine of IFN-γ (e), TNF-α (f) and Th2-type cytokine of IL-6 (g), IL-4 (h) after intramuscular injection with different vaccines formulations were measured by an ELISA kit. i The ratio of IFN-γ to IL-4 secreted by splenocytes immunized with different vaccines. j Granzyme B secretion by splenocytes immunized with different vaccines were measured by an ELISA kit. Data was expressed as Mean ± STD (n = 6). (*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001)
It was reported that cytokines secretion from splenocytes play an essential role in provoking cell-mediated immune response [30]. Th1-type cytokines as IFN-γ, TNF-α and Th2-type cytokines including IL-4, IL-6 were evaluated. It was found that the OVA@DDAB/PLGA Nv significantly promoted more IFN-γ and TNF-α secretion than OVA (P < 0.0001) and OVA@Al (P < 0.01), and improved IL-6 and IL-4 secretion compared to OVA (P < 0.001) and OVA@Al (P < 0.05) (Fig. 7e–h). This implies that DDAB/PLGA nanoparticles also functioned as vaccine adjuvants by promoting Th1 and Th2 immune responses. The IFN-γ/IL-4 ratio was an indicator of the Th1/Th2 propensity of the immune response[31]. The ratio of IFN-γ/IL-4 induced by OVA@DDAB/PLGA Nv was higher than OVA (P < 0.01) and OVA@Al (P < 0.05), which further exhibited that the OVA@DDAB/PLGA Nv were more prone to induce Th1 type immune response (Fig. 7i). Meanwhile, the Nv induced higher levels of Granzyme B (Fig. 7j). The Granzyme B is an exogenous serine protease, which is derived from cytoplasm particles released by CTLs and natural killer cells (NK). Granzyme B plays an important role in cellular immune response, which can induce DNA degradation of target cells as infected cells and then lysis by activating the chain reaction of caspases. Therefore, our analysis indicated that the OVA@DDAB/PLGA Nv increased Th1 type cytokines secretion, which regulated the activation of immune cells and further induced the production of high levels of Granzyme B. In summary, the OVA@DDAB/PLGA Nv could effectively deliver antigens to LNs and spleen to induce a stronger immune response than OVA and OVA@Al, which demonstrated that the OVA@DDAB/PLGA Nv could induced not only cellular immunity from Th1 and Th2 responses, but also humoral immunity from B cells.
OVA@DDAB/PLGA Nv promoted T-cell-mediated immune response and elicited superior antibody response in mice
The ultimate goal of vaccination is to generate immune memory that can rapidly respond to pathogens upon reinfection, and memory T cells are an important components of these memory immune responses [32]. CD44hi CD62Llow and CD44hi CD62Lhi are regarded as markers for effector-memory T cells and central-memory T cells, respectively [32,33,34]. In our study, the OVA@DDAB/PLGA Nv generated significantly more effector T cells of CD4+ T and CD8+ T cells than OVA and OVA@Al (Fig. 8a, b and Additional file 1: Fig. S11b). Meanwhile, the OVA@DDAB/PLGA Nv also increased central-memory T cells of CD4+ T and CD8+ T cells which in theory will ensure a rapid immune response that will protect against reinfection (Fig. 8c, d and Additional file 1: Fig. S11b). In addition, cytotoxic T lymphocytes (CTL), a specific type of T cell, secretes a variety of cytokines and participates in the immune response. Its most important function is the specific killing effect on the pathogens that cause cellular infection [35]. CTL activation promotes the expression of CD107 molecules on the surface of CD8+ T cells [36]. Studies have found that CTL exerts its anti-killing effect mainly through two mechanisms: the first is the release of perforin and granzyme to kill target cells and the second is FasL-mediated cell apoptosis [37]. Analysis of the expression of CTL-related molecules by flow cytometry was an important indicator for evaluating immune response induced by vaccines. Meanwhile, the OVA@DDAB/PLGA Nv increased perforin release from CD8+ T cells compared to OVA (Fig. 8e and Additional file 1: Fig. S12b; P < 0.001). Compared to OVA, the OVA@DDAB/PLGA Nv could significantly up-regulate the CD107 and FasL expression on CD8+ T cells (Fig. 8f-g and Additional file 1: Fig. S12c, d; P < 0.01). These indicated that the OVA@DDAB/PLGA Nv could promote CTL activation and enhance CD107/FasL/perforin-mediated immune killing. In combination with the increasing expression of granzymes, we confirmed that the OVA@DDAB/PLGA Nv mainly relied on the mechanisms of Granzyme B and CD107/FasL/perforin expression to induce CTL responses.
Effects of different vaccines on memory T cell responses, CTL response, and antibody levels. a Effector memory (CD44hi CD62Llow) CD4+ T cells, b Cells of effector memory (CD44hi CD62Llow) CD8+ T cells, c Central memory (CD44hi CD62Lhi) CD4+ T cells, and D Central memory (CD44hi CD62Lhi) CD8+ T cells. The expression of Perforin (e), CD107 (f), and FasL (g) on CD8+ T cell in splenocytes. h Anti-OVA IgG titers in the serum from mice after being immunized with different vaccines for 35 days. i The composition ratio of IgG1 and IgG2c in serum from mice after immunized with different vaccines for 35 days. Data were analyzed by flow cytometry and expressed as Mean ± STD (n = 6). (*p < 0.05; **p < 0.01; ***p < 0.001)
As the main immunoglobulin, Immunoglobulin G (IgG) is a key index to evaluate the level of immune response [38]. IgG1 and IgG2 are the two main subtypes of IgG. IgG2c/IgG1 can reflect the Th1/Th2 tendency in the immune response [32, 39, 40]. In this study, specific antibody titers in serum were detected by ELISA. We measured the antigen-specific IgG, IgG1, and IgG2c titers in serum from immunized mice with different vaccines. The results showed that OVA-specific antibody IgG gradually increased on day 14, 21, 28 and 35, and the IgG titers from OVA@Al and OVA@DDAB/PLGA Nv were much higher than OVA (P < 0.01), indicating that both mice groups exhibited stronger anti-OVA IgG responses (Fig. 8h). The OVA@DDAB/PLGA Nv induced higher IgG levels, while it induced stronger humoral immune response than OVA (for Day 21 and Day 35, P < 0.001; for Day 14 and Day 28, P < 0.01) (Fig. 8h), which suggested that the OVA@DDAB/PLGA Nv could induced humoral immunity. In addition, OVA@DDAB/PLGA Nv significantly increased much higher IgG2c proportion in the total IgG than OVA (P < 0.001) and OVA@Al (P < 0.01) (Fig. 8i), which indicated that the OVA@DDAB/PLGA Nv was capable of enhancing Th1 immune response (cellular immune response).
In addition to the immune effects, we also evaluated the safety of our OVA@DDAB/PLGA Nv delivery system. Blood samples were collected for the biochemistry of urea nitrogen (BUN), aspartate transaminase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), and lactate dehydrogenase (LDH). Among these parameters, the values of BUN, AST and LDH were withing normal ranges for the OVA@DDAB/PLGA Nv group (Additional file 1: Fig. S13a, b, e). In contrast, ALT and ALP were found to be abnormally enhanced by Al adjuvant (Additional file 1: Fig. S13c-d), suggesting that this treatment had effectively spared the mice from hepatic or other organ damage. This indicates that its safer using DDAB/PLGA NP than Al adjuvant for immune protection and therapy.
Conclusion
In this paper, we successfully prepared OVA-loaded DDAB-PLGA nanoparticles (OVA@DDAB/PLGA Nv) with a positive charge. We explored the signal pathways that induced DCs activation and maturation in vitro and stimulated immune responses in vivo by the OVA@DDAB/PLGA Nv. Experiments performed in vitro show that the OVA@DDAB/PLGA Nv promoted the cellular uptake of antigen and the activation of BMDCs. The antigen uptake capacity of BMDCs incubated with OVA@DDAB/PLGA Nv was improved by more than twofold relative to pure OVA and OVA@Al at 12 h. Moreover, OVA@DDAB/PLGA Nv promoted antigen escape from the lysosomes into the cytoplasm, and induced ten times higher antigen cross-presentation activity compared to those OVA and OVA@Al. It was found that OVA@DDAB/PLGA Nv and OVA@Al exhibited different pathways to activate immunity. OVA@DDAB/PLGA Nv mainly activated DCs by p38 MAPK signal pathway. Meanwhile OVA@Al did not completely rely on the p38 signal pathway. Moreover, OVA@DDAB/PLGA Nv demonstrated two antigen transport pathways: one was direct rapid transport into draining lymph nodes at 3 h, and the other was an indirect transport pathway by DCs uptake at 12 h. These transport routes ensured that the OVA@DDAB/PLGA Nv could rapidly and continuously deliver antigen to the lymph nodes, which is favorable for stimulating an immune response in vivo. The antigen+ cells in inguinal LNs and popliteal LNs for OVA@DDAB/PLGA Nv were increased more than threefold compared to those of OVA@Al and OVA at 24 h. Furthermore, the antigen of OVA@DDAB/PLGA Nv persisted in LNs for more than 7 days, which induced germinal center formation as two times higher than those of OVA@Al and OVA. After immunization, OVA@DDAB/PLGA Nv induced comparable anti-OVA IgG and much higher ratio of IgG2c/IgG1 compared to OVA@Al. It also effectively activated CD4+T, CD8+T and B cells that further induced immune memory. As expected, OVA@DDAB/PLGA Nv elicited a strong cytotoxic T lymphocytes (CTLs) response. Stimulation with OVA@DDAB/PLGA Nv increased the proportion of CD107+CD8+, Fasl+CD8+ and perforin+CD8+ T cells and the secretion of IFN-γ and Granzyme B. These results indicate that the DDAB/PLGA NP was a potent platform to improve vaccine immunogenicity by novel signaling pathway in DCs, rapid and massive transport antigens to LNs.
References
Xu P, Tang S, Jiang L, Yang L, Zhang D, Feng S, et al. Nanomaterial-dependent immunoregulation of dendritic cells and its effects on biological activities of contraceptive nanovaccines. J Control Release. 2016;225:252–68.
Verma S, Sugadev R, Kumar A, Chandna S, Ganju L, Bansal A. Multi-epitope DnaK peptide vaccine against S.Typhi: an in silico approach. Vaccine. 2018;36(28):4014–22.
Hasan M, Ghosh PP, Azim KF, Mukta S, Abir RA, Nahar J, et al. Reverse vaccinology approach to design a novel multi-epitope subunit vaccine against avian influenza A (H7N9) virus. Microb Pathog. 2019;130:19–37.
Kalita P, Padhi AK, Zhang KYJ, Tripathi T. Design of a peptide-based subunit vaccine against novel coronavirus SARS-CoV-2. Microb Pathog. 2020;145:104236.
Shah RR, Hassett KJ, Brito LA. Overview of vaccine adjuvants: introduction, history, and current status. Methods Mol Biol. 2017;1494:1–13.
Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33(4):492–503.
Wen Y, Shi Y. Alum: an old dog with new tricks. Emerg Microbes Infect. 2016;5:e25.
Hu Y, Smith D, Zhao Z, Harmon T, Pentel PR, Ehrich M, et al. Alum as an adjuvant for nanoparticle based vaccines: a case study with a hybrid nanoparticle-based nicotine vaccine. Nanomedicine. 2019;20:102023.
Zhang W, Wang L, Liu Y, Chen X, Li J, Yang T, et al. Comparison of PLA microparticles and alum as adjuvants for H5N1 influenza split vaccine: adjuvanticity evaluation and preliminary action mode analysis. Pharm Res. 2014;31(4):1015–31.
Jia J, Zhang W, Liu Q, Yang T, Wang L, Ma G. Adjuvanticity regulation by biodegradable polymeric nano/microparticle size. Mol Pharm. 2017;14(1):14–22.
Chang X, **a CQ. Administration of sulfosuccinimidyl-4-[N-maleimidomethyl] cyclohexane-1-carboxylate conjugated GP100(25–33) peptide-coupled spleen cells effectively mounts antigen-specific immune response against mouse melanoma. Biochem Biophys Res Commun. 2015;468(1–2):46–52.
Wu PK, Tao Z, Ouyang Z, Cao JY, Geng D, Liu J, Wang CM. The anti-tumor effects of cordycepin-loaded liposomes on the growth of hepatoma 22 tumors in mice and human hepatoma BEL-7402 cells in culture. Drug Dev Ind Pharm. 2016;42(9):1424–33.
Avgoustakis K. Effect of copolymer composition on the physicochemical characteristics, in vitro stability, and biodistribution of PLGA–mPEG nanoparticles. Int J Pharm. 2003;259(12):115–27.
Danhier F, Ansorena E, Silva JM, Coco R, Breton AL, Préat V. PLGA-based nanoparticles: an overview of biomedical applications. J Control Release. 2012;161(2):505–22.
Chen X, Liu Y, Wang L, Liu Y, Zhang W, Fan B, et al. Enhanced humoral and cell-mediated immune responses generated by cationic polymer-coated PLA microspheres with adsorbed HBsAg. Mol Pharm. 2014;11(6):1772–84.
Shi G, Zhang C, Rong X, Niu J, Kong D. Enhanced antitumor immunity by targeting dendritic cells with tumor cell lysate-loaded chitosan nanoparticles vaccine. Biomaterials. 2016;113:191–202.
Liu Q, Jia J, Yang T, Fan Q, Wang L, Ma G. Pathogen-mimicking polymeric nanoparticles based on dopamine polymerization as vaccines adjuvants induce robust humoral and cellular immune responses. Small. 2016;12(13):1744–57.
**a Y, Wu J, Du Y, Miao C, Su Z, Ma G. Bridging systemic immunity with gastrointestinal immune responses via oil-in-polymer capsules. Adv Mater. 2018;30(31):e1801067.
Baleeiro RB, Schweinlin M, Rietscher R, Diedrich A, Czaplewska JA, Metzger M, et al. Nanoparticle-based mucosal vaccines targeting tumor-associated antigens to human dendritic cells. J Biomed Nanotechnol. 2016;12(7):1527–43.
Qian Y, ** H, Qiao S, Dai Y, Huang C, Lu L, Luo Q, Zhang Z. Targeting dendritic cells in lymph node with an antigen peptide-based nanovaccine for cancer immunotherapy. Biomaterials. 2016;98:171–83.
Liu Q, Chen XM, JileiJia JL, Weifeng Zhang WF, Yang TY, Lianyan Wang LY, MA GH. pH-responsive Poly(D, L-lactic-co-glycolic acid) nanoparticles with rapid antigen release behavior promote immune response. ACS Nano. 2015;9(5):4925–38.
Kang L, Jiang D, England CG, Barnhart TE, Yu B, Rosenkrans ZT, et al. ImmunoPET imaging of CD38 in murine lymphoma models using (89)Zr-labeled daratumumab. Eur J Nucl Med Mol Imaging. 2018;45(8):1372–81.
Nestle FO. Dendritic cell vaccination for cancer therapy. Oncogene. 2000;19:6673–9.
Frank Liang GL, Kerrie J, Sandgren Elizabeth A, Thompson Joseph R, et al. Vaccine priming is restricted todraining lymph nodes and controlled by adjuvant-mediated antigenuptake. Sci Transl Med. 2017;9:1–10.
Chou NT, Cheng CF, Wu HC, Lai CP, Lin LT, Pan IH, Ko CH. Chlorella sorokiniana-induced activation and maturation of human monocyte-derived dendritic cells through NF-kappaB and PI3K/MAPK pathways. Evid Based Complement Alternat Med. 2012;2012:735396.
Zhang Y, Guo Z, Du T, Chen J, Wang W, Xu K, et al. Prostate specific membrane antigen (PSMA): a novel modulator of p38 for proliferation, migration, and survival in prostate cancer cells. Prostate. 2013;73(8):835–41.
Muniyappa H, Das KC. Activation of c-Jun N-terminal kinase (JNK) by widely used specific p38 MAPK inhibitors SB202190 and SB203580: a MLK-3-MKK7-dependent mechanism. Cell Signal. 2008;20(4):675–83.
Zhu G, Lynn GM, Jacobson O, Chen K, Liu Y, Zhang H, et al. Albumin/vaccine nanocomplexes that assemble in vivo for combination cancer immunotherapy. Nat Commun. 2017;8(1):1954.
Tangye SG, Pillay B, Randall KL, Avery DT, Phan TG, Gray P, et al. Dedicator of cytokinesis 8-deficient CD4(+) T cells are biased to a TH2 effector fate at the expense of TH1 and TH17 cells. J Allergy Clin Immunol Pract. 2017;139(3):933–49.
WooSikKim JSK, Seung BC, Hongmin K, Kee Woong K, So Jeong K, Seung Jung H, et al. Mycobacterium tuberculosis Rv3628 drives Th1-type T cell immunity via TLR2-mediated activation of dendritic cells and displays vaccine potential against the hyper-virulent Bei**g K strain. Oncotarget. 2016;7(18):24962–82.
Tangye SG, Pillay B, Randall KL, Avery DT, Phan TG, Gray P, et al. Dedicator of cytokinesis 8-deficient CD4+ T cells are biased to a TH2 effector fate at the expense of TH1 and TH17 cells. J Allergy Clin Immunol. 2017;139(3):933–49.
Wang L, Rubinstein R, Lines JL, Wasiuk A, Ahonen C, Guo Y, et al. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J Exp Med. 2011;208(3):577–92.
Zhang W, Wang L, Liu Y, Chen X, Liu Q, Jia J, et al. Immune responses to vaccines involving a combined antigen-nanoparticle mixture and nanoparticle-encapsulated antigen formulation. Biomaterials. 2014;35(23):6086–97.
Krishnan L, Gurnani K, Dicaire CJ, van Faassen H, Zafer A, Kirschning CJ, et al. Rapid clonal expansion and prolonged maintenance of memory CD8+ T cells of the effector (CD44highCD62Llow) and central (CD44highCD62Lhigh) phenotype by an archaeosome adjuvant independent of TLR2. J Immunol. 2007;178(4):2396–406.
Allan RS, Waithman J, Bedoui S, Jones CM, Villadangos JA, Zhan Y, et al. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25(1):0–162.
Wang J, Liu Z, Jiang LP, An YF, Zhao XD. Screening for cytotoxic defects with flow cytometric detection of CD107α on natural killer cells and cytotoxic lymphocyte cells. Zhonghua Er Ke Za Zhi. 2012;50(5):386–91.
Hsieh MH, Korngold R. Differential use of FasL- and perforin-mediated cytolytic mechanisms by T-cell subsets involved in graft-versus-myeloid leukemia responses. Blood. 2000;96(3):1047–55.
Arumugam P, Patra D, Samanta B, Agasti SS, Subramani C, Rotello VM. Self-assembly and cross-linking of FePt nanoparticles at planar and colloidal liquid-liquid interfaces. J Am Chem Soc. 2008;130(31):10046–7.
Kato H, Johnson C, Takemoto SK, Busuttil RW, Kupiec-Weglinski JW. Th2-dependent IgG2c alloantibody responses have protective effects against accelerated rejection in rat liver tranplant recipients. Transplantation. 2000;69(Supplement):S346.
Ey PL, Prowse SJ, Jenkin CR. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-Sepharose. Immunochemistry. 1978;15(7):0–436.
Acknowledgements
This work was financially supported by the Bei**g Natural Science Foundation (Grant No. L202039), the National Science Foundation of China (Grant No. 81973262, 81972446, 81871385, 81822037) and the National Science and Technology Major Project of China (Grant No.2016ZX10004001-005), Bei**g Science Foundation for Distinguished Young Scholars (JQ19028), PKU medicine-X Youth Program (PKU2021LCXQ023) and Open Funding Project of the State Key Laboratory of Biochemical Engineering (2020KF-01), the University of Wisconsin–Madison and the National Institutes of Health (P30CA014520).
Author information
Authors and Affiliations
Contributions
SH and WM completed most of all the experiments, sorted out figures and analyzed the data, and writing the manuscript. DJ worked the PET imaging and analyzed the imaging data. LS polished the language of the manuscript. JZ and YL revised the manuscript. NH and ZC assist to complete the experiment of PET imaging and analyzed the imaging data. JWE maked the Zr-89. LK designed and worked the PET imaging, revised the manuscript. WC guided PET imaging design and support imaging work. XX design and complete the experiment of Weston-blot and immune-coprecipitation, and revised the manuscript. YW and LW guided the completion of the experiment, data analysis and the writing of the manuscript, and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
Weibo Cai is scientific advisor, stockholder, and grantee of Focus-X Therapeutics, Inc. The other authors declare no competing financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Characterization of PLGA nanoparticles (PLGA NPs), DDAB/PLGA NPs, and DDAB/PLGA Nano-vaccines (OVA@DDAB/PLGA Nv) (Mean ± STD). Figure S1. Cytotoxicity of OVA@DDAB/PLGA Nv. Figure S2. Expression of co-stimulatory molecules from DCs stimulated with different formulations. (a) the gating strategies of flow cytometry of activated DC cells. (b-d) Percentages of CD40+ CD11c+ (b), CD86+ CD11c+ (c) and MHC II+ CD11c+ (d) cells were analyzed by flow cytometry. Figure S3. (a) The changes of p38 MAPK, p-AKT and p-ERK phosphorylation after being stimulated with different formulation DCs for 6 h and 12 h. (b) Change of the p38 MAPK, p-AKT and p-ERK phosphorylation level by stimulated DCs with different concentration of DDAB-PLGA Nv. (c) DDAB-PLGA Nv increased the binding of MKK3 to its substrate p38α. Figure S4. The radiochemical purity of [89Zr]-Df-Bz-NCS-OVA incubated at room temperature, saline (37℃) and fresh serum (37℃) over the course of 14 days. Figure S5. The proportion of antigen-carrying cells in different LNs as analyzed by flow cytometry. Three mice were analyzed in every group (n = 3), and data are the mean ± SEM and representative of three independent experiments. Differences between two groups were tested using an unpaired, two-tailed Student’s t-test. Differences among multiple groups were tested with one-way ANOVA followed by Tukey’s multiple comparison. Significant differences between groups are expressed as follows: *P < 0.05, **P < 0.01, or ***P < 0.001. Figure S6. The proportion of antigen-carrying cells in different LNs as analyzed by immumohistochemical staining. The data were analyzed by automatic multispectral imaging system (PerkinElmer Vectra II). Three mice were analyzed in every group (n = 3), and data are the mean ± SEM and representative of three independent experiments. Differences between two groups were tested using an unpaired, two-tailed Student’s t-test. Differences among multiple groups were tested with one-way ANOVA followed by Tukey’s multiple comparison. Significant differences between groups are expressed as follows: *P < 0.05, **P < 0.01, or ***P < 0.001. Figure S7. Activation and maturation of BMDCs in LNs in vivo. (a–c) Expression of activation markers (CD86, MHC I and MHC II) of DCs in draining LNs.Three mice were analyzed in every group (n = 3), and data are the mean ± SEM and representative of three independent experiments. Differences between two groups were tested using an unpaired, two-tailed Student’s t-test. Differences among multiple groups were tested with one-way ANOVA followed by Tukey’s multiple comparison. Significant differences between groups are expressed as follows: *P < 0.05, **P < 0.01, or ***P < 0.001. Figure S8. The OVA@DDAB/PLGA Nv induced the formation of germinal centers in draining LNs. (a) The gating strategies of flow cytometry of germinal center and follicular helper CD4+ T cells. (b) The count of germinal center (GL-7hi B220+ cells) and (c) the follicular helper CD4+ T cells (Tfh, CXCR5hi PD-1hi CD4+ T cells) in draining LNs were analyzed by flow cytometry. Figure S9. Germinal centers in draining LNs determined by immunohistochemical staining. The data were analyzed by automatic multispectral imaging system (PerkinElmer Vectra II). Three mice were analyzed in every group (n = 3), and data are the mean ± SEM and representative of three independent experiments. Differences between two groups were tested using an unpaired, two-tailed Student’s t-test. Differences among multiple groups were tested with one-way ANOVA followed by Tukey’s multiple comparison. Significant differences between groups are expressed as follows: *P < 0.05, **P < 0.01, or ***P < 0.001. Figure S10. OVA@DDAB/PLGA Nv induced splenocyte activation. (a) The gating strategies of flow cytometry of splenocyte activation. (b, c, d) The activation of CD4+ T cells (b) CD8+ T cells (c) and B cells (d) from mice after being immunized with different vaccine formulations were measured by flow cytometry. Figure S11. Effects of different vaccines on memory T cell responses. (a) The gating strategies of flow cytometry of memory T cell. (b) Effector memory (CD44hi CD62Llow) andcentral memory (CD44hi CD62Lhi) in CD4+ and CD8+ T cells were measured by flow cytometry. Figure S12. Effects of different vaccines on CTL response. (a) The gating strategies of flow cytometry of CTL cells. The expression of Perforin (b), CD107 (c), and FasL (d) on CD8+ T cell in splenocytes were measured by flow cytometry. Figure S13. In vivo toxicity evaluation of DDAB-PLGA Nv. Hematological analysis of treated mice after 35 days. The range marked by dotted lines represents the normal range of different biosafety indicators. The determination of serum biochemistry of urea nitrogen (BUN) (a), aspartate transaminase (AST) (b), alanine aminotransferase (ALT) (c), alkaline phosphatase (ALP) (d), and lactate dehydrogenase (LDH). Three mice were analyzed in every group (n = 3), and data are the mean ± SEM and representative of three independent experiments. Differences between two groups were tested using an unpaired, two-tailed Student’s t-test. Differences among multiple groups were tested with one-way ANOVA followed by Tukey’s multiple comparison. Significant differences between groups are expressed as follows: *P < 0.05, **P < 0.01, or ***P < 0.001.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Han, S., Ma, W., Jiang, D. et al. Intracellular signaling pathway in dendritic cells and antigen transport pathway in vivo mediated by an OVA@DDAB/PLGA nano-vaccine. J Nanobiotechnol 19, 394 (2021). https://doi.org/10.1186/s12951-021-01116-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12951-021-01116-8