Abstract
DANCR is an RNA gene located on chr4. This gene has several splice variants. Up-regulation of DANCR has been reported in many types of cancers. This lncRNA is mainly located in the cytoplasm and regulates genes expression at post-transcriptional level. In fact, it acts as a molecular sponge for a variety of miRNAs, including miR-874-3P, miR-335, miR-149, miR-4319, miR-758-3p, miR-216a-5p, miR-874-3p, miR-33a-5p, miR-335-5p, miR-145-3p, miR-665, miR-345-5p and miR-125b-5p. DANCR also regulates activity of PI3K/AKT/NF-κB, Wnt/β-catenin, ERK/SMAD, MAPK, IL-6/JAK1/STAT3, Smad2/3, p53, FAK/PI3K/AKT/GSK3β/Snail pathways. In the current narrative review article, we summarize the roles of DANCR in the carcinogenesis, with an especial emphasis on its role in the development of osteosarcoma and lung, liver, pancreatic and colorectal cancers.
Similar content being viewed by others
Introduction
DANCR (Differentiation Antagonizing Non-Protein Coding RNA) is an RNA gene located on chr4: 52,712,257–52,723,623, plus strand. It has a size of 11,367 bases. This gene has 14 splice variants with sizes ranging from 272 bp (DANCR-207) to 6065 bp (DANCR-203), all of them being categorized as long non-coding RNA (lncRNA). This lncRNA has been regarded as a cancer-associated lncRNA, since its up-regulation has been reported in several cancer types in association with enhancement of cell proliferation and malignant properties [1]. DANCR regulates gene expression at post-transcriptional level [1]. Based on the findings obtained from RNA fluorescence in situ hybridization and expression assays in the cellular fractions, DANCR has been found to be primarily located in the cytoplasm [2]. In the current narrative review article, we summarize the roles of DANCR in the carcinogenesis, with an especial emphasis on its role in the development of osteosarcoma and lung, liver, pancreatic and colorectal cancers.
Cell line studies
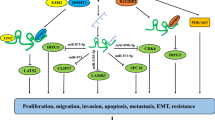
Up-regulation of DANCR has been shown to upsurge proliferation, migratory propensity, and invasiveness of osteosarcoma cells. From a functional aspect, DANCR promotes progression of osteosarcoma through induction of cancer stem cells properties. DANCR up-regulates expression of AXL through sequestering miR-33a-5p. Further, DANCR enhances activity of AXL/Akt pathway. Cumulatively, DANCR is an important regulator of osteosarcoma progression [2]. Another study in osteosarcoma cells has indicated that inhibition of DANCR leads to decrease in ROCK1-mediated proliferation and metastasis. Mechanistically, DANCR regulates expression of ROCK1 through sequestering miR-335-5p and miR-1972 [3]. Other studies have revealed the impacts of DANCR/miR-149/MSI2 axis [4] and DANCR/miR-216a-5p/SOX5 [5] axes in the pathoetiology of osteosarcoma. Moreover, METTL3 has been shown to contribute in this type of cancer through enhancement of stability of DANCR transcripts through m6A modification [6].
In bladder cancer cells, DANCR silencing has inhibited proliferation, migratory potential and invasion. DANCR has been shown to target miR-335/VEGF-C. miR-335 mimics could promote proliferation and invasive properties bladder cancer cells. In contrast, up-regulation of DANCR removes the effect of miR-335 mimics on these cells [7]. In addition, DANCR enhances metastatic and proliferative abilities of bladder cancer cells through increasing IL-11-STAT3 signals and CCND1 levels [8]. Finally, miR-149/MSI2 has been identified as another route of participation of DANCR in progression of bladder cancer [9].
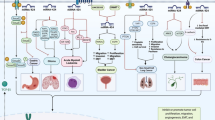
In lung cancer cells, DANCR expression levels have been negatively correlated with levels of miR-216a [10]. Another study has identified the impact of DANCR/miR-1225-3p/ErbB2 axis in the regulation of metastasis of lung cancer cells [11]. Moreover, DANCR participates in the progression of this type of cancer through sequestering miR‐496 and further modulating expression of mTOR [12]. DANCR can also regulate miR-214-5p/CIZ1 axis [13]. Moreover, invasive properties of lung cancer cells are regulated by DANCR through suppression of miR-216 and subsequent activation of Wnt/β-Catenin signals [14]. Figure 1 shows roles of DANCR in osteosarcoma, lung cancer, liver cancer, colorectal cancer, bladder cancer, and pancreatic cancer.
Hepatocellular carcinoma is another type of cancer in which DANCR has an important effect. Up-regulation of DANCR in these cells has been associated with down-regulation of miR-125b-5p. DANCR silencing or miR-125b-5p mimics could reduce cell cycle progression in HepG2 or Huh-7 cells, while promoting cell apoptosis. Both interventions could also inhibit migratory potential and invasiveness of these cells. Mechanistically, DANCR facilitates progression of this cancer through sponging miR-125b-5p and activating MAPK pathway [15]. DANCR could also contribute in the liver carcinogenesis through sponging miR‐216a‐5p and surging expression of KLF12 [16]. Another study in hepatocellular carcinoma cells has shown over-expression of DANCR and ATG7, and down-regulation of miR-222-3p. Besides, DANCR silencing has intimidated proliferation and autophagy of these cells. Mechanistically, DANCR induces proliferation, colony construction and autophagy of these cells through enhancing expression of ATG7 and decreasing expression of miR-222-3p [17]. Notably, DANCR can also affect response of hepatocellular carcinoma cells to sorafenib through enhancing activity of IL-6/STAT3 signals [18]. This lncRNA can also affect stemness and epithelial–mesenchymal transition (EMT) through modulating expression of CTNNB1 [19] and regulation of activity of ROCK1/LIMK1/COFILIN1 pathway [20], respectively.
In colorectal cancer cells, DANCR has been shown to affect activity of miR-125b-5p/HK2 axis to induce resistance to cisplatin through induction of anaerobic glycolysis [21]. In addition, DANCR/miR-518a-3p/MDMA axis has been identified as an imperative regulator of growth and malignant behavior of these malignant cells [22]. Most notably, the interaction between DANCR and the important oncogenic lncRNA MALAT1 has been found to induce resistance to doxorubicin-associated apoptosis in colorectal cancer cells [23].
In pancreatic cancer cells, DANCR regulates expression of miR-33b to promote proliferation and metastatic abilities [24]. Moreover, the invasive properties of these cells are regulated by DANCR/miR-214-5p/E2F2 [25] and DANCR/miR-135a/NLRP37 [26] axes. Figure 1 shows oncogenic roles of DANCR in osteosarcoma, lung cancer, liver cancer, colorectal cancer, bladder cancer, and pancreatic cancer. Expression of DANCR has been found to be increased in triple negative breast cancer cell lines. Notably, DANCR silencing has led to suppression of proliferation of these cells. Functional studies have detected that DANCR binding with RXRA enhances phosphorylation of this protein on its serine 49/78 via GSK3β, which subsequently leads to activation of PIK3CA transcription, and induction of PI3K/AKT signals [27]. Another study has shown over-expression of DANCR and VAPB in breast cancer cells, parallel with down-regulation of miR-4319. DANCR silencing not only has stalled proliferation, migratory potential, and invasiveness of breast cancer cells, but also has induced their apoptosis. These effects have been found to be mediated through regulation of miR-4319. This study has revealed the importance of DANCR/miR-4319/VAPB axis in development of this cancer [28]. Another mechanism of involvement of DANCR in the pathogenesis of breast cancer is mediated through enhancement of the EZH2 binding to the promoter of SOCS3, which results in suppression of expression of SOCS3. Up-regulation of SOCS3 or suppression of EZH2 has led to reversion of malignant features stimulated by DANCR [29].
Expression of DANCR has been found to be high in cisplatin-resistant gastric cancer cells. However, siRNA-mediated silencing of this lncRNA in SGC7901/DDP and BGC823/DDP cells has led to significant decrease in their survival and induction of apoptosis. Furthermore, DANCR up-regulation could up-regulate expression levels of MDR1 and MRP1 in cisplatin resistant gastric cancer cells [30]. Another study in gastric cancer cells has shown that KLF5 activates DANCR transcription. DANCR could act as a molecular sponge for miR-194 to suppress its expression and increase expression of AKT2, thus promoting gastric carcinogenesis through inhibition of autophagy [31]. Moreover, expression of DANCR in gastric cancer can be induced by SALL4 [32].
Table 1 summarizes the molecular axes mediating the effects of DANCR in the carcinogenesis, based on the results of in vitro studies.
Animal studies
Up-regulation of DANCR in osteosarcoma cells has been shown to promote xenograft tumor growth and lung metastases [2]. Critical roles of this lncRNA in induction of metastatic pathways have also been confirmed in animal models of colon cancer [22], nasopharyngeal carcinoma [73] and prostate cancer [85]. Moreover, results of experiments in animal models of cancer have suggested the impact of DANCR in resistance to sorafenib and cisplatin in hepatocellular carcinoma [18] and colon cancer [21], respectively. Moreover, bulk of evidence from investigations in xenograft models of cancer firmly supports the role of DANCR in induction of tumor growth (Table 2).
Clinical studies
Expression of DANCR has been constantly enhanced in osteosarcoma samples, and its up-regulation has been positively associated with size of tumors and their metastatic ability. In fact, it is regarded as an independent poor prognostic factor for osteosarcoma. Besides, in patient samples, DANCR expression has been positively correlated with AXL levels and negatively correlated with expression levels of miR-33a-5p [2]. DANCR over-expression has also been detected in lung cancer, principally in high-grade samples and aggressive tumors [10]. Expression assays in hepatocellular cancer tissues have revealed over-expression of DANCR and ATG7, and down-regulation of miR-222-3p. Notably, DANCR levels have been positively correlated with poor clinical outcome in these patients [17]. Another study in hepatocellular carcinoma has shown up-regulation of DANCR in tumor and plasma samples in correlation with microvascular and hepatic capsule invasion. Most remarkably, plasma levels of DANCR have shown more appropriate discriminatory power for separation of patients with hepatocellular carcinoma from healthy controls and patients with chronic hepatitis B compared to α-fetoprotein [62]. In breast cancer samples, over-expression of DANCR has been associated with involvement of lymph nodes as well as hormone receptor and HER2 expressions [90]. Cumulatively, almost all studies in clinical samples have shown up-regulation of DANCR in malignant samples compared with their non-malignant counterparts. Exceptions to this rule are few studies in renal cell carcinoma [86], papillary thyroid cancer [91] and hepatocellular carcinoma [92]. Table 3 shows dysregulation of DANCR in clinical samples.
Discussion
DANCR is regarded as an oncogene in almost all types of cancers. All conducted studies have indicated up-regulation of DANCR in cancer tissues/cell lines except for a single study in renal cell carcinoma [86]. Moreover, two studies in papillary thyroid cancer [91] and hepatocellular carcinoma [92] reported down-regulation of this lncRNA, in spite of the bulk of evidence regarding up-regulation of DANCR in these two types of cancers. In support of the oncogenic role of DANCR, several studies have indicated association between up-regulation of DANCR and poor clinical outcomes. Moreover, over-expression of DANCR has been more frequently detected in patients having advanced clinical stages and distant metastases.
Over-expression of DANCR has also been associated with resistance to anti-cancer agents such as cytarabine, sorafenib, cisplatin and docetaxel. These findings indicate that DANCR-targeting therapies might affect response of cancer cells to a wide array of drugs, possibly conquering multidrug resistance.
DANCR has also been shown to possess appropriate diagnostic power to differentiate patients with liver cancer from healthy persons or those with non-malignant liver disorders [62]. Since this expression assay has been conducted in plasma samples, it potentiates DANCR as a non-invasive marker for cancer detection.
Tens of tumor suppressor miRNAs have been shown to be sponged by DANCR, leading to release of miRNA targets from their inhibitory effects. DANCR can also regulate activity of several important cancer-related pathways such as PI3K/AKT/NF-κB, Wnt/β-catenin, ERK/SMAD, MAPK, IL-6/JAK1/STAT3, Smad2/3, p53, FAK/PI3K/AKT/GSK3β/Snail pathways. Since several signaling pathways are influenced by DANCR, drugs targeting this lncRNA are expected to affect numerous aspects of carcinogenesis, thus being effective in treatment of a wide range of cancers with different biological behaviors.
In addition, DANCR has interactions with a number of proteins including CTNNB1, RXRA, EZH2 and PRC2. Most importantly, interaction of DANCR with proteins that influence epigenetic marks shows the importance of DANCR in the regulation of gene expression.
Conclusion
Although several expression assays have assessed expression levels of DANCR in biological samples obtained from different types of cancers, the underlying cause of dysregulation of DANCR in cancer has not been identified. In addition, the impacts of genomic variants on expression of this lncRNA and possible associations between single nucleotide polymorphisms within DANCR gene and susceptibility to cancer have not been appraised yet. Thus, future investigations should focus on these aspects. High throughput sequencing techniques could facilitate answering to these questions in near future.
Availability of data and materials
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.
References
Thin KZ, Liu X, Feng X, Raveendran S, Tu JC. LncRNA-DANCR: a valuable cancer related long non-coding RNA for human cancers. Pathol Res Pract. 2018;214(6):801–5 (Epub 2018/05/08).
Jiang N, Wang X, **e X, Liao Y, Liu N, Liu J, et al. lncRNA DANCR promotes tumor progression and cancer stemness features in osteosarcoma by upregulating AXL via miR-33a-5p inhibition. Cancer Lett. 2017;405:46–55.
Wang Y, Zeng X, Wang N, Zhao W, Zhang X, Teng S, et al. Long noncoding RNA DANCR, working as a competitive endogenous RNA, promotes ROCK1-mediated proliferation and metastasis via decoying of miR-335-5p and miR-1972 in osteosarcoma. Mol Cancer. 2018;17(1):1–14.
Zhang W, Li J, Tai Q, Tang J, Huang Y, Gao S. LncRNA DANCR regulates osteosarcoma migration and invasion by targeting miR-149/MSI2 axis. Eur Rev Med Pharmacol Sci. 2020;24(12):6551–60.
Shao C, Liu G, Zhang X, Li A, Guo X. Long noncoding RNA RMRP suppresses the tumorigenesis of hepatocellular carcinoma through targeting microRNA-766. Onco Targets Ther. 2020;13:3013.
Zhou X, Yang Y, Li Y, Liang G, Kang D, Zhou B, et al. METTL3 contributes to osteosarcoma progression by increasing DANCR mRNA stability via m6A modification. Front Cell Dev Biol. 2021;9:784719.
** Q, Shi Y, Yang M, Li H, Zhong Y, Li J, et al. LncRNA DANCR regulates lymphatic metastasis of bladder cancer via the miR-335/VEGF-C axis. Transl Androl Urol. 2021;10(4):1743.
Chen Z, Chen X, **e R, Huang M, Dong W, Han J, et al. DANCR promotes metastasis and proliferation in bladder cancer cells by enhancing IL-11-STAT3 signaling and CCND1 expression. Mol Ther. 2019;27(2):326–41.
Zhan Y, Chen Z, Li Y, He A, He S, Gong Y, et al. Long non-coding RNA DANCR promotes malignant phenotypes of bladder cancer cells by modulating the miR-149/MSI2 axis as a ceRNA. J Exp Clin Cancer Res. 2018;37(1):1–12.
Zhen Q, Gao L-N, Wang R-F, Chu W-W, Zhang Y-X, Zhao X-J, et al. LncRNA DANCR promotes lung cancer by sequestering miR-216a. Cancer Control. 2018;25(1):1073274818769849.
Huang Y, Zhang Y, Fu X. Long non-coding RNA DANCR promoted non-small cell lung cancer cells metastasis via modulating of miR-1225-3p/ErbB2 signal. Eur Rev Med Pharmacol Sci. 2021;25(2):758–69.
Lu Q, Rui Z, Guo Z, **e W, Shan S, Ren T. Lnc RNA-DANCR contributes to lung adenocarcinoma progression by sponging miR-496 to modulate mTOR expression. J Cell Mol Med. 2018;22(3):1527–37.
Chen YR, Wu YS, Wang WS, Zhang JS, Wu QG. Upregulation of lncRNA DANCR functions as an oncogenic role in non-small lung cancer by regulating miR-214–5p/CIZ1 axis. Eur Rev Med Pharmacol Sci. 2020;24(5):2539–47 (Epub 2020/03/21).
Yu JE, Ju JA, Musacchio N, Mathias TJ, Vitolo MI. Long Noncoding RNA DANCR activates Wnt/β-catenin signaling through MiR-216a inhibition in non-small cell lung cancer. Biomolecules. 2020;10(12):1646 (Epub 2020/12/12).
Yang L, Jiang M-N, Liu Y, Wu C-Q, Liu H. Crosstalk between lncRNA DANCR and miR-125b-5p in HCC cell progression. Tumori J. 2021;107(6):504–13.
Wang J, Pu J, Zhang Y, Yao T, Luo Z, Li W, et al. DANCR contributed to hepatocellular carcinoma malignancy via sponging miR-216a-5p and modulating KLF12. J Cell Physiol. 2019;234(6):9408–16.
Wang X, Cheng M, Gong Y, Ma W, Li B, Jiang Y. LncRNA DANCR promotes ATG7 expression to accelerate hepatocellular carcinoma cell proliferation and autophagy by sponging miR-222-3p. Eur Rev Med Pharmacol Sci. 2020;24(17):8778–87.
Liu Y, Chen L, Yuan H, Guo S, Wu G. LncRNA DANCR promotes sorafenib resistance via activation of IL-6/STAT3 signaling in hepatocellular carcinoma cells. Onco Targets Ther. 2020;13:1145.
Yuan S, Wang J, Yang F, Tao Q, Zhang J, Wang L, et al. Long noncoding RNA DANCR increases stemness features of hepatocellular carcinoma by derepression of CTNNB1. Hepatology. 2016;63(2):499–511.
Guo D, Li Y, Chen Y, Zhang D, Wang X, Lu G, et al. DANCR promotes HCC progression and regulates EMT by sponging miR-27a-3p via ROCK1/LIMK1/COFILIN1 pathway. Cell Prolif. 2019;52(4):e12628.
Shi H, Li K, Feng J, Liu G, Feng Y, Zhang X. LncRNA-DANCR interferes with miR-125b-5p/HK2 axis to desensitize colon cancer cells to cisplatin vis activating anaerobic glycolysis. Front Oncol. 2020;10:1034.
Sun Y, Cao B, Zhou J. Roles of DANCR/microRNA-518a-3p/MDMA ceRNA network in the growth and malignant behaviors of colon cancer cells. BMC Cancer. 2020;20(1):1–13.
**ong M, Wu M, Peng D, Huang W, Chen Z, Ke H, et al. LncRNA DANCR represses doxorubicin-induced apoptosis through stabilizing MALAT1 expression in colorectal cancer cells. Cell Death Dis. 2021;12(1):1–17.
Luo Y, Wang Q, Teng L, Zhang J, Song J, Bo W, et al. LncRNA DANCR promotes proliferation and metastasis in pancreatic cancer by regulating miRNA-33b. FEBS Open Bio. 2020;10(1):18–27.
Yao Z, Chen Q, Ni Z, Zhou L, Wang Y, Yang Y, et al. Long non-coding RNA differentiation antagonizing nonprotein coding RNA (DANCR) promotes proliferation and invasion of pancreatic cancer by sponging miR-214-5p to regulate E2F2 expression. Med Sci Monit Int Med J Exper Clin Res. 2019;25:4544.
Tang Y, Cao G, Zhao G, Wang C, Qin Q. LncRNA differentiation antagonizing non-protein coding RNA promotes proliferation and invasion through regulating miR-135a/NLRP37 axis in pancreatic cancer. Invest New Drugs. 2020;38(3):714–21.
Tang J, Zhong G, Zhang H, Yu B, Wei F, Luo L, et al. LncRNA DANCR upregulates PI3K/AKT signaling through activating serine phosphorylation of RXRA. Cell Death Dis. 2018;9(12):1–12.
Jia H, Liang K, Liu G, Zhang Z, Shi Y, Liang H, et al. lncRNA DANCR promotes proliferation and metastasis of breast cancer cells through sponging miR-4319 and upregulating VAPB. Cancer Biother Radiopharm. 2020. https://doi.org/10.1089/cbr.2020.3675.
Zhang KJ, Tan XL, Guo L. The long non-coding RNA DANCR regulates the inflammatory phenotype of breast cancer cells and promotes breast cancer progression via EZH2-dependent suppression of SOCS3 transcription. Mol Oncol. 2020;14(2):309–28.
Xu Y, Shang J, Li M, Zhang Y. LncRNA DANCR accelerates the development of multidrug resistance of gastric cancer. Eur Rev Med Pharmacol Sci. 2019;23(7):2794–802.
Cheng Z, Liu G, Huang C, Zhao X. KLF5 activates lncRNA DANCR and inhibits cancer cell autophagy accelerating gastric cancer progression. NPJ Genom Med. 2021;6(1):1–10.
Pan L, Liang W, Gu J, Zang X, Huang Z, Shi H, et al. Long noncoding RNA DANCR is activated by SALL4 and promotes the proliferation and invasion of gastric cancer cells. Oncotarget. 2018;9(2):1915.
Zhang H, Liu L, Chen L, Liu H, Ren S, Tao Y. Long noncoding RNA DANCR confers cytarabine resistance in acute myeloid leukemia by activating autophagy via the miR-874-3P/ATG16L1 axis. Mol Oncol. 2021;15(4):1203–16.
Li Y-L, Wang X-M, Qiao G-D, Zhang S, Wang J, Cong Y-Z, et al. Up-regulated lnc-lung cancer associated transcript 1 enhances cell migration and invasion in breast cancer progression. Biochem Biophys Res Commun. 2020;521(2):271–8.
Tao W, Wang C, Zhu B, Zhang G, Pang D. LncRNA DANCR contributes to tumor progression via targetting miR-216a-5p in breast cancer: lncRNA DANCR contributes to tumor progression. Biosci Rep. 2019;39(4):BSR20181618.
Wu G, Zhou H, Li D, Zhi Y, Liu Y, Li J, et al. LncRNA DANCR upregulation induced by TUFT1 promotes malignant progression in triple negative breast cancer via miR-874-3p-SOX2 axis. Exp Cell Res. 2020;396(2):112331.
Sha S, Yuan D, Liu Y, Han B, Zhong N. Targeting long non-coding RNA DANCR inhibits triple negative breast cancer progression. Biol Open. 2017;6(9):1310–6.
Vaidya AM, Sun Z, Ayat N, Schilb A, Liu X, Jiang H, et al. Systemic delivery of tumor-targeting siRNA nanoparticles against an oncogenic LncRNA facilitates effective triple-negative breast cancer therapy. Bioconjug Chem. 2019;30(3):907–19.
Liang H, Zhang C, Guan H, Liu J, Cui Y. LncRNA DANCR promotes cervical cancer progression by upregulating ROCK1 via sponging miR-335–5p. J Cell Physiol. 2019;234(5):7266–78 (Epub 2018/10/27).
Tian W, Lei N, Guo R, Yuan Z, Chang L. Long non-coding RNA DANCR promotes cervical cancer growth via activation of the Wnt/β-catenin signaling pathway. Cancer Cell Int. 2020;20(1):1–12.
Hu C, Han Y, Zhu G, Li G, Wu X. Krüppel-like factor 5-induced overexpression of long non-coding RNA DANCR promotes the progression of cervical cancer via repressing microRNA-145-3p to target ZEB1. Cell Cycle. 2021;20(14):1441–54.
Cao L, ** H, Zheng Y, Mao Y, Fu Z, Li X, et al. DANCR-mediated microRNA-665 regulates proliferation and metastasis of cervical cancer through the ERK/SMAD pathway. Cancer Sci. 2019;110(3):913–25.
Zhu C, Fan C, Zhang Y, Sun Q, Yan M, Wei W, et al. LncRNA DANCR affected cell growth, EMT and angiogenesis by sponging miR-345-5p through modulating Twist1 in cholangiocarcinoma. Eur Rev Med Pharmacol Sci. 2020;24(5):2321–34.
Wang N, Zhang C, Wang W, Liu J, Yu Y, Li Y, et al. Long noncoding RNA DANCR regulates proliferation and migration by epigenetically silencing FBP1 in tumorigenesis of cholangiocarcinoma. Cell Death Dis. 2019;10(8):1–11.
Yang XJ, Zhao JJ, Chen WJ, Zhang GG, Wang W, Tao HC. Silencing long non-coding RNA, differentiation antagonizing non-protein coding RNA promotes apoptosis and inhibits tumor growth in colon cancer. Oncol Lett. 2018;16(3):2865–72.
Lu W, Huang Z, Wang J, Liu H. Long non-coding RNA DANCR accelerates colorectal cancer progression via regulating the miR-185–5p/HMGA2 axis. J Biochem. 2021. https://doi.org/10.1093/jb/mvab011.
Lian J, Zhang H, Wei F, Li Q, Lu Y, Yu B, et al. Long non-coding RNA DANCR promotes colorectal tumor growth by binding to lysine acetyltransferase 6A. Cell Signal. 2020;67:109502.
Bahreini F, Saidijam M, Mousivand Z, Najafi R, Afshar S. Assessment of lncRNA DANCR, miR-145-5p and NRAS axis as biomarkers for the diagnosis of colorectal cancer. Mol Biol Rep. 2021;48(4):3541–7.
Fang Y, Chen S, Liu Z, Ai W, He X, Wang L, et al. Endothelial stem cells attenuate cardiac apoptosis via downregulating cardiac microRNA-146a in a rat model of coronary heart disease. Exp Ther Med. 2018;16(5):4246–52.
Sun J, Gao S, Lu C. Knockdown of differentiation antagonizing non-protein coding RNA exerts anti-tumor effect by up-regulating miR-214 in endometrial carcinoma. Mol Cell Biochem. 2019;460(1):9–15.
Bi Y, Guo S, Xu X, Kong P, Cui H, Yan T, et al. Decreased ZNF750 promotes angiogenesis in a paracrine manner via activating DANCR/miR-4707-3p/FOXC2 axis in esophageal squamous cell carcinoma. Cell Death Dis. 2020;11(4):1–17.
Zhang C, Wang L, Yang J, Fu Y, Li H, **e L, et al. MicroRNA-33a-5p suppresses esophageal squamous cell carcinoma progression via regulation of lncRNA DANCR and ZEB1. Eur J Pharmacol. 2019;861:172590.
Shi H, Shi J, Zhang Y, Guan C, Zhu J, Wang F, et al. Long non-coding RNA DANCR promotes cell proliferation, migration, invasion and resistance to apoptosis in esophageal cancer. J Thorac Dis. 2018;10(5):2573.
Hao Y-P, Qiu J-H, Zhang D-B, Yu C-G. Long non-coding RNA DANCR, a prognostic indicator, promotes cell growth and tumorigenicity in gastric cancer. Tumor Biol. 2017;39(6):1010428317699798.
Feng L, Lin T, Che H, Wang X. Long noncoding RNA DANCR knockdown inhibits proliferation, migration and invasion of glioma by regulating miR-135a-5p/BMI1. Cancer Cell Int. 2020;20(1):1–13.
Yang J, Sun Y, Gao L, Meng Q, Yang B. Long non-coding RNA DANCR facilitates glioma malignancy by sponging miR-33a-5p. Neoplasma. 2018;65(5):790–8.
Li J, Zhou L. Overexpression of lncRNA DANCR positively affects progression of glioma via activating Wnt/β-catenin signaling. Biomed Pharmacother. 2018;102:602–7.
Ma Y, Zhou G, Li M, Hu D, Zhang L, Liu P, et al. Long noncoding RNA DANCR mediates cisplatin resistance in glioma cells via activating AXL/PI3K/Akt/NF-κB signaling pathway. Neurochem Int. 2018;118:233–41.
Xu D, Yu J, Gao G, Lu G, Zhang Y, Ma P. LncRNA DANCR functions as a competing endogenous RNA to regulate RAB1A expression by sponging miR-634 in glioma. Biosci Rep. 2018;38(1):BSR20171664.
Wang W, Li Y, Ma Q, Yan H, Su W. Differentiation antagonizing non-protein coding RNA modulates the proliferation, migration, and angiogenesis of glioma cells by targeting the miR-216a/LGR5 axis and the PI3K/AKT signaling pathway. Onco Targets Ther. 2019;12:2439.
Han J, Yu X, Wang S, Wang Y, Liu Q, Xu H, et al. IGF2BP2 induces U251 glioblastoma cell chemoresistance by inhibiting FOXO1-mediated PID1 expression through stabilizing lncRNA DANCR. Front Cell Dev Biol. 2021;9:659228.
Ma X, Wang X, Yang C, Wang Z, Han B, Wu L, et al. DANCR acts as a diagnostic biomarker and promotes tumor growth and metastasis in hepatocellular carcinoma. Anticancer Res. 2016;36(12):6389–98.
Zhang N, Jiang W. Long non-coding RNA DANCR promotes HMGA2-mediated invasion in lung adenocarcinoma cells. Oncol Rep. 2019;41(2):1083–90.
Guo L, Gu J, Hou S, Liu D, Zhou M, Hua T, et al. Long non-coding RNA DANCR promotes the progression of non-small-cell lung cancer by inhibiting p21 expression. Onco Targets Ther. 2019;12:135.
Bai Y, Zhang G, Chu H, Li P, Li J. The positive feedback loop of lncRNA DANCR/miR-138/Sox4 facilitates malignancy in non-small cell lung cancer. Am J Cancer Res. 2019;9(2):270.
Wang S, Jiang M. The long non-coding RNA-DANCR exerts oncogenic functions in non-small cell lung cancer via miR-758-3p. Biomed Pharmacother. 2018;103:94–100.
Wang B, Chen W, Zhao Z, Sun Y, Huang Y. LncRNA-DANCR promotes growth and metastasis of colorectal cancer via activating epithelial-mesenchymal transition process. Transl Cancer Res. 2019;8(7):2517.
Wu L, **a L, Jiang H, Hu Y, Li L, Xu L, et al. Long non-coding RNA DANCR represses the viability, migration and invasion of multiple myeloma cells by sponging miR-135b-5p to target KLF9. Mol Med Rep. 2021;24(3):1–12.
Jia X, Shi L, Wang X, Luo L, Ling L, Yin J, et al. KLF5 regulated lncRNA RP1 promotes the growth and metastasis of breast cancer via repressing p27kip1 translation. Cell Death Dis. 2019;10(5):1–16.
Li Q, Jiang Y, Zhong G, Lu Y, Song T, Zhang Y, et al. Long noncoding RNA DANCR regulates cell proliferation by stabilizing SOX2 mRNA in nasopharyngeal carcinoma. Am J Pathol. 2020;190(12):2343–54.
Hao Y, Zhao H, ** X, He P, Zhang J, Dong Q, et al. Long non-coding RNA DANCR promotes nasopharyngeal carcinoma cell proliferation and migration. Mol Med Rep. 2019;19(4):2883–9.
Zhang J, ** X, Zhou C, Zhao H, He P, Hao Y, et al. Resveratrol suppresses human nasopharyngeal carcinoma cell growth via inhibiting differentiation antagonizing non-protein coding RNA (DANCR) expression. Med Sci Monit Int Med J Exp Clin Res. 2020;26:e923622–31.
Wen X, Liu X, Mao Y-P, Yang X-J, Wang Y-Q, Zhang P-P, et al. Long non-coding RNA DANCR stabilizes HIF-1α and promotes metastasis by interacting with NF90/NF45 complex in nasopharyngeal carcinoma. Theranostics. 2018;8(20):5676.
Bi C, Shan J, Li M, Zhang Q, Li C, Tong J, et al. Long noncoding RNA differentiation antagonizing nonprotein coding RNA promotes the proliferation, invasion and migration of neuroblastoma cells via targeting β-1, 4-galactosyltransferase III by sponging miR-338-3p. NeuroReport. 2021;32(12):965–74.
Qu X-H, Shi Y-L, Ma Y, Bao W-W, Yang L, Li J-C, et al. LncRNA DANCR regulates the growth and metastasis of oral squamous cell carcinoma cells via altering miR-216a-5p expression. Hum Cell. 2020;33(4):1281–93.
Cui PH, Li ZY, Li DH, Han SY, Zhang YJ. SP1-induced lncRNA DANCR contributes to proliferation and invasion of ovarian cancer. Kaohsiung J Med Sci. 2021;37(5):371–8.
Huang P, Qi B, Yao H, Zhang L, Li Y, Li Q. Knockdown of DANCR suppressed the biological behaviors of ovarian cancer cells treated with transforming growth factor-β (TGF-β) by sponging MiR-214. Med Sci Monit Int Med J Exp Clin Res. 2020;26:e922760–1.
Lin X, Yang F, Qi X, Li Q, Wang D, Yi T, et al. LncRNA DANCR promotes tumor growth and angiogenesis in ovarian cancer through direct targeting of miR-145. Mol Carcinog. 2019;58(12):2286–96.
Pei C, Fei K, Yuan X, Gong X. LncRNA DANCR aggravates the progression of ovarian cancer by downregulating UPF1. Eur Rev Med Pharmacol Sci. 2019;23(24):10657–63.
Chen L, Liu J, Tang T, Zhang Y-C, Liu M-Z, Xu L-Y, et al. lncRNA differentiation antagonizing nonprotein coding RNA overexpression accelerates progression and indicates poor prognosis in pancreatic ductal adenocarcinoma. Onco Targets Ther. 2018;11:7955.
Zhao H, Zhang Z, Shi B, Jiang X. DANCR sponges miR-135a to regulate paclitaxel sensitivity in prostate cancer. Eur Rev Med Pharmacol Sci. 2019;23(16):6849–57.
Sun W, Zu S, Shao G, Wang W, Gong F. Long non-coding DANCR targets miR-185-5p to upregulate LIM and SH3 protein 1 promoting prostate cancer via the FAK/PI3K/AKT/GSK3β/snail pathway. J Gene Med. 2021;23(7):e3344.
Deng H, Zhu B, Dong Z, Jiang H, Zhao X, Wu S. miR-214-5p targeted by LncRNA DANCR mediates TGF-β signaling pathway to accelerate proliferation, migration and inhibit apoptosis of prostate cancer cells. Am J Transl Res. 2021;13(4):2224.
Ma Y, Fan B, Ren Z, Liu B, Wang Y. Long noncoding RNA DANCR contributes to docetaxel resistance in prostate cancer through targeting the miR-34a-5p/JAG1 pathway. Onco Targets Ther. 2019;12:5485.
Jia J, Li F, Tang X-S, Xu S, Gao Y, Shi Q, et al. Long noncoding RNA DANCR promotes invasion of prostate cancer through epigenetically silencing expression of TIMP2/3. Oncotarget. 2016;7(25):37868.
** L, Fu H, Quan J, Pan X, He T, Hu J, et al. Overexpression of long non-coding RNA differentiation antagonizing non-protein coding RNA inhibits the proliferation, migration and invasion and promotes apoptosis of renal cell carcinoma. Mol Med Rep. 2017;16(4):4463–8.
Wang JX, Yang Y, Li K. Long noncoding RNA DANCR aggravates retinoblastoma through miR-34c and miR-613 by targeting MMP-9. J Cell Physiol. 2018;233(10):6986–95.
Zheng Y, Zheng B, Meng X, Yan Y, He J, Liu Y. LncRNA DANCR promotes the proliferation, migration, and invasion of tongue squamous cell carcinoma cells through miR-135a-5p/KLF8 axis. Cancer Cell Int. 2019;19(1):1–14.
Liu Y, Li J, Yue B, Liang L, Zhang S, Chen Y. Long non-coding RNA DANCR regulate MLL3 and thereby it determines the progression of pancreatic cancer. J BUON. 2020;25:1954–9.
Shi W, ** X, Wang Y, Zhang Q, Yang L. High serum exosomal long non-coding RNA DANCR expression confers poor prognosis in patients with breast cancer. J Clin Lab Anal. 2022;36:e24186.
Zhang K, Lv J, Peng X, Liu J, Li C, Li J, et al. Down-regulation of DANCR acts as a potential biomarker for papillary thyroid cancer diagnosis. Biosci Rep. 2019;39(4):BSR20181616.
Mu X, Chen W, Shi J, Li X, Wang Y. Development of a GeXP-based multiplex RT-PCR assay for detection of long noncoding RNA in hepatocellular carcinoma. Lab Med. 2019;50(2):180–8.
Kamaliyan Z, Mirfakhraie R, Azizi-Tabesh G, Darbeheshti F, Omranipour R, Ahmadinejad N, et al. The role of FOXC1/FOXCUT/DANCR axis in triple negative breast cancer: a bioinformatics and experimental approach. Mol Biol Rep. 2022. https://doi.org/10.1007/s11033-021-07093-3.
Tuluhong D, Dunzhu W, Wang J, Chen T, Li H, Li Q, et al. Prognostic value of differentially expressed LncRNAs in triple-negative breast cancer: a systematic review and meta-analysis. Crit Rev Eukaryot Gene Expr. 2020;30(5):447–56.
Shen X, Xue Y, Cong H, Wang X, Fan Z, Cui X, et al. Circulating lncRNA DANCR as a potential auxillary biomarker for the diagnosis and prognostic prediction of colorectal cancer. Biosci Rep. 2020;40(3):BSR20191481.
Liu Y, Zhang M, Liang L, Li J, Chen Y-X. Over-expression of lncRNA DANCR is associated with advanced tumor progression and poor prognosis in patients with colorectal cancer. Int J Clin Exp Pathol. 2015;8(9):11480.
Icduygu FM, Akgun E, Sengul D, Ozgoz A, Alp E. Expression of SOX2OT, DANCR and TINCR long non-coding RNAs in papillary thyroid cancer and its effects on clinicopathological features. Mol Med Rep. 2022;25(4):1–10.
Acknowledgements
This study was financially supported by Grant from Medical School of Shahid Beheshti University of Medical Sciences.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
SGF wrote the manuscript and revised it. MT and AB supervised and designed the study. TK, MS and BMH collected the data and designed the figures and tables. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent forms were obtained from all study participants. The study protocol was approved by the ethical committee of Shahid Beheshti University of Medical Sciences. All methods were performed in accordance with the relevant guidelines and regulations.
Consent of publication
Not applicable.
Competing interests
The authors declare they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ghafouri-Fard, S., Khoshbakht, T., Hussen, B.M. et al. A review on the role of DANCR in the carcinogenesis. Cancer Cell Int 22, 194 (2022). https://doi.org/10.1186/s12935-022-02612-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12935-022-02612-z