Abstract
Tumor organoids, especially patient-derived organoids (PDOs) exhibit marked similarities in histopathological morphology, genomic alterations, and specific marker expression profiles to those of primary tumour tissues. They are applied in various fields including drug screening, gene editing, and identification of oncogenes. However, CAR-T therapy in the treatment of solid tumours is still at an exploratory stage. Tumour organoids offer unique advantages over other preclinical models commonly used for CAR-T therapy research, which the preservation of the biological characteristics of primary tumour tissue is critical for the study of early-stage solid tumour CAR-T therapies. Although some investigators have used this co-culture model to validate newly targeted CAR-T cells, optimise existing CAR-T cells and explore combination therapy strategies, there is still untapped potential in the co-culture models used today. This review introduces the current status of the application of tumour organoid and CAR-T cell co-culture models in recent years and commented on the limitations of the current co-cultivation model. Meanwhile, we compared the tumour organoid model with two pre-clinical models commonly used in CAR-T therapy research. Eventually, combined with the new progress of organoid technologies, optimization suggestions were proposed for the co-culture model from five perspectives: preserving or reconstructing the tumor microenvironment, systematization, vascularization, standardized culture procedures, and expanding the tumor organoids resource library, aimed at assisting related researchers to better utilize co-culture models.
Similar content being viewed by others
Introduction
Since the pioneering work of Sato et al. [1] in constructing colorectal cancer organoid models, there has been significant progress in the development of tumor organoids. The establishment and advancement of tumor organoids have provided a novel approach for creating more physiologically relevant human tumor models. In comparison to commonly used cancer models like cancer cell lines and primary patient-derived tumor xenografts (PDTXs), tumor organoids offer distinct advantages. The advantages have resulted in their utilization across diverse domains, encompassing drug screening, genome editing, and oncogene identification [2].
Chimeric Antigen Receptor T-Cell Immunotherapy (CAR-T therapy) has garnered significant interest as a burgeoning treatment for tumors, demonstrating promising outcomes in the management of specific hematological tumors such as B-cell acute lymphoblastic leukemia (B-ALL), B-cell non-Hodgkin lymphoma (B-NHL), and multiple myeloma (MM) [3,4,5]. The scientific community has shown considerable attention towards the efficacy of CAR-T therapy in addressing hematological tumors, perceiving it as a potential remedy for solid tumors. Nevertheless, the effectiveness of this therapy is constrained by factors such as tumor heterogeneity, limited transport and infiltration of CAR-T cells into tumor tissues, and the presence of immunosuppressive microenvironments within the tumor [6]. These crucial concerns emphasise the necessity of preclinical research models for CAR-T therapy.
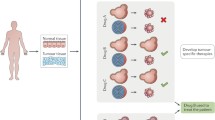
Tumor organoids can preserve primary tumour tissue characteristics more completely, allowing for a realistic simulation of the interaction between tumour and CAR-T cells in vitro. Researchers have co-cultured CAR-T cells with tumor organoids to validate the anti-tumour effects of new target CAR-T cells, modified CAR-T cells, and CAR-T combination therapies.
However, there remains a significant amount of unexplored research potential within the currently utilized co-culture models of tumor organoids and CAR-T cells. This review summarizes a total of 10 research papers on the use of tumor organoids for CAR-T therapy from March 2019 to June 2023. At the same time, based on the new advances in organoid technologies from September 2014 to June 2023, optimization suggestions were proposed for this co-culture model from five perspectives: preserving or reconstructing the immune microenvironment, systematization, vascularization, standardized culture procedures, and expanding the tumor organoid resource library. All cited articles are sourced from the Pubmed database. We hope that this review will offer a fresh perspective to assist CAR-T therapeutics researchers in the successful implementation of this co-culture model in both fundamental and translational CAR-T therapies.
A basic model for co-culture of tumor organoids with CAR-T cells
As of June 2020, there are currently over 500 ongoing clinical trials investigating CAR-T therapy for various tumor types, with numerous additional trials in the developmental phase. The development of a practical, cost-effective, and realistic in vitro model for early CAR-T research is of utmost importance as it allows for the evaluation of the anti-tumor activity of CAR-T cells, a detailed exploration of the mechanism of action of CAR-T cells, and an enhancement of the structural design of CAR-T cells. Furthermore, there is a growing clinical demand for personalized medicine models that can predict the efficacy of CAR-T treatments and identify viable combinations of CAR-T therapy strategies. Co-culture models of tumor organoids have become a prominent choice owing to their remarkable resemblance to the original tumor in terms of histopathological morphology, genomic alterations, and expression profiles of specific markers.
Submerged Matrigel culture is a classic approach for organoid culture and is applicable to tumor organoids [7]. This method involves dissociating tumor tissue into a dispersed suspension of tumor cells using enzymatic or physical techniques. These cells are then embedded in a gel and placed in a culture medium to facilitate the growth of tumor organoids. The culture medium not only contains essential components for organoid growth but also includes pathway inhibitors and/or growth factors. And the additives and culture conditions being adapted based on the tumor organoids type [8,9,10]. Common additions comprise Wnt3a, R-spondin, epidermal growth factor (EGF), and Noggin—a bone morphogenesis (BMP) inhibitor which together promote stem cell growth, differentiation, and self-renewal [11] (Fig. 1)
It should be emphasised that the application of the Submerged Matrigel culture approach for tumor organoids is not fixed and should be customized to suit the unique attributes of the tumor organoids. Jacob et al. [12] generated glioblastomas organoids by directly culturing micro-dissected tumor fragments, as opposed to dispersing the tumor tissues into cell suspensions. Tumour fragments were manipulated directly to ensure the survival of sensitive cells and reproduced the hypoxic gradient, whilst preserving part of the mesenchymal component in tumor organoids. This facilitated the study of tumour cell-mesenchymal cell interactions. Fujiii et al. [10] discovered that genetic mutations in tumour cells had a tendency to impact the nature of the additive. The majority of colorectal cancer (CRC) organoids and all adenoma organoids were capable of autonomous growth without the need for exogenous R-spondin/Wnt3a. This can be attributed to the existence of mutations in one of the pivotal protein genes involved in the Wnt signalling pathway, which sustains the activation of said pathway. For example, variation-induced activation of TCF7L2, CTNNB1, APC. Hence, it is imperative to establish the requirement for growth factor/pathway inhibitors based on the genetic characteristics of the tumor organoids. Incorrect application of growth factor/pathway grafts may result in induced clonal selection of the tumor organoids, while the incorporation of incorrect components may complicate the interpretation of drug therapy [12]. The instructions/literature should be read in detail to determine the appropriate culture procedure and additives for the use of Submerged Matrigel culture for tumor organoids.
How can the anti-tumour activity of CAR-T cells be accurately analyzed in a co-culture model? Three studies offer valuable insights into this inquiry. Zou et al. [13] utilised a Caspase3/7 green fluorescent probe to label tumor organoid cells undergoing apoptosis, and also employed flow cytometry to quantitatively analyse CAR-T cells with elevated killing virulence expressing CD107a, IFN-γ. Schnalzger et al. [14] performed lentiviral transduction of luciferase/GFP into tumor organoids. They quantified the anti-tumour activity of CAR-T cells based on the remaining fluorescence intensity, which decreased as tumor organoid cells died. Additionally, Yu et al. [15] labelled CAR-T cells with CD8 and granzyme B, observing differences between the two markers in experimental and control groups. LDH, IL-2, TNF-α, and IFN-γ in the cell matrix were quantified. In brief, the evaluation of the anti-tumour activity of CAR-T cells comprises three perspectives: apoptosis of tumor organoids, killing activity of CAR-T cells, and the content of relevant cytokines in the cell matrix.
Current application of tumor organoids and CAR-T cell co-culture models
Validation of the anti-tumour effect of newly targeted CAR-T cells
CAR-T therapy has demonstrated promising outcomes in specific haematological malignancies, but its efficacy in solid tumours is considerably limited. Despite the identification of several potential targets, it is crucial to investigate novel targets in order to advance the field [16]. Therefore, there is an urgent need for a preclinical model that can faithfully replicate the unique surface markers found on human cells and can be readily constructed to assess the therapeutic efficacy of CAR-T on new targets. In this regard, tumor organoids offer a viable solution.
Yu [15] and Jacob [12] independently developed organoid models for bladder cancer and glioblastoma, and proved the degree of preservation of tumor organoids on biological characteristics of primary tumor tissue. Subsequently, they conducted co-culturing experiments by introducing MUC1-CAR-T cells and EGFR VIII-CAR-T cells to their respective tumor organoids, thereby verified the anti-tumorigenic effect of these two innovative CAR-T cell targets. Tumors overexpressing MET are generally insensitive to small molecule targeted drugs therapy. In light of this, Chiriaco et al. [17] devised two MET-CAR constructs with distinct structures specifically for MET-overexpressing tumours, and assess the anti-tumour effect of these two constructs by utilising different tumour organoid models that overexpress MET. Both types of MET-CAR-T cells can overcome resistance to small molecule targeted drugs against MET, and their anti-tumor activity is correlated with MET expression levels. Li et al. [18] co-cultured NSCLC organoids expressing B7-H3 with B7-H3-CAR-T cells to verify their anti-tumor activity prior to brain metastasis. Then, CCR2b was expressed on the CAR-T cell surface. Finally, it was confirmed in the patient-derived tumor xenograft (PDTX) model that its binding with CCR2 on the surface of tumor cells can promote CCR2b-B7-H3-CAR-T cells to penetrate the blood–brain barrier.
Optimise existing CAR-T cells
The identification of novel targets broadens the potential applicability of CAR-T therapy, and on this basis, it is also meaningful to optimise existing CAR-T cells in multiple ways to enhance their killing effect. The presence of tumor organoids also provides a platform for evaluating the anti-tumour effect of CAR-T after optimisation.
The optimization of CAR-T cells can be undertaken from various perspectives, often involving the modification of the CAR structure to enhance the cytotoxicity of CAR-T cells. Thokala et al. [19] replaced the single stranded fragment variable region (scFv) of CD19-CAR-T cells with monoclonal antibody (mAb) 806, which can target various EGFR mutants. In order to evaluate the anti-tumor efficacy of improved CAR-T cells, researchers co-cultured them with GBM organoid containing multiple EGFR mutations. The improved CAR-T cells successfully targeted and eliminated multiple tumor copies, significantly reducing the possibility of esca** tumors through antigen loss. Wang et al. [20] constructed a modified CAR-T cell targeting glypican-3 (GPC3) in hepatocellular carcinoma (HCC).This modification involved substituting the cd8α-derived hinge region in the conventional CAR structure with a 4-1bb-derived hinge region containing 11 cysteine residues. By co-culturing with HCC organoids, it was observed that modified CAR-T cells showed stronger effectiveness in inhibiting tumor growth.
Zou et al. [13] attempted to optimize CAR-T therapy by screening a subset of CAR-T cells with strong anti-tumor activity. Researchers divided HBVs CAR-T cells into two groups based on whether express CD39. Subsequently, co-culturing these populations with HBV + HCC organoid models revealed that CD39 + HBVs CAR-T cells exhibited more significant apoptosis induction in HCC organoids. Therefore, CD39 can serve as an indicator to distinguish the subgroups of CAR-T cells with stronger activity in HBV + HCC.
Qiao et al. [44] developed microorganoids spheroids (MOSs) using emulsion microfluidics. Additionally, an automated MOS seeding, processing and imaging system has also been developed. Based on this efficient therapeutic analysis platform, I believe that the aforementioned prospects will soon be achievable. Due to the presence of TME, which leads to a poor response of CAR-T therapy to solid tumours, our knowledge of the mechanisms by which TME affects CAR-T cells is currently limited. However, tumor organoids co-culture models with preserved TME offer a realistic platform for further in-depth research in this area. Inadequate local infiltration of CAR-T cells following infusion is a frequent issue encountered by patients undergoing CAR-T cell therapy. It is closely related to the presence of TME, but there is also a correlation between the physiological process of CAR-T cells drilling out of blood vessels. The phenomenon of vascular mimicry has been confirmed in various malignant tumors [45], so what impact will this phenomenon have on the infiltration of CAR-T cells? This presents an interesting area for exploration. The construction of a co-culture model for vascularization provides strong support for the study of the interaction between CAR-T cells and tumor blood vessels.
Finally, the utilisation of tumor organoids co-culture models in scientific research in oncology treatments is not limited to CAR-T therapies. Schnalzger et al. [14] utilised colorectal cancer organoids to carry out killing toxicity assessments of CAR-NK. Recently, CD19-CAR-T cells have been used for the treatment of systemic lupus erythematosus, achieving encouraging therapeutic effects [46, 47]. At the same time, some research teams have successfully used organ chip technology to construct and simulate organoid models of autoimmune diseases [48, 49]. The good news from these two aspects has given us great inspiration—let's imagine that with the development of autoimmune disease organoid models, the autoimmune disease organoids and CAR-T cell co-culture models will become a preclinical model with great potential, just like the tumor organoids and CAR-T cell co culture models, widely used in preclinical research, pre infusion efficacy evaluation, and other aspects, which will benefit a large patient population. In brief, co-culture models are expected to see extensive use in future scientific research on adoptive cell therapy (ACT). The potential for growth in co-culture models remains high, and researchers are encouraged to conduct thorough explorations of such opportunities.
Availability of data and materials
Not applicable.
References
Sato T, Stange DE, Ferrante M, Vries RG, Van Es JH, Van den Brink S, Van Houdt WJ, Pronk A, Van Gorp J, Siersema PD, et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology. 2011;141:1762–72.
Drost J, Clevers H. Organoids in cancer research. Nat Rev Cancer. 2018;18:407–18.
Fry TJ, Shah NN, Orentas RJ, Stetler-Stevenson M, Yuan CM, Ramakrishna S, Wolters P, Martin S, Delbrook C, Yates B, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med. 2018;24:20–8.
Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, Braunschweig I, Oluwole OO, Siddiqi T, Lin Y, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377:2531–44.
Raje N, Berdeja J, Lin Y, Siegel D, Jagannath S, Madduri D, Liedtke M, Rosenblatt J, Maus MV, Turka A, et al. Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N Engl J Med. 2019;380:1726–37.
Maalej KM, Merhi M, Inchakalody VP, Mestiri S, Alam M, Maccalli C, Cherif H, Uddin S, Steinhoff M, Marincola FM, et al. CAR-cell therapy in the era of solid tumor treatment: current challenges and emerging therapeutic advances. Mol Cancer. 2023;22:20.
Lancaster MA, Knoblich JA. Organogenesis in a dish: modeling development and disease using organoid technologies. Science. 2014;345:1247125.
Drost J, van Jaarsveld RH, Ponsioen B, Zimberlin C, van Boxtel R, Buijs A, Sachs N, Overmeer RM, Offerhaus GJ, Begthel H, et al. Sequential cancer mutations in cultured human intestinal stem cells. Nature. 2015;521(7550):43–7.
Seino T, Kawasaki S, Shimokawa M, Tamagawa H, Toshimitsu K, Fujii M, Ohta Y, Matano M, Nanki K, Kawasaki K, et al. Human pancreatic tumor organoids reveal loss of stem cell niche factor dependence during disease progression. Cell Stem Cell. 2018;22:454–67.
Fujii M, Shimokawa M, Date S, Takano A, Matano M, Nanki K, Ohta Y, Toshimitsu K, Nakazato Y, Kawasaki K, et al. A Colorectal tumor organoid library demonstrates progressive loss of niche factor requirements during tumorigenesis. Cell Stem Cell. 2016;18:827–38.
Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–5.
Jacob F, Ming GL, Song H. Generation and biobanking of patient-derived glioblastoma organoids and their application in CAR T cell testing. Nat Protoc. 2020;15:4000–33.
Zou F, Tan J, Liu T, Liu B, Tang Y, Zhang H, Li J. The CD39+ HBV surface protein-targeted CAR-T and personalized tumor-reactive CD8+ T cells exhibit potent anti-HCC activity. Mol Ther. 2021;29:1794–807.
Schnalzger TE, de Groot MH, Zhang C, Mosa MH, Michels BE, Röder J, Darvishi T, Wels WS, Farin HF. 3D model for CAR-mediated cytotoxicity using patient-derived colorectal cancer organoids. EMBO J. 2019;38(12): e100928.
Yu L, Li Z, Mei H, Li W, Chen D, Liu L, Zhang Z, Sun Y, Song F, Chen W, et al. Patient-derived organoids of bladder cancer recapitulate antigen expression profiles and serve as a personal evaluation model for CAR-T cells in vitro. Clin Transl Immunol. 2021;10: e1248.
Ma S, Li X, Wang X, Cheng L, Li Z, Zhang C, Ye Z, Qian Q. Current progress in CAR-T cell therapy for solid tumors. Int J Biol Sci. 2019;15:2548–60.
Chiriaco C, Donini C, Cortese M, Ughetto S, Modica C, Martinelli I, Proment A, Vitali L, Fontani L, Casucci M, et al. Efficacy of CAR-T immunotherapy in MET overexpressing tumors not eligible for anti-MET targeted therapy. J Exp Clin Cancer Res. 2022;41:309.
Li H, Harrison EB, Li H, Hirabayashi K, Chen J, Li QX, Gunn J, Weiss J, Savoldo B, Parker JS, et al. Targeting brain lesions of non-small cell lung cancer by enhancing CCL2-mediated CAR-T cell migration. Nat Commun. 2022;13:2154.
Thokala R, Binder ZA, Yin Y, Zhang L, Zhang JV, Zhang DY, Milone MC, Ming GL, Song H, Oourke DM. High-affinity chimeric antigen receptor with cross-reactive scFv to clinically relevant EGFR oncogenic isoforms. Front Oncol. 2021;11: 664236.
Wang Y, Gao Y, Niu C, Wang B, Zhao S, Roex G, Qian J, Qie J, Chen L, Yi C, et al. Chimeric antigen receptor clustering via cysteines enhances T-cell efficacy against tumor. Cancer Immunol Immunother. 2022;71:2801–14.
Qiao Y, Chen J, Wang X, Yan S, Tan J, **a B, Chen Y, Lin K, Zou F, Liu B, et al. Enhancement of CAR-T cell activity against cholangiocarcinoma by simultaneous knockdown of six inhibitory membrane proteins. Cancer Commun (Lond). 2023;43:788–807.
Song EZ, Wang X, Philipson BI, Zhang Q, Thokala R, Zhang L, Assenmacher CA, Binder ZA, Ming GL, O’Rourke DM, et al. The IAP antagonist birinapant enhances chimeric antigen receptor T cell therapy for glioblastoma by overcoming antigen heterogeneity. Mol Ther Oncolytics. 2022;27:288–304.
de Visser KE, Joyce JA. The evolving tumor microenvironment: from cancer initiation to metastatic outgrowth. Cancer Cell. 2023;41(3):374–403.
Dijkstra KK, Cattaneo CM, Weeber F, Chalabi M, van de Haar J, Fanchi LF, Slagter M, van der Velden DL, Kaing S, Kelderman S, et al. Generation of tumor-reactive T cells by co-culture of peripheral blood lymphocytes and tumor organoids. Cell. 2018;174:1586-1598.e12.
Neal JT, Li X, Zhu J, Giangarra V, Grzeskowiak CL, Ju J, Liu IH, Chiou SH, Salahudeen AA, Smith AR, et al. Organoid modeling of the tumor immune microenvironment. Cell. 2018;175:1972-1988.e16.
Sontheimer-Phelps A, Hassell BA, Ingber DE. Modelling cancer in microfluidic human organs-on-chips. Nat Rev Cancer. 2019;19:65–81.
Kennedy LB, Salama AKS. A review of cancer immunotherapy toxicity. CA Cancer J Clin. 2020;70:86–104.
Brudno JN, Kochenderfer JN. Recent advances in CAR T-cell toxicity: mechanisms, manifestations and management. Blood Rev. 2019;34:45–55.
Baulu E, Gardet C, Chuvin N, Depil S. TCR-engineered T cell therapy in solid tumors: state of the art and perspectives. Sci Adv. 2023;9: eadf3700.
Kumari R, Ouyang X, Wang J, Xu X, Zheng M, An X, Li QX. Preclinical pharmacology modeling of chimeric antigen receptor T therapies. Curr Opin Pharmacol. 2021;61:49–61.
Skardal A, Murphy SV, Devarasetty M, Mead I, Kang HW, Seol YJ, Shrike Zhang Y, Shin SR, Zhao L, Aleman J, et al. Multi-tissue interactions in an integrated three-tissue organ-on-a-chip platform. Sci Rep. 2017;7:8837.
Nowak-Sliwinska P, van Beijnum JR, Griffioen CJ, Huinen ZR, Sopesens NG, Schulz R, Jenkins SV, Dings RPM, Groenendijk FH, Huijbers EJM, et al. Proinflammatory activity of VEGF-targeted treatment through reversal of tumor endothelial cell anergy. Angiogenesis. 2023;26:279–93.
Watson CL, Mahe MM, Múnera J, Howell JC, Sundaram N, Poling HM, Schweitzer JI, Vallance JE, Mayhew CN, Sun Y, et al. An in vivo model of human small intestine using pluripotent stem cells. Nat Med. 2014;20:1310–4.
Sun XY, Ju XC, Li Y, Zeng PM, Wu J, Zhou YY, Shen LB, Dong J, Chen YJ, Luo ZG. Generation of vascularized brain organoids to study neurovascular interactions. Elife. 2022;11: e76707.
Cakir B, **ang Y, Tanaka Y, Kural MH, Parent M, Kang YJ, Chapeton K, Patterson B, Yuan Y, He CS, et al. Engineering of human brain organoids with a functional vascular-like system. Nat Methods. 2019;16:1169–75.
Tan SY, Feng X, Cheng LKW, Wu AR. Vascularized human brain organoid on-chip. Lab Chip. 2023;23:2693–709.
Dekkers JF, Whittle JR, Vaillant F, Chen HR, Dawson C, Liu K, Geurts MH, Herold MJ, Clevers H, Lindeman GJ, et al. Modeling breast cancer using CRISPR-Cas9-mediated engineering of human breast organoids. J Natl Cancer Inst. 2020;112:540–4.
Wang S, Wang Y, Xun X, Zhang C, **ang X, Cheng Q, Hu S, Li Z, Zhu J. Hedgehog signaling promotes sorafenib resistance in hepatocellular carcinoma patient-derived organoids. J Exp Clin Cancer Res. 2020;39:22.
Shi R, Radulovich N, Ng C, Liu N, Notsuda H, Cabanero M, Martins-Filho SN, Raghavan V, Li Q, Mer AS, et al. Organoid cultures as preclinical models of non-small cell lung cancer. Clin Cancer Res. 2020;26(5):1162–74.
Gao D, Vela I, Sboner A, Iaquinta PJ, Karthaus WR, Gopalan A, Dowling C, Wanjala JN, Undvall EA, et al. Organoid cultures derived from patients with advanced prostate cancer. Cell. 2014;159:176–87.
Schutgens F, Rookmaaker MB, Margaritis T, Rios A, Ammerlaan C, Jansen J, Gijzen L, Vormann M, Vonk A, Viveen M, et al. Tubuloids derived from human adult kidney and urine for personalized disease modeling. Nat Biotechnol. 2019;37(3):303–13.
Sachs N, Papaspyropoulos A, Zomer-van Ommen DD, Heo I, Böttinger L, Klay D, Weeber F, Huelsz-Prince G, Iakobachvili N, Amatngalim GD, et al. Long-term expanding human airway organoids for disease modeling. EMBO J. 2019;38: e100300.
Yuan J, Li X, Yu S. Cancer organoid co-culture model system: novel approach to guide precision medicine. Front Immunol. 2023;13:1061388.
Wang Z, Boretto M, Millen R, Natesh N, Reckzeh ES, Hsu C, Negrete M, Yao H, Quayle W, Heaton BE, et al. Rapid tissue prototy** with micro-organospheres. Stem Cell Rep. 2022;17:1959–75.
Wei X, Chen Y, Jiang X, Peng M, Liu Y, Mo Y, Ren D, Hua Y, Yu B, Zhou Y, et al. Mechanisms of vasculogenic mimicry in hypoxic tumor microenvironments. Mol Cancer. 2021;20:7.
Müller F, Boeltz S, Knitza J, Aigner M, Völkl S, Kharboutli S, Reimann H, Taubmann J, Kretschmann S, Rösler W, et al. CD19-targeted CAR T cells in refractory antisynthetase syndrome. Lancet. 2023;401:815–8.
Mackensen A, Müller F, Mougiakakos D, Böltz S, Wilhelm A, Aigner M, Völkl S, Simon D, Kleyer A, Munoz L, et al. Anti-CD19 CAR T cell therapy for refractory systemic lupus erythematosus. Nat Med. 2022;28(10):2124–32.
Li ZA, Sant S, Cho SK, Goodman SB, Bunnell BA, Tuan RS, Gold MS, Lin H. Synovial joint-on-a-chip for modeling arthritis: progress, pitfalls, and potential. Trends Biotechnol. 2023;41:511–27.
Smith VM, Nguyen H, Rumsey JW, Long CJ, Shuler ML, Hickman JJ. A functional human-on-a-chip autoimmune disease model of myasthenia gravis for development of therapeutics. Front Cell Dev Biol. 2021;9: 745897.
Acknowledgements
Thanks to Zi-Ming Lu for the art direction of the figures and tables used in this article.
Funding
The Disciplinary Construction of Posts for Zhujiang Scholars (No: 4SG21005G).
Author information
Authors and Affiliations
Contributions
R-XN participated in literature search and manuscript writing work; C-YL, S-QW, and W-KL drafted some key content in the article and revised it according to the revision suggestions; thank you to the corresponding authors, Z-WH and XK, for their valuable feedback on the article.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ning, RX., Liu, CY., Wang, SQ. et al. Application status and optimization suggestions of tumor organoids and CAR-T cell co-culture models. Cancer Cell Int 24, 98 (2024). https://doi.org/10.1186/s12935-024-03272-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12935-024-03272-x