Abstract
Mucosal-associated invariant T (MAIT) cells are an atypical subset of T lymphocytes, which have a highly conserved semi-constant αβ chain of T-cell receptor (TCR) and recognize microbe-derived vitamin B metabolites via major histocompatibility complex class I related-1 molecule (MR1). MAIT cells get activated mainly through unique TCR-dependent and TCR-independent pathways, and express multiple functional and phenotypic traits, including innate-like functionality, T helper (Th) 1 cell immunity, Th 17 cell immunity, and tissue homing. Given the functions, MAIT cells are extensively reported to play a key role in mucosal homeostasis and infectious diseases. In the current work, we review the basic characteristics of MAIT cells and their roles in mucosal homeostasis and development of respiratory infectious diseases as well as their potential therapeutic targets.
Similar content being viewed by others
Introduction
Mucosal-associated invariant T (MAIT) cells, an innate subset of T cell, express a semi-invariant T cell receptor (TCR), which is widely found in mucosal tissues and plays a vital role in the mucosal barrier. Due to its unique mode of activation and phenotype, MAIT cells demonstrate special antibacterial and immunomodulatory activities. With the recent research advancements in MAIT cells and development of novel activators, their roles in a variety of conditions are gradually revealed, especially infectious diseases.
About MAIT cells
Porcelli et al. reported a class of T cells with a constant TCR chain as early as in 1993 [1] before Treiner et al. introduced the concept of MAIT cells in 2003, who found major histocompatibility complex class I related-1 molecule (MR1) to be the limiting structure for MAIT cell activation [2]. In 2012 Kjer-Nielsen et al. demonstrated that the metabolic products of vitamin B could act as ligands for MR1 and bind to MAIT cells. Interestingly, the metabolite of vitamin B2 can effectively activate MAIT cells via the TCR pathway when bound to MR1. In contrast, 6-formylteropterin (6-FP), the metabolite of vitamin B9, fails to activate MAIT cells although it binds to MR1 as well [3], an interesting and specific activation modality that set the stage for numerous subsequent studies. Classical MAIT cells are currently considered to be a class of lymphocytes with dual functions of innate and adaptive immunity, with a specific phenotype of TCRVa7.2+ CD3+ CD161+, which can be expressed as different subtypes depending on the activation status of the cells. In addition to classical MAIT cells, some TRAV1-2 negative cells can also function through MR1 activation, which can be classified as “classical MAIT, non-classical MAIT and MR1-T” according to surface markers and activation characteristics [4, 5], but there are fewer studies and the definition is not standardised, and their function still needs further study. However, there are few studies and the definitions are not standardised and their functions need further investigation. In this section, we focus on the number, distribution, and cellular phenotype of classical MAIT cells.
Number and distribution
The sequence homology between the α1 and α2 structural domains of human and mouse MR1 is high at 90%. However, the number of MAIT cells in SPF experimental mice is relatively small -approximately 0.01% to 5% of αβ T cells [6]. This indicates a possible dependence of MAIT cell expansion on bacteria. Prevalence of MAIT cells in specific tissues and organs also varies due to factors such as activation mode and phenotype (Fig. 1).
Phenotype
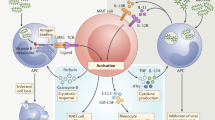
MAIT cells develop in the thymus, where immature thymocytes expressing the MAIT TCR interact with MR1 thymocytes expressing CD4+ CD8+ double positivity to trigger the intrathoracic developmental pathway [25]. MAIT cells are divided into three groups based on the expression of the phenotypic markers of CD4 and CD8: CD4+ cells, CD8+ cells, and CD8− CD4− double-negative cells [26]. The mature MAIT cells then enter the peripheral circulation and tissues to function accordingly. MAIT cells have a unique genetic rearrangement of the restricted TCR, which in humans is encoded by TRAV1-2 with the TCR α chain bound to TRAJ33, TRAJ12, or TRAJ20 and paired with a limited TCR β chain, usually encoded by TRBV6 or TRBV20. In mice TRAV1 is linked to TRAJ33 and paired with a TCR β consisting of a variable region of TRBV19 or TRBV13 [5, 27, 28].According to their phenotypic characteristics, the following functions of MAIT cells have been reported, including functions related to chemokines (C–C motif chemokine ligands: CCL-3, CCL-4) and chemokine receptors (C–C motif chemokine receptors: CCR-5, CCR6, CCR9; C-X-C motif chemokine receptor: CXCR-6), T helper (Th)-type 1 cells (Interferon-γ [IFN-γ]; tumor necrosis factor-a [TNF-α]; IL-2Rβ), Th17-type cells (IL-17, IL-22), and other cytokines [29,30,31] (Fig. 2).
Phenotypic characteristics of MAIT cells. Note: Functionalities are as follows: The BASIC part of this figure shows the markers of the TCR-dependent functions; The other parts show the TCR-independent functions (Innate-like, Th1-type cell, Th17-type cell, tissue homing functions) via the experience phenotype genes
Activation pathways
MAIT cells are activated via two pathways. Similar to other T cells, MAIT cells can be activated through the TCR pathway. Ligands such as microorganisms may use the riboflavin biosynthesis pathway to activate MAIT cells in an MR1-dependent TCR pathway, a process in which co-stimulation of CD28, Toll-like receptors (TLR) enhances TCR-mediated activation of MAIT cells [32]. Recently reported noval ligands, such as 5-(2-oxoethylideneamino)-5-dribitylaminouracil (5-OE-RU) and 5-(2-oxopropylideneamino)-5-dribitylaminouracil (5-OP-RU), which demonstrated the most potent activating effects to date, have drawn extensive attention [33, 34]. And 5-OP-RU-loaded MR1 tetramers are also routinely used to identify MAIT cells by flow cytometry. In contrast, prolonged signaling in the normal organismal environment fails to induce proliferation or production of sequential cytokines different from conventional T cells. This regulation may be critical in preventing inappropriate MAIT cell activation. High expression of CD25 and CD69, and high or low expression of CD161 as well as secretion of Th1 cytokines such as IFN-γ and TNF-α, and Th17-type cytokines are often noted in MAIT cell activation. In addition to pro-inflammatory cytokines, MAIT cells secrete granzymes (GzmA, GzmB, and GzmK) and perforin to lysate infected cells [6, 29].
In addition to the TCR pathway where MAIT cells are rapidly activated by MR1-expressing antigen-presenting cells (APCs) combined with ligands formed by vitamin B metabolites, there is a non-TCR-dependent pathway. Increasing evidence has indicated that these two activation pathways combined may play an important role in optimizing the functionality of MAIT cells [35, 36].
Roles in maintaining immune barrier
Microbial communities may colonize the skin, oral cavity, respiratory tract, digestive tract, and genitourinary tract, and interact with, for example, immune cells on the mucosal surface to maintain homeostasis at the mucosal barrier [37, 38]. Growing evidence has shown that MAIT cells, which are widely present at mucosal sites, are key to protecting the mucosa from external microbial threats and maintaining the immune barrier [39]. In this section, we will discuss the functions of MAIT cells in protecting and maintaining the integrity of the mucosal barrier by reviewing their multifaceted roles and interactions with other mucosal cells. In this section, we will further clarify the function of MAIT cells in the protection and maintenance of the mucosal barrier by their functions and their interactions with other mucosal cells.
Autonomous function
Microbial flora play a vital role in the maintenance of the mucosal immune barrier. Normal flora are in a dynamic balance in terms of species and numbers, interacting with host immune system to maintain homeostasis at the mucosal barrier, in which state MAIT cells have low cytotoxicity and release mostly GzmA and GzmK while GzmB and perforin are expressed at lower levels [40, 41]. MAIT cells can also show basal activity characterized by TCR-dependent activation by producing such cytokines as IL-17 and IL-22 to promote tissue repair and mucosal barrier function [42, 43]. Additionally, they may promote the expression of VEGF, TGF-β, CCL3, and GM-CSF at their mucosal barriers via both TCR-dependent and non-TCR-dependent pathways for tissue repair [44, 45]. Both approaches may be essential for maintaining mucosal barrier functionality (Fig. 3, left).
In contrast, at the onset of infection, significant increases in the frequency of MAIT cells are seen at the site of infection whereas the number of MAIT cells decreases significantly in the peripheral blood [46]. This observation may be closely related to the type of pathogen, where the alteration in the number and function of MAIT cells are more pronounced when the pathogen contains enzymes related to riboflavin metabolism [47]. In addition to the quantitative changes, MAIT cells exhibited a significant elevation in their cytotoxicity, primarily releasing GzmB with a greater killing capacity. They also release TNF-α, IL-17, and IFN-γ to inhibit pathogen-associated functions. MAIT cells can secrete chemokines, which enables them to recruit other immune cells to the infection site rapidly after activation. This facilitates the formation of an interaction network between cells to play their parts at the mucosal barrier (Fig. 3, right). MAIT cells can exhibit diverse manifestations at different stages of infection. For instance, during the early stage of infection onset,they demonstrate an early cytotoxic response characterized by high expression of Forkhead box protein 3 (FOXP3) and GzmB. As the infection prolongs, they may display more heterogeneous phenotypic subpopulations, including proliferation, late activation, mast cell degranulation, and even depleted phenotypes [48].
Similar to other immunizations, excessive MAIT cell activation in the organism can lead to the development of local inflammatory storms, further damaging tissues at the site of the lesion. Interestingly, MAIT cells demonstrate the ability to self-adjust. While they typically exhibit pro-inflammatory features in most cases, an increasing number of studies have revealed potential immunosuppressive functions of MAIT cells. Several studies indicate that MAIT cells can exert immunosuppressive effects through IL-10, TGF-β, etc. [49, 50], suggesting a possible role of MAIT cells in the development of infection. The transition between pro- and anti-inflammatory states suggest that MAIT cells may have an essential function in the process of infection by adjusting their phenotype. How to exploit this mechanism to develop the function of MAIT cells in the prevention and treatment of infectious diseases are key to current research.
Interaction with other immune cells in mucosal barrier
As mentioned above, the TCR activation pathway relies on APCs expressing MR1 receptors, such as dendritic cells, macrophages, and monocytes. These APCs activate MAIT cells by binding to riboflavin products produced by bacterial metabolism. The types of APCs responsible for MAIT cell activation vary between pathogens, potentially related to differences in cytokine expression and immune cells at the onset of infectious diseases.
For instance, at the site of infection, various cytokines, such as IL-7, IL-12, IL-15, and IL-18, directly activate MAIT cells [32, 51, 52]. IL-23, on the other hand, stimulates the accumulation of MAIT cells at the site of inflammation [53], and IL-2 further promotes the activation and leads to increased release of IFN-γ and GzB [54]. This implies that the degree of MAIT cell activation might be influenced not only by the pathogen but also by the status of other immune cells and cytokines [55,56,57,58].
In addition to activation by professional APCs, non-professional APCs can also activate MAIT cells in a TCR-dependent manner in certain diseases. For instance, during tuberculosis and typhoid infections, lung epithelial cells express MR1, which directly activate MAIT cells through a TCR-dependent pathway [17, 59].
The evidence presented above underscores the significance of MAIT cell interactions with other immune cells in mucosal tissue, which plays a key role in mucosal barrier homeostasis and the development of infections. Gaining a deeper understanding of the reciprocal immune relationships between MAIT cells, other cells, and cytokines within the mucosal barrier is crucial for further exploring the function of MAIT cells.
Roles in infectious diseases of respiratory system
Due to their unique characteristics, MAIT cells hold a crucial function in preserving the integrity of the mucosal barrier in various tissues and organs, both in healthy states and disease processes [60]. These cells exert their functions through self-activation and interaction with other immune cells. And the microbiota in the normal respiratory system work together with immune cells, etc. to build up an immune balance [61, 62] to which MAIT cells can respond when this balance is disrupted by infection. Notably, lung MAIT cells secrete the directly antimicrobial molecule IL-26 and selectively express cytokine and chemokine-related molecules, such as IFN-γ and IL-12R [63]. These attributes further support MAIT cells as an early sensor in defending the respiratory barrier.
The activation of MAIT cells during bacterial infection primarily involves TCR-dependent and TCR-independent pathways. When antigen-presenting cells engulf and process pathogenic bacteria, the antigen binds to MR1, triggering the activation of MAIT cells in a TCR-dependent manner. This rapidly stimulates the releases of gamma interferon, TNF-a, and cytotoxic factors.
It is essential to note that not all infected cells can be activated through the non-TCR-dependent pathway. In some cases of infectious diseases, certain pathogenic bacteria may not activate MAIT cells to perform their physiological functions. However, the activation of MAIT cells is strongly correlated with the increased production of riboflavin intermediates in microorganisms. It has been reported that bacteria with riboflavin metabolic pathways can activate MAIT cells more effectively [47].
Interestingly, the immune microenvironment at the site of infection often exhibits variations at the onset of different infections. As a result, MAIT cells frequently demonstrate different activation states depending on factors such as the type of pathogen infection and the severity of the disease.. This diversity in activation states may suggest that MAIT cells do not uniformly perform the same function in different infectious diseases or at different stages of the disease. Notably, viruses do not synthesize riboflavin, and as a result, the regulation of MAIT cell function in viral infections is believed to be achieved through a TCR-independent mechanism [64, 65].
This section offers an overview of the significance of MAIT cells in respiratory diseases linked to bacterial, fungal, and viral infections.
Bacterial infections
Mycobacterium tuberculosis
The role of MAIT cells in Mycobacterium tuberculosis (MTB) infection has been extensively studied in the last decade. MTB has been shown to specifically activate MAIT cells via the TCR pathway, and the overexpression of genes in the riboflavin-biosynthesis pathway has been found to attenuate the virulence of MTB [66]. It is widely recognized that MTB infection can activate MAIT cells not only through the TCR pathway but also the non-TCR pathways. Several studies [67, 68] have demonstrated that the proportion and absolute number of MAIT cells in the peripheral blood of patients with MTB are significantly lower than that of healthy volunteers. However, MAIT cells exhibit an increased capacity to release IFN-γ and GzB. The number of MAIT cells is significantly higher at the site of infection and in the respiratory tract. This correlates with their chemotactic ability to rapidly reach the site of infection and recruit other immune cells to perform their functions jointly. Interestingly, the glycolytic capacity of stimulated MAIT cells is significantly enhanced, possibly as an adaptive feedback to the low glucose levels in the diseased tissues [69, 70].
Further studies have indicated that the activity of MAIT cells varies during the different stages of MTB, where inactive MTB MAIT cells are relatively stable which perform functions similar to tissue repair. In contrast, upon the onset of active MTB infection, MAIT cells undergo prolonged activation, leading to a significantly increase in the number of PD-1-positive MAIT cells. This suggests that possible over-activation may further induce apoptosis of MAIT cells [68].
Based on the activation mode of MAIT cells, some scholars have proposed that specific activation of MAIT cells could be effective in preventing MTB infection or mitigating its severity. To this end, 5-OP-RU, a potent activation compound for MAIT cells, has been extensively studied in recent preclinical investigations.In one study, researchers utilized 5-OP-RU as an agonist to specifically activate MAIT cells after MTB exposure in mice. Surprisingly, the intranasal intervention with 5-OP-RU induced early activation and expansion of MAIT cells but did not attenuate the growth of MTB or the infection in the organism [71]. Similarly, another study exploring the preventive effect of 5-OP-RU vaccination after MTB infection found that MAIT cells failed to prevent MTB infection, and the intervention even delayed the initiation of specific CD4 + T cells after MTB infection. Consequently, this affected network interactions between MAIT cells and other cells, impacting further activation of MAIT cells [72].
Since the number, phenotype, and function of MAIT cells are different between mice and humans, some studies were conducted in rhesus monkeys. After 5-OP-RU treatment, MAIT cells did not expand but upregulated the expression of PD-1 which reduced their release of granzymes, cytokines, and other functions [73]. Several outcomes from this study indicate that treatment with 5-OP-RU does not improve infection control. Also noteworthy is the possibility that the application of 5-OP-RU for nasal dip induction may lead to bronchoconstriction, which complicates its therapeutic potential in the course of treatment.
In conclusion, existing preventive interventions may not be fully effective in preventing MTB infection. Further research is needed to develop and explore more targeted and efficient interventions that address the underlying mechanisms.
Legionella pneumophila
Legionella pneumophila is a parthenogenic intracellular pathogen known to cause Legionella pneumonia and Pontiac fever [74]. Notably, Legionella pneumophila possesses an intact riboflavin metabolic pathway that effectively activates MAIT cells. During the onset of infection, MAIT cells rapidly accumulate and activate at the site of infection during the onset of infection, displaying a cytotoxic function and exerting immune protective effects. This response is dependent on MR1, IFN-γ, and GM-CSF, while not relying on IL-17A, TNF, or perforin [8, 75].
Studies have explored the application of 5-OP-RU to activate MAIT cells, followed by re-inoculation with Legionella, which led to a significantly reducion in bacterial load and infection in the mouse organism. While IL-23 plus 5-OP-RU vaccine enhanced MAIT cell-mediated control of pulmonary Legionella infection and these findings provide evidence for the development of MAIT cells against infectious diseases [53], such findings need to be confirmed by further studies.
Other bacterial infections
Numerous studies have demonstrated that bacteria capable of metabolizing riboflavin can activate MAIT cells effectively, such as Escherichia coli, and Staphylococcus aureus [39]. Notably, Pseudomonas aeruginosa infection leads to a significant decrease in the number of MAIT cells in the peripheral blood, where the severity of the lung infection is negatively correlated with the number of MAIT cells [76].
In patients with community-acquired pneumonia, MAIT cell counts in peripheral blood have shown a correlation with disease severity. During antibiotic treatment and recovery from symptomatic remission, MAIT cell counts gradually recover [77]. Additionally, studies on sputum samples have revealded interesting findings. Rachel et al. [78] observed a higherabundance of MAIT cells in sputum in patients with mild community acquired pneumonia (CAP) compared to healthy controls. This abundance was associated with the presence of IFN-α, IFN-γ, and sputum neutrophil abundance. In Bronchoalveolar lavage fluid (BALF), MAIT cells expressing PLZF were significantly more prevalent in CAP patients, and their ability to secrete IL-17 was notably higher, suggesting possible functions at the site of infection [79].
Regarding Streptococcus pneumoniae infection, research has shown that the number of MAIT cells at the mucosa significantly increased after infection [80]. Nevertheless, despite these promising findings, more comprehensive basic and clinical evidence is still required to fully understand and support how MAIT cells can be effectively integrated into the prevention or treatment of infectious diseases.
Fungal infections
Notably, some common fungal infection pathogens also possess the riboflavin metabolic pathway, such as Candida albicans, Candida smoothie, and Aspergillus, which can effective activate MAIT cells [81, 82]. Nevertheless, it is essential to acknowledge that fungal infections exhibit distinct MR1-dependent and TCR β-chain deviations compared to bacterial infections [83].
For instance, in the case of Mucorales, the activation of MAIT cells by Trichoderma reesei also requires the presence of riboflavin metabolites and was dependent on TCR involvement. This activation results in increased expression of CD69 and CD107a, whereas intracellular expression of perforin and GzmA was reduced [84]. However, despite the significance of these findings, there is currently limited literature exploring the relevance of fungi to MAIT cells. The association of MAIT cells with commensal fungal colonization or pathogenic fungal infections remains understudied, and further investigations are warranted to elucidate their potential relevance.
Viral infections
Respiratory diseases caused by viral infections encompass a wide range of illnesses, including influenza virus, cytomegalovirus infections, and novel coronavirus infections. This section focuses on organismal infections with novel coronaviruses as a representative example. During infection with novel coronaviruses, the number of MAIT cells in peripheral blood decreases significantly, while MAIT cells in the respiratory tract become significantly enriched [85,86,87]. As the body recovers, the number and activation status of MAIT cells in peripheral blood gradually return to normal [88].
However, it is worth noting that the two phenotypes of MAIT cells, CD69high and CXCR3low, have been associated with poor clinical outcomes in patients with novel neocoronary pneumonia [89]. Moreover, an imbalance in the IFN-a-IL-18 axis has been shown to induce alterations in MAIT cell function, leading to cytotoxicity [90]. Notably, the level of IL-18 activation in vivo is positively correlated with the severity of infection and mortality. Consequently, over-activation of MAIT cells may result in an imbalance between protective and pathological immune responses.
Given the critical role that MAIT cells play in novel coronavirus infections, several studies have investigated the expression and activation of MAIT cells in relation to the likelihood and severity of Coronavirus Disease 2019 (COVID-19) infection in various populations. A study examining gender differences in MAT cell profiles following COVID-19 infection revealed a negative correlation between the frequency of infected circulating MAIT cells and the severity of infection. Interestingly, infected women had significantly fewer circulating MAIT cells but a significantly higher proportion of MAIT cells in the respiratory tract, with the cells showing greater activation [91]. This suggests that the specific protective MAIT cell profile observed in women may be associated with the lower severity of their infection.
Pregnant women, as a special group, have been suggested several studies to be generally more susceptible to viral infections. However, evidence indicates that risk factors for severe COVID 19 during pregnancy are similar to those of the general population. One study found that pregnant patients exhibit a stronger inflammatory response (elevated C-reactive protein and IL-6) and increased activation and chemotaxis of MAIT cells compared to non-pregnant infected women of the same age [92]. This suggests that pregnant women may possess a distinct immune capacity to cope with the onset of infection.
COVID-19 is an ever-evolving situation, and the remarkable effector and modulatory functions of MAIT cells make them a subject of significant interest in the context of the disease [93]. The wealth of evidence supporting their key role in COVID-19 underscores the potential of MAIT cells as targets for disease prevention and treatment. However, effectively harnessing MAIT cell immunity against pathogens requires a delicate balance to avoid excessive, premature, or dysregulated MAIT cell activation, which may lead to detrimental effects and organismal damage. Thus, unraveling the precise mechanisms of MAIT cell activation and regulation becomes crucial for develo** strategies that maximize their protective functions while minimizing potential adverse outcomes.
Conclusions and future perspectives
The evidence presented above highlights the significant role of MAIT cells in the body's innate and adaptive immunity. In respiratory infectious diseases, MAIT cells primarily function by releasing pro-inflammatory factors and lysis. The development of MAIT cell activation ligands such as 5-OP-RU, has brought light to this work. Nevertheless, current research has yet to identify an effective method of activating MAIT cells for the prevention or treatment of diseases. Combined with the specific mode of activation of MAIT cells that it may be able to play a relevant role in the prevention of infectious diseases, and by improving the time point of administration (pre-infection administration) or screening for better concentration and mode of administration may provide an opportunity for the realisation of this conjecture. Moving forward, it is essential to explore the delicate balance between the maintaining the mucosal barrier and managing the pro-inflammatory, cytotoxic profile of MAIT cells. This balance is crucial for the development MAIT cells as potential targets for disease prevention and treatment. It is worth noting that during an infectious disease, the organism may experience changes in the metabolic function of multiple tissues and organs, for example, in combination with metabolism-related disorders during an infection, a complexity that creates difficulties in intervening in the development of the disease. And many current mechanistic studies are based on in vitro or clean mice models, where animals are not exposed to pathogenic stimuli prior to the experiments. This scenario differs from real-life human infections, and strategies aimed at expanding MAIT cells through vaccination may not be as effective in humans as they are in clean conditions. Therefore, further research is necessary to elucidate the efficiency and safety of MAIT cell vaccination.
Availability of data and materials
Not applicable.
Abbreviations
- MAIT:
-
Mucosal-associated invariant T
- TCR:
-
T-cell receptor
- MR1:
-
Major histocompatibility complex class I related-1 molecule
- IL:
-
Interleukin
- 6-FP:
-
6-Formylteropterin
- CCL:
-
C-C motif chemokine ligand
- CCR:
-
C-C motif chemokine receptor
- CXCR:
-
C-X-C motif chemokine receptor
- Th:
-
T helper
- IFN-γ:
-
Interferon-γ
- T-Bet:
-
T-box transcription factor 21
- EOMES:
-
Eomesodermin
- Blimp-1:
-
PR/SET domain 1
- RORγt:
-
RAR related orphan receptor C
- STAT3:
-
Signal transducer and activator of transcription 3
- BALF:
-
Bronchoalveolar lavage fluid
- PLZF:
-
Zinc finger and BTB domain containing 16
- CAP:
-
Community acquired pneumonia
- COVID-19:
-
Coronavirus Disease 2019
- C/EBPδ:
-
CCAAT/Enhancer Binding Protein δ
- TLR:
-
Toll-like receptors
- 5-OE-RU:
-
5-(2-Oxoethylideneamino)-5-dribitylaminouracil
- 5-OP-RU:
-
5-(2-Oxopropylideneamino)-5-dribitylaminouracil
- Gzm:
-
Granzymes
- APCs:
-
Antigen-presenting cells
- TNF-a:
-
Tumour necrosis factor-a
- VEGF:
-
Vascular endothelial growth factor
- TGF-β:
-
Transforming growth factor-β
- GM-CSF:
-
Granulocyte-macrophage colony stimulating factor
- FOXP3:
-
Forkhead box protein 3
- MTB:
-
Mycobacterium tuberculosis
References
Porcelli S, Yockey CE, Brenner MB, Balk SP. Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4-8- alpha/beta T cells demonstrates preferential use of several V beta genes and an invariant TCR alpha chain. J Exp Med. 1993;178(1):1–16. https://doi.org/10.1084/jem.178.1.1.
Treiner E, Duban L, Bahram S, Radosavljevic M, Wanner V, Tilloy F, Affaticati P, Gilfillan S, Lantz O. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature. 2003;422(6928):164–9. https://doi.org/10.1038/nature01433.
Kjer-Nielsen L, Patel O, Corbett AJ, et al. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature. 2012;491(7426):717–23. https://doi.org/10.1038/nature11605.
Gherardin NA, Keller AN, Woolley RE, et al. Diversity of T Cells Restricted by the MHC Class I-Related Molecule MR1 Facilitates Differential Antigen Recognition. Immunity. 2016;44(1):32–45. https://doi.org/10.1016/j.immuni.2015.12.005.
Corbett AJ, Awad W, Wang H, et al. Antigen Recognition by MR1-Reactive T Cells; MAIT Cells, Metabolites, and Remaining Mysteries. Front Immunol. 2020;27(11):1961. https://doi.org/10.3389/fimmu.2020.01961. PMID:32973800;PMCID:PMC7482426.
Provine NM, Klenerman P. MAIT Cells in Health and Disease. Annu Rev Immunol. 2020;26(38):203–28. https://doi.org/10.1146/annurev-immunol-080719-015428.
Murugesan A, Ibegbu C, Styles TM, et al. Functional MAIT Cells Are Associated With Reduced Simian-Human Immunodeficiency Virus Infection. Front Immunol. 2020;10:3053. https://doi.org/10.3389/fimmu.2019.03053. Published 2020 Jan 17.
Wang H, D’Souza C, Lim XY, et al. MAIT cells protect against pulmonary Legionella longbeachae infection. Nat Commun. 2018;9(1):3350. https://doi.org/10.1038/s41467-018-05202-8. Published 2018 Aug 22.
Yu H, Yang A, Liu L, Mak JYW, Fairlie DP, Cowley S. CXCL16 Stimulates Antigen-Induced MAIT Cell Accumulation but Trafficking During Lung Infection Is CXCR6-Independent. Front Immunol. 2020;11:1773. https://doi.org/10.3389/fimmu.2020.01773. Published 2020 Aug 7.
Rahimpour A, Koay HF, Enders A, et al. Identification of phenotypically and functionally heterogeneous mouse mucosal-associated invariant T cells using MR1 tetramers. J Exp Med. 2015;212(7):1095–108. https://doi.org/10.1084/jem.20142110.
Sobkowiak MJ, Davanian H, Heymann R, et al. Tissue-resident MAIT cell populations in human oral mucosa exhibit an activated profile and produce IL-17. Eur J Immunol. 2019;49(1):133–43. https://doi.org/10.1002/eji.201847759.
Hegde P, Weiss E, Paradis V, et al. Mucosal-associated invariant T cells are a profibrogenic immune cell population in the liver. Nat Commun. 2018;9(1):2146. https://doi.org/10.1038/s41467-018-04450-y. Published 2018 Jun 1.
Murayama G, Chiba A, Suzuki H, et al. A critical role for mucosal-associated invariant t cells as regulators and therapeutic targets in systemic lupus erythematosus. Front Immunol. 2019;10:2681. https://doi.org/10.3389/fimmu.2019.02681. Published 2019 Nov 29.
Reantragoon R, Corbett AJ, Sakala IG, et al. Antigen-loaded MR1 tetramers define T cell receptor heterogeneity in mucosal-associated invariant T cells. J Exp Med. 2013;210(11):2305–20. https://doi.org/10.1084/jem.20130958.
Chiba A, Tajima R, Tomi C, et al. Mucosal-associated invariant T cells promote inflammation and exacerbate disease in murine models of arthritis. Arthritis Rheum. 2012;64(1):153–61. https://doi.org/10.1002/art.33314.
Hinks TS, Wallington JC, Williams AP, et al. Steroid-induced Deficiency of Mucosal-associated Invariant T Cells in the Chronic Obstructive Pulmonary Disease Lung. Implications for Nontypeable Haemophilus influenzae Infection. Am J Respir Crit Care Med. 2016;194(10):1208–18. https://doi.org/10.1164/rccm.201601-0002OC.
Gold MC, Cerri S, Smyk-Pearson S, et al. Human mucosal associated invariant T cells detect bacterially infected cells. PLoS Biol. 2010;8(6):e1000407. https://doi.org/10.1371/journal.pbio.1000407. Published 2010 Jun 29.
Tang XZ, Jo J, Tan AT, et al. IL-7 licenses activation of human liver intrasinusoidal mucosal-associated invariant T cells. J Immunol. 2013;190(7):3142–52. https://doi.org/10.4049/jimmunol.1203218.
Böttcher K, Rombouts K, Saffioti F, et al. MAIT cells are chronically activated in patients with autoimmune liver disease and promote profibrogenic hepatic stellate cell activation. Hepatology. 2018;68(1):172–86. https://doi.org/10.1002/hep.29782.
D’Souza C, Pediongco T, Wang H, et al. Mucosal-Associated Invariant T Cells Augment Immunopathology and Gastritis in Chronic Helicobacter pylori Infection. J Immunol. 2018;200(5):1901–16. https://doi.org/10.4049/jimmunol.1701512.
Booth JS, Salerno-Goncalves R, Blanchard TG, et al. Mucosal-associated invariant T cells in the human gastric mucosa and blood: role in helicobacter pylori Infection. Front Immunol. 2015;6:466. https://doi.org/10.3389/fimmu.2015.00466. Published 2015 Sep 17.
Provine NM, Binder B, FitzPatrick MEB, Schuch A, Garner LC, et al. Unique and common features of innate-like human Vδ2+ γδT cells and mucosal-associated invariant T cells. Front Immunol. 2018;9:756.
Serriari NE, Eoche M, Lamotte L, et al. Innate mucosal-associated invariant T (MAIT) cells are activated in inflammatory bowel diseases. Clin Exp Immunol. 2014;176(2):266–74. https://doi.org/10.1111/cei.12277.
Haga K, Chiba A, Shibuya T, et al. MAIT cells are activated and accumulated in the inflamed mucosa of ulcerative colitis. J Gastroenterol Hepatol. 2016;31(5):965–72. https://doi.org/10.1111/jgh.13242.
Koay HF, Gherardin NA, Enders A, et al. A three-stage intrathymic development pathway for the mucosal-associated invariant T cell lineage. Nat Immunol. 2016;17(11):1300–11. https://doi.org/10.1038/ni.3565.
Gherardin NA, Souter MN, Koay HF, et al. Human blood MAIT cell subsets defined using MR1 tetramers. Immunol Cell Biol. 2018;96(5):507–25. https://doi.org/10.1111/imcb.12021.
Lepore M, Kalinichenko A, Colone A, et al. Parallel T-cell cloning and deep sequencing of human MAIT cells reveal stable oligoclonal TCRβ repertoire. Nat Commun. 2014;15(5):3866. https://doi.org/10.1038/ncomms4866.
Eckle SB, Birkinshaw RW, Kostenko L, et al. A molecular basis underpinning the T cell receptor heterogeneity of mucosal-associated invariant T cells. J Exp Med. 2014;211(8):1585–600. https://doi.org/10.1084/jem.20140484.
Jiang Q, Wang F, Yang JY, Zhou G. MAIT cells and their implication in human oral diseases. Inflamm Res. 2022;71(9):1041–54. https://doi.org/10.1007/s00011-022-01600-3. Epub 2022 Jul 4 PMID: 35781343.
Ussher JE, Bilton M, Attwod E, et al. CD161++ CD8+ T cells, including the MAIT cell subset, are specifically activated by IL-12+IL-18 in a TCR-independent manner. Eur J Immunol. 2014;44(1):195–203. https://doi.org/10.1002/eji.201343509.
Rouxel O, Da Silva J, Beaudoin L, et al. Cytotoxic and regulatory roles of mucosal-associated invariant T cells in type 1 diabetes. Nat Immunol. 2017;18(12):1321–31. https://doi.org/10.1038/ni.3854.
Pavlovic M, Gross C, Chili C, et al. MAIT Cells Display a Specific Response to Type 1 IFN Underlying the Adjuvant Effect of TLR7/8 Ligands. Front Immunol. 2020;8(11):2097. https://doi.org/10.3389/fimmu.2020.02097. PMID:33013883;PMCID:PMC7509539.
Yvorra T, Steinmetz A, Retailleau P, Lantz O, Schmidt F. Synthesis, biological evaluation and molecular modelling of new potent clickable analogues of 5-OP-RU for their use as chemical probes for the study of MAIT cell biology. Eur J Med Chem. 2021;211: 113066. https://doi.org/10.1016/j.ejmech.2020.113066.
Li K, Vorkas CK, Chaudhry A, Bell DL, Willis RA, Rudensky A, Altman JD, Glickman MS, Aubé J. Synthesis, stabilization, and characterization of the MR1 ligand precursor 5-amino-6-D-ribitylaminouracil (5-A-RU). PLoS One. 2018;13(2):e0191837. https://doi.org/10.1371/journal.pone.0191837.
Godfrey DI, Koay HF, McCluskey J, et al. The biology and functional importance of MAIT cells. Nat Immunol. 2019;20(9):1110–28. https://doi.org/10.1038/s41590-019-0444-8.
Jenkins MK, Pardoll DM, Mizuguchi J, et al. T-cell unresponsiveness in vivo and in vitro: fine specificity of induction and molecular characterization of the unresponsive state. Immunol Rev. 1987;95:113–35. https://doi.org/10.1111/j.1600-065x.1987.tb00502.x.
Poletti M, Treveil A, Csabai L, Gul L, Modos D, Madgwick M, Olbei M, Bohar B, Valdeolivas A, Turei D, Verstockt B, Triana S, Alexandrov T, Saez-Rodriguez J, Stanifer ML, Boulant S, Korcsmaros T. Map** the epithelial-immune cell interactome upon infection in the gut and the upper airways. NPJ Syst Biol Appl. 2022;8(1):15. https://doi.org/10.1038/s41540-022-00224-x.
Shen S, Gong M, Wang G, Dua K, Xu J, Xu X, Liu G. COVID-19 and Gut Injury. Nutrients. 2022;14(20):4409. https://doi.org/10.3390/nu14204409.
Constantinides MG, Link VM, Tamoutounour S, Wong AC, Perez-Chaparro PJ, Han SJ, Chen YE, Li K, Farhat S, Weckel A, Krishnamurthy SR, Vujkovic-Cvi** I, Linehan JL, Bouladoux N, Merrill ED, Roy S, Cua DJ, Adams EJ, Bhandoola A, Scharschmidt TC, Aubé J, Fischbach MA, Belkaid Y. MAIT cells are imprinted by the microbiota in early life and promote tissue repair. Science. 2019;366(6464):eaax6624. https://doi.org/10.1126/science.aax6624.
Kurioka A, Ussher JE, Cosgrove C, Clough C, Fergusson JR, Smith K, Kang YH, Walker LJ, Hansen TH, Willberg CB, Klenerman P. MAIT cells are licensed through granzyme exchange to kill bacterially sensitized targets. Mucosal Immunol. 2015;8(2):429–40. https://doi.org/10.1038/mi.2014.81.
Lamichhane R, Schneider M, de la Harpe SM, Harrop TWR, Hannaway RF, Dearden PK, Kirman JR, Tyndall JDA, Vernall AJ, Ussher JE. TCR- or Cytokine-Activated CD8+ Mucosal-Associated Invariant T cells are rapid Polyfunctional effectors that can coordinate immune responses. Cell Rep. 2019;28(12):3061–3076.e5. https://doi.org/10.1016/j.celrep.2019.08.054.
Hinks TSC, Marchi E, Jabeen M, Olshansky M, Kurioka A, Pediongco TJ, Meehan BS, Kostenko L, Turner SJ, Corbett AJ, Chen Z, Klenerman P, McCluskey J. Activation and In Vivo Evolution of the MAIT Cell Transcriptome in Mice and Humans Reveals Tissue Repair Functionality. Cell Rep. 2019;28(12):3249–3262.e5. https://doi.org/10.1016/j.celrep.2019.07.039.
Leng T, Akther HD, Hackstein CP, Powell K, King T, Friedrich M, Christoforidou Z, McCuaig S, Neyazi M, Arancibia-Cárcamo CV, Hagel J, Powrie F, Oxford IBD, Investigators, Peres RS, Millar V, Ebner D, Lamichhane R, Ussher J, Hinks TSC, Marchi E, Willberg C, Klenerman P. TCR and Inflammatory Signals Tune Human MAIT Cells to Exert Specific Tissue Repair and Effector Functions. Cell Rep. 2019Sep 17;28(12):3077–3091.e5. https://doi.org/10.1016/j.celrep.2019.08.050.
Du Halgouet A, Darbois A, Alkobtawi M, Mestdagh M, Alphonse A, Premel V, Yvorra T, Colombeau L, Rodriguez R, Zaiss D, El Morr Y, Bugaut H, Legoux F, Perrin L, Aractingi S, Golub R, Lantz O, Salou M. Role of MR1-driven signals and amphiregulin on the recruitment and repair function of MAIT cells during skin wound healing. Immunity. 2023;56(1):78–92.e6. https://doi.org/10.1016/j.immuni.2022.12.004. PMID:36630919;PMCID:PMC9839364.
Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83(3):835–70. https://doi.org/10.1152/physrev.2003.83.3.835.
Berkson JD, Prlic M. The MAIT conundrum - how human MAIT cells distinguish bacterial colonization from infection in mucosal barrier tissues. Immunol Lett. 2017;192:7–11. https://doi.org/10.1016/j.imlet.2017.09.013.
Tastan C, Karhan E, Zhou W, Fleming E, Voigt AY, Yao X, Wang L, Horne M, Placek L, Kozhaya L, Oh J, Unutmaz D. Tuning of human MAIT cell activation by commensal bacteria species and MR1-dependent T-cell presentation. Mucosal Immunol. 2018;11(6):1591–605. https://doi.org/10.1038/s41385-018-0072-x.
Vorkas CK, Krishna C, Li K, Aubé J, Fitzgerald DW, Mazutis L, Leslie CS, Glickman MS. Single-Cell Transcriptional Profiling Reveals Signatures of Helper, Effector, and Regulatory MAIT Cells during Homeostasis and Activation. J Immunol. 2022;208(5):1042–56. https://doi.org/10.4049/jimmunol.2100522.
Croxford JL, Miyake S, Huang YY, Shimamura M, Yamamura T. Invariant V(alpha)19i T cells regulate autoimmune inflammation. Nat Immunol. 2006;7(9):987–94. https://doi.org/10.1038/ni1370.
Yu H, Yang A, Derrick S, Mak JYW, Liu L, Fairlie DP, Cowley S. Artificially induced MAIT cells inhibit M. bovis BCG but not M. tuberculosis during in vivo pulmonary infection. Sci Rep. 2020;10(1):13579. https://doi.org/10.1038/s41598-020-70615-9.
Wallington JC, Williams AP, Staples KJ, Wilkinson TMA. IL-12 and IL-7 synergize to control mucosal-associated invariant T-cell cytotoxic responses to bacterial infection. J Allergy Clin Immunol. 2018;141(6):2182–2195.e6. https://doi.org/10.1016/j.jaci.2017.08.009. Epub 2017 Sep 1 PMID: 28870466.
Jo J, Tan AT, Ussher JE, et al. Toll-like receptor 8 agonist and bacteria trigger potent activation of innate immune cells in human liver. PLoS Pathog. 2014;10(6):e1004210. https://doi.org/10.1371/journal.ppat.1004210. PMID:24967632;PMCID:PMC4072808.
Wang H, Kjer-Nielsen L, Shi M, et al. IL-23 costimulates antigen-specific MAIT cell activation and enables vaccination against bacterial infection. Sci Immunol. 2019;4(41):eaaw0402. https://doi.org/10.1126/sciimmunol.aaw0402. PMID: 31732518.
Tao H, Pan Y, Chu S, et al. Differential controls of MAIT cell effector polarization by mTORC1/mTORC2 via integrating cytokine and costimulatory signals. Nat Commun. 2021;12(1):2029. https://doi.org/10.1038/s41467-021-22162-8. PMID:33795689;PMCID:PMC8016978.
Jouan Y, Guillon A, Gonzalez L, et al. Phenotypical and functional alteration of unconventional T cells in severe COVID-19 patients. J Exp Med. 2020;217(12):e20200872. https://doi.org/10.1084/jem.20200872. PMID:32886755;PMCID:PMC7472174.
Kurioka A, van Wilgenburg B, Javan RR, et al. Diverse Streptococcus pneumoniae Strains Drive a Mucosal-Associated Invariant T-Cell Response Through Major Histocompatibility Complex class I-Related Molecule-Dependent and Cytokine-Driven Pathways. J Infect Dis. 2018;217(6):988–99. https://doi.org/10.1093/infdis/jix647. PMID:29267892;PMCID:PMC5854017.
Shaler CR, Choi J, Rudak PT, et al. MAIT cells launch a rapid, robust and distinct hyperinflammatory response to bacterial superantigens and quickly acquire an anergic phenotype that impedes their cognate antimicrobial function: Defining a novel mechanism of superantigen-induced immunopathology and immunosuppression. PLoS Biol. 2017;15(6):e2001930. https://doi.org/10.1371/journal.pbio.2001930. PMID:28632753;PMCID:PMC5478099.
Mabire M, Hegde P, Hammoutene A, Wan J, Caër C, Sayegh RA, Cadoux M, Allaire M, Weiss E, Thibault-Sogorb T, Lantz O, Goodhardt M, Paradis V, de la Grange P, Gilgenkrantz H, Lotersztajn S. MAIT cell inhibition promotes liver fibrosis regression via macrophage phenotype reprogramming. Nat Commun. 2023;14(1):1830. https://doi.org/10.1038/s41467-023-37453-5.
Le Bourhis L, Dusseaux M, Bohineust A, et al. MAIT cells detect and efficiently lyse bacterially-infected epithelial cells. PLoS Pathog. 2013;9(10):e1003681. https://doi.org/10.1371/journal.ppat.1003681. Epub 2013 Oct 10. PMID: 24130485; PMCID: PMC3795036.
Toubal A, Nel I, Lotersztajn S, Lehuen A. Mucosal-associated invariant T cells and disease. Nat Rev Immunol. 2019;19(10):643–57. https://doi.org/10.1038/s41577-019-0191-y. PMID: 31308521.
Charlson ES, Bittinger K, Haas AR, et al. Topographical continuity of bacterial populations in the healthy human respiratory tract. Am J Respir Crit Care Med. 2011;184(8):957–63. https://doi.org/10.1164/rccm.201104-0655OC. Epub 2011 Jun 16. PMID: 21680950; PMCID: PMC3208663.
Faner R, Sibila O, Agustí A, et al. The microbiome in respiratory medicine: current challenges and future perspectives. Eur Respir J. 2017;49(4):1602086. https://doi.org/10.1183/13993003.02086-2016. PMID: 28404649.
Meermeier EW, Zheng CL, Tran JG, et al. Human lung-resident mucosal-associated invariant T cells are abundant, express antimicrobial proteins, and are cytokine responsive. Commun Biol. 2022;5(1):942. https://doi.org/10.1038/s42003-022-03823-w.
Loh L, Wang Z, Sant S, et al. Human mucosal-associated invariant T cells contribute to antiviral influenza immunity via IL-18-dependent activation. Proc Natl Acad Sci U S A. 2016;113(36):10133–8. https://doi.org/10.1073/pnas.1610750113. Epub 2016 Aug 19. PMID: 27543331; PMCID: PMC5018778.
Harriff MJ, Karamooz E, Burr A, et al. Endosomal MR1 Trafficking Plays a Key Role in Presentation of Mycobacterium tuberculosis Ligands to MAIT Cells. PLoS Pathog. 2016;12(3):e1005524. https://doi.org/10.1371/journal.ppat.1005524. PMID:27031111;PMCID:PMC4816560.
Dey RJ, Dey B, Harriff M, et al. Augmentation of the Riboflavin-Biosynthetic Pathway Enhances Mucosa-Associated Invariant T (MAIT) Cell Activation and Diminishes Mycobacterium tuberculosis Virulence. mBio. 2021;13(1):e0386521. https://doi.org/10.1128/mbio.03865-21. Epub 2022 Feb 15. PMID: 35164552; PMCID: PMC8844931.
Suliman S, Murphy M, Musvosvi M, et al. MR1-Independent Activation of Human Mucosal-Associated Invariant T Cells by Mycobacteria. J Immunol. 2019;203(11):2917–27. https://doi.org/10.4049/jimmunol.1900674. Epub 2019 Oct 14. PMID: 31611259; PMCID: PMC6859375.
Vorkas CK, Wipperman MF, Li K, et al. Mucosal-associated invariant and γδ T cell subsets respond to initial Mycobacterium tuberculosis infection. JCI Insight. 2018;3(19):e121899. https://doi.org/10.1172/jci.insight.121899. PMID:30282828;PMCID:PMC6237486.
Zinser ME, Highton AJ, Kurioka A, et al. Human MAIT cells show metabolic quiescence with rapid glucose-dependent upregulation of granzyme B upon stimulation. Immunol Cell Biol. 2018;96(6):666–74. https://doi.org/10.1111/imcb.12020. Epub 2018 Mar 9. PMID: 29423939; PMCID: PMC6055666.
Amini A, Pang D, Hackstein CP, et al. MAIT Cells in Barrier Tissues: Lessons from Immediate Neighbors. Front Immunol. 2020;30(11):584521. https://doi.org/10.3389/fimmu.2020.584521. PMID:33329559;PMCID:PMC7734211.
Vorkas CK, Levy O, Skular M, et al. Efficient 5-OP-RU-Induced Enrichment of Mucosa-Associated Invariant T Cells in the Murine Lung Does Not Enhance Control of Aerosol Mycobacterium tuberculosis Infection. Infect Immun. 2020;89(1):e00524–e620. https://doi.org/10.1128/IAI.00524-20. PMID:33077620;PMCID:PMC7927919.
Sakai S, Kauffman KD, Oh S, et al. MAIT cell-directed therapy of Mycobacterium tuberculosis infection. Mucosal Immunol. 2021;14(1):199–208. https://doi.org/10.1038/s41385-020-0332-4. Epub 2020 Aug 18. PMID: 32811991; PMCID: PMC7790750.
Sakai S, Lora NE, Kauffman KD, et al. Functional inactivation of pulmonary MAIT cells following 5-OP-RU treatment of non-human primates. Mucosal Immunol. 2021;14(5):1055–66. https://doi.org/10.1038/s41385-021-00425-3. Epub 2021 Jun 22. PMID: 34158594; PMCID: PMC8217205.
Gonçalves IG, Simões LC, Simões M. Legionella pneumophila. Trends Microbiol. 2021;29(9):860–1. https://doi.org/10.1016/j.tim.2021.04.005. Epub 2021 May 13.
Chen Z, Wang H, D’Souza C, et al. Characterization and Purification of Mouse Mucosal-Associated Invariant T (MAIT) Cells. Curr Protoc Immunol. 2019;127(1):e89. https://doi.org/10.1002/cpim.89. PMID: 31763782.
Smith DJ, Hill GR, Bell SC, Reid DW. Reduced mucosal associated invariant T-cells are associated with increased disease severity and Pseudomonas aeruginosa infection in cystic fibrosis. PLoS One. 2014;9(10):e109891. https://doi.org/10.1371/journal.pone.0109891. PMID:25296025;PMCID:PMC4190362.
Grimaldi D, Le Bourhis L, Sauneuf B, Dechartres A, Rousseau C, Ouaaz F, Milder M, Louis D, Chiche JD, Mira JP, Lantz O, Pène F. Specific MAIT cell behaviour among innate-like T lymphocytes in critically ill patients with severe infections. Intensive Care Med. 2014;40(2):192–201. https://doi.org/10.1007/s00134-013-3163-x. Epub 2013 Dec 10 PMID: 24322275.
Hannaway RF, Wang X, Schneider M, Slow S, Cowan J, Brockway B, Schofield MR, Morgan XC, Murdoch DR, Ussher JE. Mucosal-associated invariant T cells and Vδ2+ γδ T cells in community acquired pneumonia: association of abundance in sputum with clinical severity and outcome. Clin Exp Immunol. 2020;199(2):201–15. https://doi.org/10.1111/cei.13377. PMID: 31587268; PMCID: PMC6954682.
Lu B, Liu M, Wang J, Fan H, Yang D, Zhang L, Gu X, Nie J, Chen Z, Corbett AJ, Zhan MJ, Zhang S, Bryant VL, Lew AM, McCluskey J, Luo HB, Cui J, Zhang Y, Zhan Y, Lu G. IL-17 production by tissue-resident MAIT cells is locally induced in children with pneumonia. Mucosal Immunol. 2020;13(5):824–35. https://doi.org/10.1038/s41385-020-0273-y. Epub 2020 Feb 28. PMID: 32112047; PMCID: PMC7434594.
Jochems SP, de Ruiter K, Solórzano C, Voskamp A, Mitsi E, Nikolaou E, Carniel BF, Pojar S, German EL, Reiné J, Soares-Schanoski A, Hill H, Robinson R, Hyder-Wright AD, Weight CM, Durrenberger PF, Heyderman RS, Gordon SB, Smits HH, Urban BC, Rylance J, Collins AM, Wilkie MD, Lazarova L, Leong SC, Yazdanbakhsh M, Ferreira DM. Innate and adaptive nasal mucosal immune responses following experimental human pneumococcal colonization. J Clin Invest. 2022;132(11):e161565. https://doi.org/10.1172/JCI161565. Erratumfor:JClinInvest.2019Jul30;129(10):4523-4538.PMID:35642639;PMCID:PMC9151695.
Jahreis S, Boettcher S, von Lilienfeld-Toal M. MAIT Cell Activation by Fungal Pathogens. Methods Mol Biol. 2020;2098:167–77. https://doi.org/10.1007/978-1-0716-0207-2_11. PMID: 31792822.
Böttcher S, Hartung S, Meyer F, Rummler S, Voigt K, Walther G, Hochhaus A, von Lilienfeld-Toal M, Jahreis S. Human mucosal-associated invariant T cells respond to Mucorales species in a MR1-dependent manner. Med Mycol. 2021;59(5):505–9. https://doi.org/10.1093/mmy/myaa103. PMID: 33336238.
Dias J, Leeansyah E, Sandberg JK. Multiple layers of heterogeneity and subset diversity in human MAIT cell responses to distinct microorganisms and to innate cytokines. Proc Natl Acad Sci U S A. 2017;114(27):E5434–43. https://doi.org/10.1073/pnas.1705759114. Epub 2017 Jun 19. PMID: 28630305; PMCID: PMC5502643.
Jahreis S, Böttcher S, Hartung S, Rachow T, Rummler S, Dietl AM, Haas H, Walther G, Hochhaus A, von Lilienfeld-Toal M. Human MAIT cells are rapidly activated by Aspergillus spp. in an APC-dependent manner. Eur J Immunol. 2018;48(10):1698–706. https://doi.org/10.1002/eji.201747312. Epub 2018 Aug 13. PMID: 30059139.
Deschler S, Kager J, Erber J, et al. Mucosal-Associated Invariant T (MAIT) Cells Are Highly Activated and Functionally Impaired in COVID-19 Patients. Viruses. 2021;13(2):241. https://doi.org/10.3390/v13020241. PMID:33546489;PMCID:PMC7913667.
Yang Q, Wen Y, Qi F, et al. Suppressive Monocytes Impair MAIT Cells Response via IL-10 in Patients with Severe COVID-19. J Immunol. 2021;207(7):1848–56. https://doi.org/10.4049/jimmunol.2100228. Epub 2021 Aug 27 PMID: 34452933.
Björkström NK, Ponzetta A. Natural killer cells and unconventional T cells in COVID-19. Curr Opin Virol. 2021;49:176–82. https://doi.org/10.1016/j.coviro.2021.06.005. Epub 2021 Jun 19. PMID: 34217135; PMCID: PMC8214213.
Shi J, Zhou J, Zhang X, et al. Single-Cell Transcriptomic Profiling of MAIT Cells in Patients With COVID-19. Front Immunol. 2021;30(12):700152. https://doi.org/10.3389/fimmu.2021.700152. PMID:34394094;PMCID:PMC8363247.
Parrot T, Gorin JB, Ponzetta A, et al. MAIT cell activation and dynamics associated with COVID-19 disease severity. Sci Immunol. 2020;5(51):eabe1670. https://doi.org/10.1126/sciimmunol.abe1670. PMID: 32989174; PMCID: PMC7857393.
Flament H, Rouland M, Beaudoin L, et al. Outcome of SARS-CoV-2 infection is linked to MAIT cell activation and cytotoxicity. Nat Immunol. 2021;22(3):322–35. https://doi.org/10.1038/s41590-021-00870-z. Epub 2021 Feb 2 PMID: 33531712.
Yu C, Littleton S, Giroux NS, et al. Mucosal-associated invariant T cell responses differ by sex in COVID-19. Med. 2021;2(6):755–772.e5. https://doi.org/10.1016/j.medj.2021.04.008. Epub 2021 Apr 13. PMID: 33870241; PMCID: PMC8043578.
Chen G, Zhang Y, Zhang Y, et al. Differential immune responses in pregnant patients recovered from COVID-19. Signal Transduct Target Ther. 2021;6(1):289. https://doi.org/10.1038/s41392-021-00703-3. PMID:34326311;PMCID:PMC8320317.
Haeryfar SMM. MAIT Cells in COVID-19: Heroes, Villains, or Both? Crit Rev Immunol. 2020;40(2):173–84. https://doi.org/10.1615/CritRevImmunol.2020034943. PMID: 32749095.
Acknowledgements
Not applicable.
Funding
The study was funded by Sichuan Natural Science Foundation project (No. 2022NSFSC0786).
Author information
Authors and Affiliations
Contributions
All authors contributed to gathering of data, writing, editing, and revising of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lin, X., Wang, Y. & He, Y. Mucosal-associated invariant T cells in infectious diseases of respiratory system: recent advancements and applications. J Inflamm 21, 6 (2024). https://doi.org/10.1186/s12950-024-00376-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12950-024-00376-z