Abstract
Background
Evidence suggests organophosphate esters (OPEs) are neurotoxic; however, the epidemiological literature remains scarce. We investigated whether prenatal exposures to OPEs were associated with child neurobehavior in the MADRES cohort.
Methods
We measured nine OPE metabolites in 204 maternal urine samples (gestational age at collection: 31.4 ± 1.8 weeks). Neurobehavior problems were assessed among 36-month-old children using the Child Behavior Checklist’s (CBCL) three composite scales [internalizing, externalizing, and total problems]. We examined associations between tertiles of prenatal OPE metabolites (> 50% detection) and detect/non-detect categories (< 50% detection) and CBCL composite scales using linear regression and generalized additive models. We also examined mixtures for widely detected OPEs (n = 5) using Bayesian kernel machine regression.
Results
Maternal participants with detectable versus non-detectable levels of bis(2-methylphenyl) phosphate (BMPP) had children with 42% (95% CI: 4%, 96%) higher externalizing, 45% (-2%, 114%) higher internalizing, and 35% (3%, 78%) higher total problems. Participants in the second versus first tertile of bis(butoxethyl) phosphate (BBOEP) had children with 43% (-1%, 109%) higher externalizing scores. Bis(1-chloro-2-propyl) phosphate (BCIPP) and child sex had a statistically significant interaction in internalizing (p = 0.02) and total problems (p = 0.03) models, with 120% (23%, 295%) and 57% (6%, 134%) higher scores in the third versus first BCIPP tertile among males. Among females, detectable vs non-detectable levels of prenatal BMPP were associated with 69% higher externalizing scores (5%, 170%) while the third versus first tertile of prenatal BBOEP was associated with 45% lower total problems (-68%, -6%). Although the metabolite mixture and each CBCL outcome had null associations, we observed marginal associations between di-n-butyl phosphate and di-isobutyl phosphate (DNBP + DIBP) and higher internalizing scores (0.15; 95% CrI: -0.02, 0.32), holding other metabolites at their median.
Conclusions
Our results generally suggest adverse and sex-specific effects of prenatal exposure to previously understudied OPEs on neurobehavioral outcomes in 36-month children, providing evidence of potential OPE neurotoxicity.
Similar content being viewed by others
Background
Neurobehavioral development is a lifelong, dynamic process which encompasses a host of psychosocial and biological processes that influence behavior, emotion, and learning [1, 2]. Environmental chemical exposures are increasingly recognized as major risk factors for adverse neurobehavioral outcomes, ranging in effects from subclinical deficits in neurobehavioral functioning to increased risks of neurobehavioral disorders [2,3,4]. The prenatal period is a particularly susceptible window for neurobehavioral development given the rapid cascade of tightly controlled and sequenced biological processes that occur in utero, resulting in heightened susceptibility to environmental exposures [2]. Even minor, incremental disruptions to prenatal biological processes from low-level chronic exposures to environmental chemicals have the potential to result in lifelong health effects [3, 5].
Flame retardants are anthropogenic chemical additives incorporated into materials to prevent or delay fires and to meet flammability regulations in the United States, particularly in California [6, 7]. For many decades, legacy flame retardants, such as polybrominated diphenyl ethers (PBDEs), were the most frequently used [8, 9]. However, due to their bioaccumulation in the environment, persistence, and neurotoxicity to children, PBDEs have been phased out of the US market and banned from production in the European Union [10]. As a result, organophosphate esters (OPEs) have dramatically increased in use as replacement flame retardants in recent years [11,12,13]. However, emerging literature suggests that OPEs may be a regrettable substitution for PBDEs and may also adversely impact neurobehavioral and neurodevelopmental outcomes [14].
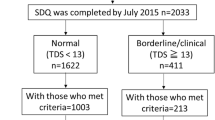
OPEs are commonly used as plasticizers and lubricants, contributing to their environmental ubiquity [7]. OPEs are also applied as additives to various consumer, industrial, and electronic products, such as polyurethane foam, textiles, and building materials [7, 15]. Due to their physical incorporation within a product matrix and their semivolatile nature, OPEs easily volatize and leach into surrounding environments, commonly settling into dust particles in homes and environmental media such as soil, surface water, sediment, and agricultural products and facilitating human exposure to OPEs [ The MADRES study is an ongoing prospective pregnancy cohort of predominately low-income Hispanic/Latino mother-child pairs living in urban Los Angeles, CA. A detailed description of the MADRES study population and protocol have been previously described [41]. In brief, participants were recruited into the study prior to 30 weeks’ gestation at three partner community health clinics, one private obstetrics and gynecology practice in Los Angeles, and through self-referrals from community meetings and local advertisements. Eligible participants at time of recruitment were: (1) less than 30 weeks’ gestation, (2) over 18 years of age, and (3) fluent in English or Spanish. Exclusion criteria included: (1) multiple gestation, (2) having a physical, mental, or cognitive disability that prevented participation or ability to provide consent, (3) current incarceration, and (4) HIV positive status. Written informed consent was obtained at study entry for each participant and the study was approved by the University of Southern California’s Institutional Review Board. Nine urinary OPE metabolite concentrations were measured in 426 participants’ urine samples provided during the third trimester study visit (mean GA at sample collection ± SD = 31.4 ± 1.8 weeks) from 2017 to 2019. Child neurobehavior was assessed using the Child Behavioral Checklist 1.5–5 years (CBCL 1.5–5) composite scales, including the internalizing problems, externalizing problems, and total problems scales, administered at the 36-month timepoint. As shown in the consort diagram (Fig. 1), mother-child participants with complete information on the exposure, outcome, and key covariates of interest were included in the final analytic sample. A total of 204 mother-child dyads with available data on OPE metabolite concentrations, the CBCL administered at 36 months, and key covariates were included in this study. Single spot urine samples were collected in 90 mL sterile specimen containers during a third trimester study visit. Urine specimens were aliquoted into 1.5 mL aliquot cryovials and specific gravity was measured in room temperature urine samples using a digital handheld refractometer (ATAGO PAL-10s pocket refractometer). Samples were stored at -80 ºC prior to shipment and sent to the Wadsworth Center’s Human Health Exposure Analysis Resource (HHEAR) lab hub for the analysis of the following nine OPE metabolites: diphenyl phosphate (DPHP), composite of di-n-butyl phosphate and di-isobutyl phosphate (DNBP + DIBP), bis(1,3,-dichloro-2-propyl) phosphate (BDCIPP), bis(2-chloroethyl) phosphate (BCEP), bis(butoxethyl) phosphate (BBOEP), bis(1-chloro-2-propyl) phosphate (BCIPP), bis(2-ethylhexyl) phosphate (BEHP), bis(2-methylphenyl) phosphate (BMPP), and dipropyl phosphate (DPRP). Additional information on each metabolite, the corresponding parent compound, and common uses are described in Table S1. Urinary OPE metabolites were quantified following methods similar to those previously described, with some slight modifications [42]. In brief, urine samples (0.5 mL) were aliquoted into pre-baked glass tubes and spiked with 1 ng of deuterated internal standard (IS) mixtures of OPEs and 1 mL of 10 mM ammonium acetate buffer (pH 5). The samples were passed through solid phase extraction (SPE) cartridges (STRATA-X-AW: 60 mg, 3 cc, Phenomenex, Torrance, CA, USA) which were conditioned by successive passage with 2 mL of 5% (v/v) ammonia/methanol, 2 mL of methanol, and 2 mL of water. The samples were loaded with the valves partially opened. The SPE cartridges were then dried under vacuum for 3 min after washing with 1.0 mL of water. Analytes were eluted with 2 times 0.5 mL of 5% (v/v) ammonia/methanol, concentrated under a gentle stream of nitrogen at 37 °C to near dryness, and reconstituted with 0.1 mL of acetonitrile. High-performance liquid chromatography (HPLC, ExionLC™ system; SCIEX, Redwood City, CA, USA), coupled with an AB SCIEX QTRAP 5500+ triple quadrupole mass spectrometer (TQMS, Applied Biosystems, Foster City, CA, USA), was used in the identification and quantification of target compounds. Nine OPE diester metabolites and corresponding 9 internal standards were separated by a Kinetex HILIC column (100 mm × 2.1 mm, 2.6 μm particle size; Phenomenex) serially connected to a Betasil C18 guard column (20 mm × 2.1 mm, 5 μm particle size; Thermo Scientific). The analytes were quantified by isotopic dilution method and an 11-point calibration curve (at concentrations ranging from 0.02 to 50 ng/mL) with the regression coefficient ≥ 0.998. Matrix spikes (synthetic and urine pool spiked with 1 ng of native standards and 1 ng of internal standards) were analyzed with real samples as quality control (QC) samples. For each batch of samples, replicates of reagent blanks, matrix blanks, and matrix spiked samples were processed. Replicates of HHEAR Urine Quality Control (QC) Pools Standard Reference Materials (SRM3672 and SRM3673, NIST, Gaithersburg, MD, USA) were analyzed with every batch of samples. Trace levels of all OPE diester metabolites were found in procedural blanks. OPE diester metabolite concentrations measured in blanks were subtracted from sample values. Matrix spiked samples had average recoveries of 70.4–133% (CV: ± 9–19%). Repeated analysis of HHEAR Urine QC Pools A and B among batches showed coefficients of variation of ± 12–31% and ± 12–30% respectively. SRM3672 and SRM3673 had coefficients of variation of ± 12–40% and ± 12–27% respectively. Target analytes limit of detection (LOD) ranged from 0.012 to 0.044 ng/mL. Due to poor chromatographic separation and co-elution of peaks accompanying a similar mass transition for DNBP and DIBP, these two isomers were reported as sum concentration of di-n-butyl phosphate and di-isobutyl phosphate (DNBP + DIBP). OPE metabolites with concentrations below the LOD were imputed using the LOD/\(\surd 2\) [43]. Metabolites were then specific gravity (SG) adjusted using the following formula: Pc = P[(SGm-1)/(SG-1)], where Pc is the specific gravity corrected toxicant concentration (ng/mL), P is the observed toxicant concentration (ng/mL), SGm is the median SG value among the study population (median = 1.016), and SG = the SG value of the sample. The Child Behavior Checklist for ages 1½ through 5 years old (CBCL 1.5–5) is a 99-item questionnaire which has been validated and widely used to assess a broad range of emotional and behavioral problems in children [44]. The questionnaire was orally administered to maternal participants during the 36 month study visit who indicated the frequency of behaviors in their child within the prior 2 months on a 3-point Likert scale (not true = 0, sometimes true = 1, or very often true = 2), with each raw scale created by summing together relevant items and t-scores and corresponding borderline (t-scores: 60–63) and clinical symptom categories (t-scores: ≥ 64) calculated based on previously described criteria to quantify areas that may warrant evaluation by a professional [45]. Higher scores across all CBCL scales indicate increasing problems. The CBCL consists of seven scored syndrome scales (emotionally reactive (9 items), anxious/depressed (8 items), somatic complaints (11 items), withdrawn (8 items), sleep problems (7 items), attention problems (5 items), aggressive behavior (19 items), and other problems (33 items)). These syndrome scales can be summed to create two composite scales, internalizing problems (emotionally reactive, anxious/depressed, somatic complaints, and withdrawn) and externalizing problems (attention problems and aggressive behavior). The CBCL additionally includes a total problems score which is the summed total of all 99 questionnaire items, plus the highest score on any additional problems listed under an open-ended item, question 100 (score range = 0–200). For the purposes of this analysis, the raw internalizing problems, externalizing problems, and total problems scores were each analyzed to encapsulate the breadth of potential behavioral and emotional developmental problems experienced by participants and to facilitate comparisons to prior studies similarly examining impacts of OPEs on raw CBCL scores [40]. However, sensitivity analyses examining associations between OPEs and CBCL t-scores were also evaluated to assess the robustness of our results after standardizing raw scores to a normative US sample of children. Covariates assessed in this analysis were study design or sample collection variables or were identified based on previous literature which examined impacts of neurotoxic chemicals on early neurobehavioral development [3, 31, 39, 40]. Relationships between prenatal OPE metabolites and neurobehavioral development were visualized using a Directed Acyclic Graph (DAG) created using DAGitty (Fig. S1) [46]. All models were adjusted for variables identified in the DAG’s minimal sufficient adjustment set (maternal age, parity, pre-pregnancy BMI, race/ethnicity, income, and education) and study design or sample collection variables whose inclusion in models changed the effect estimate of our exposure of interest by 10% or more (recruitment site, specimen collection season, GA at sample collection, and child adjusted age at CBCL administration). The only exception to these criteria was adjustment for maternal-reported smoking during pregnancy. Prenatal smoking was identified in the minimal sufficient adjustment set, but, given the small frequency of maternal smoking (n = 5, 2.5%), we instead evaluated its impact in sensitivity analyses by removing participants who reported smoking during pregnancy. Additionally, child sex was adjusted for in all models since it is an important predictor of neurobehavioral outcomes and was also evaluated as an effect modifier in adjusted models. Maternal age (years), household annual income during pregnancy (< $50,000, ≥ $50,000, do not know), education (≤ 12th grade, > 12th grade), race/ethnicity (White non-Hispanic, Black non-Hispanic, Hispanic, Multiracial non-Hispanic/Other non-Hispanic), maternal smoking during pregnancy (yes, no), and parity (first born, ≥ second born, missing) were collected via interviewer administered questionnaires in the participant’s preferred language (English or Spanish). Pre-pregnancy BMI was calculated using participant-reported pre-pregnancy weight and standing height measured by study staff at the first study visit using a commercial stadiometer (Perspectives Enterprises model P-AIM-101). Child sex assigned at birth was primarily abstracted from electronic medical records (n = 200, 98.0%), followed by maternal-reported child sex (n = 4, 2.0%) for cases in which abstracted sex could not be obtained. Child adjusted age at time of questionnaire administration was calculated in weeks using date of birth and date of questionnaire administration, corrected for premature birth (< 37 weeks). We examined participant demographic characteristics using means and frequencies. OPE metabolite distributions were explored using histograms, geometric means, percentile distributions, and metabolite detect frequencies. Given the generally right skewed distribution of OPE metabolites, Kruskal Wallis tests were conducted to evaluate bivariate associations between categorical covariates and OPE concentrations and Spearman correlations were performed to evaluate associations between OPE metabolites. The distribution of CBCL raw scores was right skewed with 7.4% and 2.5% of scores with a 0 on the internalizing and externalizing problems scales, respectively; therefore, CBCL scores were offset by 0.1 and natural log transformed prior to linear regression modeling. Locally Weighted Scatterplot Smoothing (LOWESS) plots between prenatal OPEs and CBCL composite scales were then evaluated, and due to non-linear associations that persisted after natural log transformation, OPE metabolites were categorized into exposure tertiles prior to linear regression modeling. For OPE biomarkers detected in > 80% of participants (DPHP, DNBP + DIBP, BDCIPP), OPE metabolites were categorized into tertiles of specific gravity adjusted exposure concentrations. For OPE metabolites detected in 50–80% of participants (BCEP, BBOEP, BCIPP), a three-level categorical variable was created, with the lowest category defined as concentrations < LOD, and the remaining detected values categorized as < median or ≥ median. For OPE biomarkers detected in < 50% of participants (BMPP, BEHP, DPRP), we modeled OPE biomarkers as binary variables that were detected (> LOD) or not detected (≤ LOD). Modeling assumptions for all linear regressions were evaluated and met. A statistical interaction between each OPE metabolite and child sex was also tested in linear regression models. Data were managed and linear regression models were analyzed using SAS v9.4 (SAS Institute, Inc., Cary, NC, USA). Generalized Additive Models (GAMs) with a smoothing term for natural log transformed OPE metabolites were also performed to evaluate possible non-linear associations between OPE metabolites and neurobehavioral outcomes using the R package “mgcv”. Consistent with prior literature, only metabolites with a detect frequency > 60% (DPHP, DNBP + DIBP, BDCIPP, BCEP, BBOEP) were evaluated using GAMs [47,48,37, 38]. This study has several important strengths. Its prospective design provided us with the opportunity to collect urine samples during potentially sensitive periods (i.e., pregnancy) to measure OPEs prior to our outcome of interest. An additional strength of this study was the use of prenatal urinary metabolites as a measure of in utero exposure to OPEs, given that maternal urinary OPE metabolites are considered reliable indicators of potential fetal OPE exposures [15]. We also measured various previously understudied OPE metabolites, including DNBP + DIBP, BCIPP, BCEP, BBOEP, DRPR, BMPP, and BEHP, which advances opportunities for risk assessment and subsequent interventions. Furthermore, the population evaluated in this study was largely comprised of pregnant individuals of Latin American origin, who are historically underrepresented in U.S. biomedical and population health research and disproportionally burdened by environmental exposures [75], providing us with the opportunity to inform environmental justice solutions. An additional strength of this study is the use of a flexible environmental mixture modeling approach to assess the association between mixtures of OPE metabolites and neurobehavioral outcomes at 36 months. However, our study also has some limitations. Since single spot urine samples collected during the third trimester were used to assess OPE exposures throughout pregnancy, there may have been some exposure misclassification. However, previous studies indicate moderate to good reproducibility for DPHP and BDCIPP levels throughout pregnancy [76, 77]. Additionally, although many key covariates identified in the literature were adjusted for, residual confounding could still be present, especially for postnatal OPE exposures, which could impact neurobehavioral outcomes. The relatively modest analytical sample analyzed in this study is another limitation since we may have been underpowered to detect associations between OPE mixtures and neurobehavioral outcomes. Furthermore, although our use of a flexible environmental mixture modeling approach was used to assess joint OPE exposures, we were unable to explore the impacts of joint OPE exposures among metabolites with low detect frequencies, such as BMPP, which we found to adversely impact neurobehavioral development. In this prospective pregnancy cohort of predominately low-income and Hispanic pregnant individuals living in Los Angeles, we found adverse associations between prenatal exposures to multiple previously understudied OPEs and children’s neurobehavioral outcomes at 36 months. There was also suggestive evidence of interactions between metabolites, highlighting the importance of evaluating OPEs beyond the effects of a single metabolite, along with non-linear and sex-specific associations between OPEs and children’s neurobehavioral development. Given the scarcity of studies evaluating associations between prenatal OPE metabolites and early neurobehavioral outcomes, additional studies exploring these associations, for exposures during both the prenatal and postnatal periods, are warranted.Methods
Study design
OPE metabolites
Health outcome assessment
Covariates
Statistical analysis
Conclusion
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request and after approval by the USC Institutional Review Board.
Abbreviations
- OPEs:
-
Organophosphate Esters
- MADRES:
-
Maternal And Developmental Risks from Environmental and Social Stressors
- CBCL:
-
Child Behavior Checklist
- DPHP:
-
Diphenyl phosphate
- DNBP + DIBP:
-
Di-n-butyl phosphate and di-isobutyl phosphate
- BDCIPP:
-
Bis(1,3,-dichloro-2-propyl) phosphate
- BCEP:
-
Bis(2-chloroethyl) phosphate
- BBOEP:
-
Bis(butoxethyl) phosphate
- BCIPP:
-
Bis(1-chloro-2-propyl) phosphate
- BMPP:
-
Bis(2-methylphenyl) phosphate
- BEHP:
-
Bis(2-ethylhexyl) phosphate
- DPRP:
-
Dipropyl phosphate
- BKMR:
-
Bayesian kernel Machine Regression
- PBDEs:
-
Polybrominated diphenyl ethers
- DAG:
-
Directed Acyclic Graph
- BMI:
-
Body mass index
- GA:
-
Gestational age
- LOWESS:
-
Locally Weighted Scatterplot Smoothing
- GAM:
-
Generalized additive model
- CrI:
-
Credible Interval
- CI:
-
Confidence Interval
- MCMC:
-
Markov chain Monte Carlo
- WAIC:
-
Watanabe-Akaike information criterion
- PIPs:
-
Posterior Inclusion Probabilities
References
Salisbury AL, Fallone MD, Lester B. Neurobehavioral assessment from fetus to infant: the NICU network neurobehavioral scale and the fetal neurobehavior coding scale. Ment Retard Dev Disabil Res Rev. 2005;11:14–20. https://doi.org/10.1002/mrdd.20058.
Monk C, Hane A. Fetal and infant brain–behavior development: milestones & environmental influences. 2014.
Grandjean P, Landrigan PJ. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014;13:330–8. https://doi.org/10.1016/S1474-4422(13)70278-3.
Rauh VA, Margolis AE. Research review: environmental exposures, neurodevelopment, and child mental health - new paradigms for the study of brain and behavioral effects. J Child Psychol Psychiatry. 2016;57:775–93. https://doi.org/10.1111/jcpp.12537.
Barker DJP. The origins of the developmental origins theory. J Intern Med. 2007;261:412–7. https://doi.org/10.1111/j.1365-2796.2007.01809.x.
Pantelaki I, Voutsa D. Organophosphate flame retardants (OPFRs): a review on analytical methods and occurrence in wastewater and aquatic environment. Sci Total Environ. 2019;649:247–63. https://doi.org/10.1016/j.scitotenv.2018.08.286.
Yang J, Zhao Y, Li M, et al. A review of a class of emerging contaminants: the classification, distribution, intensity of consumption, synthesis routes, environmental effects and expectation of pollution abatement to organophosphate flame retardants (OPFRs). Int J Mol Sci. 2019;20(12):2874. https://doi.org/10.3390/ijms20122874.
Blum A, Behl M, Birnbaum L, et al. Organophosphate ester flame retardants: are they a regrettable substitution for polybrominated diphenyl ethers? Environ Sci Technol Lett. 2019;6:638–49. https://doi.org/10.1021/acs.estlett.9b00582.
Dodson RE, Perovich LJ, Covaci A, et al. After the PBDE phase-out: a broad suite of flame retardants in repeat house dust samples from California. Environ Sci Technol. 2012;46:13056–66. https://doi.org/10.1021/es303879n.
Bergman A, Rydén A, Law RJ, et al. A novel abbreviation standard for organobromine, organochlorine and organophosphorus flame retardants and some characteristics of the chemicals. Environ Int. 2012;49:57–82. https://doi.org/10.1016/j.envint.2012.08.003.
Stapleton HM, Klosterhaus S, Eagle S, et al. Detection of organophosphate flame retardants in furniture foam and U.S. house dust. Environ Sci Technol. 2009;43:7490–5. https://doi.org/10.1021/es9014019.
Stapleton HM, Klosterhaus S, Keller A, et al. Identification of flame retardants in polyurethane foam collected from baby products. Environ Sci Technol. 2011;45:5323–31. https://doi.org/10.1021/es2007462.
Hoffman K, Butt CM, Webster TF, et al. Temporal trends in exposure to organophosphate flame retardants in the United States. Environ Sci Technol Lett. 2017;4:112–8. https://doi.org/10.1021/acs.estlett.6b00475.
Vuong AM, Yolton K, Cecil KM, et al. Flame retardants and neurodevelopment: an updated review of epidemiological literature. Curr Epidemiol Rep. 2020;7:220–36. https://doi.org/10.1007/s40471-020-00256-z.
Hou R, Xu Y, Wang Z. Review of OPFRs in animals and humans: absorption, bioaccumulation, metabolism, and internal exposure research. Chemosphere. 2016;153:78–90. https://doi.org/10.1016/j.chemosphere.2016.03.003.
Bollmann UE, Möller A, **e Z, et al. Occurrence and fate of organophosphorus flame retardants and plasticizers in coastal and marine surface waters. Water Res. 2012;46:531–8.
Dodson RE, Rodgers KM, Carey G, et al. Flame retardant chemicals in college dormitories: flammability standards influence dust concentrations. Environ Sci Technol. 2017;51:4860–9.
Giulivo M, Capri E, Kalogianni E, et al. Occurrence of halogenated and organophosphate flame retardants in sediment and fish samples from three European river basins. Sci Total Environ. 2017;586:782–91.
Ali N, Malik RN, Mehdi T, et al. Organohalogenated contaminants (OHCs) in the serum and hair of pet cats and dogs: biosentinels of indoor pollution. Sci Total Environ. 2013;449:29–36.
Yang F, Ding J, Huang W, et al. Particle size-specific distributions and preliminary exposure assessments of organophosphate flame retardants in office air particulate matter. Environ Sci Technol. 2014;48:63–70.
Möller A, Sturm R, **e Z, et al. Organophosphorus flame retardants and plasticizers in airborne particles over the Northern Pacific and Indian Ocean toward the polar regions: evidence for global occurrence. Environ Sci Technol. 2012;46:3127–34.
Kim J-W, Isobe T, Chang K-H, et al. Levels and distribution of organophosphorus flame retardants and plasticizers in fishes from Manila Bay, the Philippines. Environ Pollut. 2011;159:3653–9.
Bika SH, Adeniji AO, Okoh AI, et al. Spatiotemporal distribution and analysis of organophosphate flame retardants in the environmental systems: a review. Molecules. 2022;27:573. https://doi.org/10.3390/molecules27020573.
National Academies of Sciences E and Medicine. Why indoor chemistry matters. Washington, DC: The National Academies Press; 2022. p. 190.
Varshavsky JR, Robinson JF, Zhou Y, et al. Organophosphate flame retardants, highly fluorinated chemicals, and biomarkers of placental development and disease during mid-gestation. Toxicol Sci. 2021;181:215–28. https://doi.org/10.1093/toxsci/kfab028.
Ding J, Xu Z, Huang W, et al. Organophosphate ester flame retardants and plasticizers in human placenta in Eastern China. Sci Total Environ. 2016;554–555:211–7. https://doi.org/10.1016/j.scitotenv.2016.02.171.
Zhao F, Chen M, Gao F, et al. Organophosphorus flame retardants in pregnant women and their transfer to chorionic villi. Environ Sci Technol. 2017;51:6489–97. https://doi.org/10.1021/acs.est.7b01122.
Bai XY, Lu SY, **e L, et al. A pilot study of metabolites of organophosphorus flame retardants in paired maternal urine and amniotic fluid samples: potential exposure risks of tributyl phosphate to pregnant women. Environ Sci Process Impacts. 2019;21:124–32. https://doi.org/10.1039/c8em00389k.
Doherty BT, Hammel SC, Daniels JL, et al. Organophosphate esters: are these flame retardants and plasticizers affecting children’s health? Curr Environ Health Rep. 2019;6:201–13. https://doi.org/10.1007/s40572-019-00258-0.
Wang X, Chen P, Zhao L, et al. Transplacental behaviors of organophosphate tri- and diesters based on paired human maternal and cord whole blood: efficiencies and impact factors. Environ Sci Technol. 2021;55:3091–100. https://doi.org/10.1021/acs.est.0c06095.
Hoffman K, Lorenzo A, Butt CM, et al. Predictors of urinary flame retardant concentration among pregnant women. Environ Int. 2017;98:96–101. https://doi.org/10.1016/j.envint.2016.10.007.
Ospina M, Jayatilaka NK, Wong LY, et al. Exposure to organophosphate flame retardant chemicals in the U.S. general population: data from the 2013–2014 National Health and Nutrition Examination Survey. Environ Int. 2018;110:32–41. https://doi.org/10.1016/j.envint.2017.10.001.
Dishaw LV, Powers CM, Ryde IT, et al. Is the PentaBDE replacement, tris (1,3-dichloro-2-propyl) phosphate (TDCPP), a developmental neurotoxicant? Studies in PC12 cells. Toxicol Appl Pharmacol. 2011;256:281–9. https://doi.org/10.1016/j.taap.2011.01.005.
Program NT. NTP toxicology and carcinogenesis studies of tris (2-chloroethyl) phosphate (CAS no. 115–96–8) in F344/N rats and B6C3F1 mice (gavage studies). Natl Toxicol Program Tech Rep Ser. 1991;391:1–233.
Tilson H, Veronesi B, McLamb R, et al. Acute exposure to tris (2-chloroethyl) phosphate produces hippocampal neuronal loss and impairs learning in rats. Toxicol Appl Pharmacol. 1990;106:254–69.
Wang Q, Lai NL-S, Wang X, et al. Bioconcentration and transfer of the organophorous flame retardant 1,3-dichloro-2-propyl phosphate causes thyroid endocrine disruption and developmental neurotoxicity in zebrafish larvae. Environ Sci Technol. 2015;49:5123–32. https://doi.org/10.1021/acs.est.5b00558.
Patisaul HB, Behl M, Birnbaum LS, et al. Beyond cholinesterase inhibition: developmental neurotoxicity of organophosphate ester flame retardants and plasticizers. Environ Health Perspect. 2021;129:105001. https://doi.org/10.1289/ehp9285.
Doherty BT, Hoffman K, Keil AP, et al. Prenatal exposure to organophosphate esters and behavioral development in young children in the Pregnancy, Infection, and Nutrition Study. Neurotoxicology. 2019;73:150–60. https://doi.org/10.1016/j.neuro.2019.03.007.
Castorina R, Bradman A, Stapleton HM, et al. Current-use flame retardants: maternal exposure and neurodevelopment in children of the CHAMACOS cohort. Chemosphere. 2017;189:574–80. https://doi.org/10.1016/j.chemosphere.2017.09.037.
Sugeng EJ, de Cock M, Leonards PEG, et al. Association of exposure to organophosphate flame retardants and children’s behavior at a median age of 18 months. Environ Adv. 2021;5:100077. https://doi.org/10.1016/j.envadv.2021.100077.
Bastain TM, Chavez T, Habre R, et al. Study design, protocol and profile of the Maternal And Developmental Risks from Environmental and Social Stressors (MADRES) pregnancy cohort: a prospective cohort study in predominantly low-income Hispanic women in urban Los Angeles. BMC Pregnancy Childbirth. 2019;19:189–189. https://doi.org/10.1186/s12884-019-2330-7.
Wang Y, Li W, Martínez-Moral MP, et al. Metabolites of organophosphate esters in urine from the United States: concentrations, temporal variability, and exposure assessment. Environ Int. 2019;122:213–21. https://doi.org/10.1016/j.envint.2018.11.007.
Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg. 1990;5:46–51. https://doi.org/10.1080/1047322X.1990.10389587.
Achenbach TM. Manual for the ASEBA preschool forms & profiles : an integrated system of multi-informant assessment. 2000.
Achenbach. Manual for the ASEBA preschool forms & profiles: an integrated system of multi-informant assessment. 2000.
Textor J, van der Zander B, Gilthorpe MS, et al. Robust causal inference using directed acyclic graphs: the R package ‘dagitty.’ Int J Epidemiol. 2016;45:1887–94. https://doi.org/10.1093/ije/dyw341.
Howe CG, Claus Henn B, Eckel SP, et al. Prenatal metal mixtures and birth weight for gestational age in a predominately lower-income Hispanic pregnancy cohort in Los Angeles. Environ Health Perspect. 2020;128:117001. https://doi.org/10.1289/ehp7201.
Howe CG, Claus Henn B, Farzan SF, et al. Prenatal metal mixtures and fetal size in mid-pregnancy in the MADRES study. Environ Res. 2021;196:110388. https://doi.org/10.1016/j.envres.2020.110388.
Liu W, Luo D, **a W, et al. Prenatal exposure to halogenated, aryl, and alkyl organophosphate esters and child neurodevelopment at two years of age. J Hazard Mater. 2021;408:124856. https://doi.org/10.1016/j.jhazmat.2020.124856.
Bobb JF, Valeri L, Claus Henn B, et al. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics. 2015;16:493–508. https://doi.org/10.1093/biostatistics/kxu058.
Bobb JF, Claus Henn B, Valeri L, et al. Statistical software for analyzing the health effects of multiple concurrent exposures via Bayesian kernel machine regression. Environ Health. 2018;17:67. https://doi.org/10.1186/s12940-018-0413-y.
Antonelli J, Mazumdar M, Bellinger D, et al. Estimating the health effects of environmental mixtures using Bayesian semiparametric regression and sparsity inducing priors. Ann Appl Stat. 2020;14:257–75.
Choi G, Keil AP, Richardson DB, et al. Pregnancy exposure to organophosphate esters and the risk of attention-deficit hyperactivity disorder in the Norwegian mother, father and child cohort study. Environ Int. 2021;154:106549. https://doi.org/10.1016/j.envint.2021.106549.
Lipscomb ST, McClelland MM, MacDonald M, et al. Cross-sectional study of social behaviors in preschool children and exposure to flame retardants. Environ Health. 2017;16:23. https://doi.org/10.1186/s12940-017-0224-6.
Hausherr V, van Thriel C, Krug A, et al. Impairment of glutamate signaling in mouse central nervous system neurons in vitro by tri-ortho-cresyl phosphate at noncytotoxic concentrations. Toxicol Sci. 2014;142:274–84. https://doi.org/10.1093/toxsci/kfu174.
Hogberg HT, de Cássia da Silveira ESR, Kleensang A, et al. Organophosphorus flame retardants are developmental neurotoxicants in a rat primary brainsphere in vitro model. Arch Toxicol. 2021;95:207–28. https://doi.org/10.1007/s00204-020-02903-2.
Yang W, Zhao F, Fang Y, et al. (1)H-nuclear magnetic resonance metabolomics revealing the intrinsic relationships between neurochemical alterations and neurobehavioral and neuropathological abnormalities in rats exposed to tris(2-chloroethyl)phosphate. Chemosphere. 2018;200:649–59. https://doi.org/10.1016/j.chemosphere.2018.02.056.
Liu X, Zhao X, Wang Y, et al. Triphenyl phosphate permeates the blood brain barrier and induces neurotoxicity in mouse brain. Chemosphere. 2020;252:126470. https://doi.org/10.1016/j.chemosphere.2020.126470.
Gant DB, Eldefrawi ME, Eldefrawi AT. Action of organophosphates on GABAA receptor and voltage-dependent chloride channels. Fundam Appl Toxicol. 1987;9:698–704. https://doi.org/10.1016/0272-0590(87)90176-x.
Shi Q, Wang M, Shi F, et al. Developmental neurotoxicity of triphenyl phosphate in zebrafish larvae. Aquat Toxicol. 2018;203:80–7. https://doi.org/10.1016/j.aquatox.2018.08.001.
Zhong X, Wu J, Ke W, et al. Neonatal exposure to organophosphorus flame retardant TDCPP elicits neurotoxicity in mouse hippocampus via microglia-mediated inflammation in vivo and in vitro. Arch Toxicol. 2020;94:541–52. https://doi.org/10.1007/s00204-019-02635-y.
Slotkin TA, Skavicus S, Stapleton HM, et al. Brominated and organophosphate flame retardants target different neurodevelopmental stages, characterized with embryonic neural stem cells and neuronotypic PC12 cells. Toxicology. 2017;390:32–42. https://doi.org/10.1016/j.tox.2017.08.009.
Wu S, Ji G, Liu J, et al. TBBPA induces developmental toxicity, oxidative stress, and apoptosis in embryos and zebrafish larvae (Danio rerio). Environ Toxicol. 2016;31:1241–9. https://doi.org/10.1002/tox.22131.
Behl M, Hsieh J-H, Shafer TJ, et al. Use of alternative assays to identify and prioritize organophosphorus flame retardants for potential developmental and neurotoxicity. Neurotoxicol Teratol. 2015;52:181–93. https://doi.org/10.1016/j.ntt.2015.09.003.
Ryan KR, Sirenko O, Parham F, et al. Neurite outgrowth in human induced pluripotent stem cell-derived neurons as a high-throughput screen for developmental neurotoxicity or neurotoxicity. Neurotoxicology. 2016;53:271–81. https://doi.org/10.1016/j.neuro.2016.02.003.
Shafer TJ, Brown JP, Lynch B, et al. Evaluation of chemical effects on network formation in cortical neurons grown on microelectrode arrays. Toxicol Sci. 2019;169:436–55. https://doi.org/10.1093/toxsci/kfz052.
Phillips AL, Chen A, Rock KD, et al. Editor’s highlight: transplacental and lactational transfer of Firemaster® 550 components in dosed Wistar rats. Toxicol Sci. 2016;153:246–57.
Baldwin KR, Phillips AL, Horman B, et al. Sex specific placental accumulation and behavioral effects of developmental Firemaster 550 exposure in Wistar rats. Sci Rep. 2017;7:1–13.
Rock KD, Horman B, Phillips AL, et al. EDC IMPACT: molecular effects of developmental FM 550 exposure in Wistar rat placenta and fetal forebrain. Endocr Connect. 2018;7:305.
Rock KD, St Armour G, Horman B, et al. Effects of prenatal exposure to a mixture of organophosphate flame retardants on placental gene expression and serotonergic innervation in the fetal rat brain. Toxicol Sci. 2020;176:203–23.
Ghassabian A, Henrichs J, Tiemeier H. Impact of mild thyroid hormone deficiency in pregnancy on cognitive function in children: lessons from the Generation R Study. Best Pract Res Clin Endocrinol Metab. 2014;28:221–32.
Yao Y, Li M, Pan L, et al. Exposure to organophosphate ester flame retardants and plasticizers during pregnancy: thyroid endocrine disruption and mediation role of oxidative stress. Environ Int. 2021;146:106215. https://doi.org/10.1016/j.envint.2020.106215.
Demeneix BA. Evidence for prenatal exposure to thyroid disruptors and adverse effects on brain development. Europ Thyroid J. 2019;8:283–92. https://doi.org/10.1159/000504668.
Luo K, Liu J, Wang Y, et al. Associations between organophosphate esters and sex hormones among 6–19-year old children and adolescents in NHANES 2013–2014. Environ Int. 2020;136:105461. https://doi.org/10.1016/j.envint.2020.105461.
Buckley JP, Kuiper JR, Bennett DH, et al. Exposure to Contemporary and emerging chemicals in commerce among pregnant women in the United States: the Environmental influences on Child Health Outcome (ECHO) program. Environ Sci Technol. 2022;56:6560–73. https://doi.org/10.1021/acs.est.1c08942.
Hoffman K, Daniels JL, Stapleton HM. Urinary metabolites of organophosphate flame retardants and their variability in pregnant women. Environ Int. 2014;63:169–72. https://doi.org/10.1016/j.envint.2013.11.013.
Romano ME, Hawley NL, Eliot M, et al. Variability and predictors of urinary concentrations of organophosphate flame retardant metabolites among pregnant women in Rhode Island. Environ Health. 2017;16:1–11.
Acknowledgements
We are indebted to the MADRES study families, nurses, midwives, physicians, and staff at each of our study sites for their cooperation and participation and especially to the members of the MADRES study team for their efforts to improve the health of underserved communities.
Funding
This work was supported by the Maternal and Developmental Risks from Environmental and Social Stressors (MADRES) Center (grant #s P50ES026086, 83615801, P50MD015705) funded by the National Institute of Environmental Health Sciences, the National Institute for Minority Health and Health Disparities and the Environmental Protection Agency; the Southern California Environmental Health Sciences Center (grant # 5P30ES007048) funded by the National Institute of Environmental Health Sciences, and the Life course Approach to Developmental Repercussions of Environmental Agents on Metabolic and Respiratory health (LA DREAMERs) (grant #s UH3OD023287) funded by the National Institutes of Health Office of the Director ECHO Program. Research reported in this publication was supported by the National Institute of Environmental Health Sciences Award Number U2CES026542. Dr. Howe is supported by an NIH Pathway to Independence Award (R00 ES030400).
Author information
Authors and Affiliations
Contributions
IHC carried out the analysis, drafted the initial manuscript, and reviewed and revised the manuscript. SPE and CGH contributed to the study design, statistical approach, verified the analytical methods and statistical interpretations, and revised the manuscript. ZN contributed to the statistical methods and manuscript editing. KK and MR led exposure quantification and contributed to manuscript editing. HBF managed sample collection and contributed to manuscript editing. TY, MJV, and XC supported data curation and manuscript editing. BG, DL, NL, and LA provided clinical input and contributed to manuscript editing. MTA contributed to statistical interpretations and manuscript editing. RH, GFD, SFF, and CVB led and conceptualized the cohort design, investigation, and manuscript editing. TMB led and conceptualized the cohort design, investigation, conceptualization, and contributed to manuscript editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Written informed consent was obtained at study entry for each participant and the study was approved by the University of Southern California’s Institutional Review Board (ethics approval number = HS-15-00498).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplemental Figure 1.
Directed Acyclic Graph (DAG) of Prenatal OPE metabolites and Child Neurobehavioral Development. Supplemental Figure 2. Associations Between Urinary Prenatal OPE Metabolite Concentrations (ng/mL) and Internalizing Scores by Child Sex, Using Generalized Additive Models (N = 204). Supplemental Figure 3. Associations Between Urinary Prenatal OPE Metabolite Concentrations (ng/mL) and Externalizing Scores by Child Sex, Using Generalized Additive Models (N = 204). Supplemental Figure 4. Associations Between Urinary Prenatal OPE Metabolite Concentrations (ng/mL) and Total Problems Scores by Child Sex, Using Generalized Additive Models (N = 204). Supplemental Figure 5. Associations Between Urinary Prenatal OPE Metabolite Concentrations (ng/mL) and CBCL Composite T-Scores, Using Generalized Additive Models (N = 204). Supplemental Figure 6. Associations Between Urinary Prenatal OPE Metabolite Concentrations (ng/mL) and CBCL Composite Raw Scores Among Participants Who Reported No In-Utero Smoking, Using Generalized Additive Models (N = 199). Supplemental Figure 7. Posterior Inclusion Probabilities (PIPs) for Pairwise Interactions Between OPE Metabolites and CBCL Composite Raw Scores Using NLinteraction Method. Supplemental Figure 8. Prenatal DNBP+DIBP Exposures and Children’s Total Problems Scores by Tertiles of BCEP, Using Generalized Additive Models. Supplemental Figure 9. Prenatal OPE Urinary Metabolite Mixtures (ng/mL) and CBCL Composite T-Scores, Using BKMR (N = 204). Supplemental Figure 10. Prenatal OPE Urinary Metabolite Mixtures (ng/mL) and CBCL Composite Raw Scores Among Participants Who Reported No In-Utero Smoking, Using BKMR (N = 199). Supplemental Figure 11. Prenatal OPE Urinary Metabolite Mixtures (ng/mL) and CBCL Composite Raw Scores, Using BKMR Varying the Smoothing Parameter to b = 50. Supplemental Figure 12. Prenatal OPE Urinary Metabolite Mixtures (ng/mL) and CBCL Composite Raw Scores, Using BKMR Varying the Smoothing Parameter to b = 1000. Supplemental Figure 13. Posterior Inclusion Probabilities (PIPs) for Pairwise Interactions Between OPE Metabolites and CBCL Composite Raw Scores Using NLinteraction Method and Increasing the Threshold to 0.25. Supplemental Figure 14. Prenatal OPE Urinary Metabolite Mixtures and CBCL Composite Raw Scores, Using BKMR and Metabolites with Detect Frequency >80% Only (N = 204). Supplemental Figure 15. Bivariate Associations Between Prenatal OPE Urinary Metabolite Mixtures (ng/mL) and CBCL Composite Raw Scores, Using BKMR and Metabolites with Detect Frequency >80% Only (N = 204). Supplemental Table 1. Parent Compounds of OPE Metabolites Analyzed and Common Applications. Supplemental Table 2. Distribution of Specific Gravity Adjusted OPE Concentrations (ng/mL) in Urine for Maternal Participants Analyzed (N = 204) vs the Full Sample of Maternal Participants with OPEs Available (N = 426). Supplemental Table 3. Comparison of Participant Characteristics Analyzed in the Analytical Dataset (N = 204) to Subset with OPE Metabolite Concentrations Available (N = 426) and Full MADRES Participants Who Delivered Children in the Study by August 28th, 2022 (N = 774). Supplemental Table 4. Individual Associations Between Third Trimester Urinary OPE Metabolites (ng/mL) and CBCL Raw Composite Scores by Child Sex (N = 204). Supplemental Table 5. Individual Associations Between Third Trimester Urinary OPE Metabolites (ng/mL) and CBCL Composite T-Scores (N = 204). Supplemental Table 6. Individual Associations Between Third Trimester Urinary OPE Metabolites (ng/mL) and CBCL Raw Composite Scores Among Participants Who Reported No In-Utero Smoking (N = 199). Supplemental Table 7. Median Concentrations (ng/mL) of Urinary OPE Metabolites Across Published Studies.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hernandez-Castro, I., Eckel, S.P., Howe, C.G. et al. Prenatal exposures to organophosphate ester metabolite mixtures and children’s neurobehavioral outcomes in the MADRES pregnancy cohort. Environ Health 22, 66 (2023). https://doi.org/10.1186/s12940-023-01017-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12940-023-01017-3