Abstract
Background
B cell lymphoma-2 (Bcl-2) family members play important roles in cell survival as well as cell death. The role of myeloid cell leukemia-1 (Mcl-1), an important member of the Bcl-2 family, is well established in hematopoietic malignancies. However, the association between Mcl-1 and oral cavity, cancers is not clearly defined.
Methods
A sco** review was conducted until June 30, 2021, using four major databases, PubMed, Scopus, Web of Science, and Embase. Medical subject headings keywords for Mcl-1, along with its other identifiers, and head and neck cancers (only oral cavity tumors) were used to evaluate the expression, function, molecular association, and therapeutic approach of Mcl-1 in oral cavity cancers and precancers.
Findings
Mcl-1 expression was associated with the progression of oral cavity cancers. The molecular mechanism and pathways of Mcl-1 in oral cavity cancers established via experimental results have been highlighted in this review. Moreover, the various synthetic and naturally derived therapeutic agents targeting Mcl-1 have been documented.
Novelty/Improvement
Based on our present review, Mcl-1 appears to be an effective anticancer target that can be used in the therapeutic management of oral cancers.
Similar content being viewed by others
Background
Cancerous lesions in the oral cavity
Oral cancers are a malignant tumors that occur in the mouth, and oropharynx and on the lips; oral cancers account for approximately 2% of all malignancies worldwide [1]. More than 90% of these cancers are squamous cell carcinomas (SCC) [2], and approximately 3 ~ 5% salivary gland tumors (SGTs) [3]. The potentially malignant lesions of the oral cavity (OPML) include conditions such as leukoplakia, erythroplakia, and submucous fibrosis [4]. Despite various advancements in therapeutic regimens, survival of patients with oral cancers has not significantly improved, and most chemotherapeutic or combination interventions have not been proven successful [5]. Thus, the identification of predictive molecules that preempt the malignant transformation to oral squamous cell carcinomas(OSCC) might prove to be useful in the development of effective therapies.
Myeloid cell leukemia-1
Myeloid cell leukemia-1 (Mcl-1) was first identified in a myeloid leukemia cell line by Kozopas et al. in 1993 [6]. It is located at 1q.21, which is frequently amplified in cases of multiple myeloma [7]. Mcl-1 is involved in normal cell homeostasis and function. Under normal conditions, it protects the cells from apoptosis and plays an important role in cell survival. It also plays a significant role during embryogenesis. The deletion of this gene in murine embryonic stem cells resulted in peri-implantation embryonic lethality [8]. Mcl-1 also promotes the maintenance of normal mitochondrial morphology and energy production by exerting both anti-apoptotic and mitochondrial effects [9]. Just as anti-apoptotic Bcl-2 family members antagonize pro-apoptotic BH3-only proteins to inhibit the essential apoptosis effectors Bak/BAX [10], Mcl-1 exerts its anti-apoptotic function by sequestering the pro-apoptotic proteins Bak/BAX [11]. Mcl-1 is regulated via modifications at the transcriptional, post-transcriptional, translational, or post-translational levels, and the functional activity and stability of Mcl-1 is determined by its post-translational modifications [12,13,14]. Notably, alternative splicing can specifically affect Mcl-1 function by yielding a longer isoform, which is anti-apoptotic, or a shorter isoform, which is pro-apoptotic [13].
Mcl-1 overexpression has been associated with poor outcomes and therapeutic responses in hematologic malignancies [15] and breast [16, 17], lung [18], and gastric cancers [19]. Its overexpression in different cancers, particularly in leukemia, has resulted in an increased focus on the therapeutic targeting of this protein [20] leading to the development and identification of various synthetically produced, naturally occurring, or synthetically derived natural analogous compounds targeting Mcl-1 [21,22,23,24]. In addition to single compounds, combination therapies that target Mcl-1 reportedly show promising effects [24]. On the basis of the information currently available, we hypothesize that Mcl-1 can be a promising target for anticancer therapy.
The aim of the current review was to evaluate the expression, regulation, function, associated features, and potential therapeutic agents of Mcl-1 in oral cancers.
Methods
A previously established method was used to conduct a sco** review by applying the Preferred Reporting Items for Systematic Reviews and Meta-Analyses for Sco** Reviews guidelines.
Search strategy
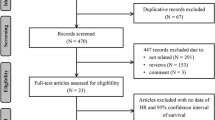
A literature search was conducted using the PubMed, Scopus, Embase, and Web of Science databases as well as a gray literature search using Research Gate and Google Scholar until June 30, 2021. Medical subject headings (MeSH) terms were used to explore Mcl-1 along with other aliases, such as oral cancer, SGT, precancerous lesion, head and neck SCCs; other tumors were not included for this review. Only the studies published in English were evaluated, and duplicated records, posters, and abstracts were excluded (Fig. 1).
Eligibility criteria
The articles were reviewed by two authors of this study (SJC and NS) for eligibility and included after evaluation using the SPIDER criteria (Table 1).
Mcl-1 expression in clinical oral cancer samples
OPMLs have a high likelihood to progress to cancer, and the identification of oncogenic proteins that aid in the progression to oral cancer can be extremely helpful for better therapeutic planning. Several authors have verified that Mcl-1 is overexpressed in OPMLs. Ribeiro et al. [25] observed gains in Mcl-1 in two patients with leukoplakia and erythroleukoplakia. Similarly, Mallick et al. [26] reported the upregulation of Mcl-1 in malignant and premalignant tissues in vivo, interestingly indicating that the expression of Mcl-1 in homogeneous leukoplakia tended to be higher than in non-homogeneous leukoplakia. Our group also previously showed that Mcl-1 is overexpressed in oral lichen planus compared with the normal oral mucosa [27]. Sulkshane et al. reported that Mcl-1 was upregulated in OPMLs and demonstrated a positive correlation between Mcl-1 and USP9X in leukoplakia [28]. Moreover, Yu et al. found that an increase in the Bak/Mcl-1 ratio had favorable therapeutic outcomes after on photodynamic therapy for oral verrucous hyperplasia and leukoplakia [29]. These results indicate the essential role of Mcl-1 in the malignant transformation of OPMLs.
Mcl-1 overexpression is well documented in various solid and hematological tumors, including oral cancer, and has been demonstrated as genetic amplifications [25] and in mRNA [26, 30, 31] and protein [26, 28, 32, 33] levels. According to a study by Nagata et al., strong Mcl-1 expression was observed in tongue SCC (SCCKN and SAS) cell lines compared with fibroblasts from normal lips [32]. The results of a study by Shin et al. [33] were valuable in terms of Mcl-1 expression through analysis of normal oral mucosa, human OSCC tissues (n = 14 and 25, respectively) and various OSCC cell lines (HSC2, HSC3, HSC4, HN22, OSC-20, Ca9.22, and SAS). In addition, Sulkshane et al. [28] confirmed the strong expression of Mcl-1 in other OSCC cell lines (AW8507, AW13516, and SCC029B). SGTs form a heterogeneous group of tumors that can be aggressive in nature; their gene expression patterns are similar to those of OPMLs and OSCC. The ubiquitous overexpression of Mcl-1 was reported in various types of malignant parotid gland tumors; the highest expression was observed in SCC of the parotid gland [34]. Although an isolated finding, Mcl-1 amplification was observed in high-grade stage III adenoid cystic carcinoma [35]. Determining the associations between Mcl-1 and the various categories and stages of oral cancer can enhance our understanding of its potential impact on the clinical progression of the disease. Studies on the associations between Mcl-1 overexpression and advanced tumor stages [28, 30] and lymph node metastasis [30] are limited. Mcl-1 overexpression has been reported more in recurrent tumors than in primary tumors [28]. In addition, increased Mcl-1 expression has been associated with reduced overall survival [28, 30], disease-free survival, and survival time [31, 36]. Various histopathological indicators have been used to predict the progression of OSCC. Interestingly, increased Mcl-1 expression was associated with well-differentiated tumors [26, 32]. Mcl-1 plays an important role in keratinocyte differentiation as it helps to maintain mitochondrial function [37]. These findings indicate a complex interaction, wherein histological function is maintained despite the poor clinicopathological stages. Taken together, the consistent findings of Mcl-1 overexpression in cancers indicates its association with carcinogenesis, and it is suggested that Mcl-1 has a significant impact on the development and progression of oral cancer. The associations between Mcl-1 and the different features of OPMLs, OSCC, and SGTs are summarized in Fig. 2; Table 2.
Molecular associations of Mcl-1
Despite evidence on the role of Mcl-1 as an important molecular target in oral cancer, the molecular mechanisms involved in oral cancer have not been well documented compared with those in other cancers. Isolated reports on the regulation and interactions of Mcl-1 in oral cancer have been identified [32, 38, 39]. The activity of Mcl-1 in oral cancer is found to be regulated by paracrine signaling mechanisms, physical forces, or intracellular regulatory mechanisms [28, 39, 40].
The Mcl-1 mRNA expression was upregulated by STAT3 activation and stabilized by Akt-mediated GSK3β inactivation in chemotherapy-resistant OSCC [38]. The tumorigenesis regulating gene MYB is capable of upregulating Mcl-1 in adenoid cystic carcinoma cell lines [41]. FBW7 stabilizes Mcl-1 and promotes Mcl-1 addiction in oral cancer [42]. USP9X modulates the stability of Mcl-1 and prevents its degradation by deubiquitinating the protein [28]. Hyperosmotic stress has been shown to counteract Mcl-1 in head and neck SCC [39]. The upregulation of Noxa acts as a link between the osmotic pressure in the tumor environment and mitochondrial priming, thereby counteracting the anti-apoptotic properties of Mcl-1 in head and neck SCC. LncRNA FGD-AS1 inhibited the proliferation and migration/invasion of oral cancer, acting as a sponge for miR-153-3p and miR-153-3p to inhibit Mcl-1 expression [43]. Furthermore, the non-coding RNA HOXA10 AS was found to increase Mcl-1 mRNA levels [44]. Mcl-1 function can be also regulated through alternative splicing; a study demonstrated that Mcl-1 L transcripts were highly expressed compared with those of Mcl-1 S and Mcl-1ES in oral cells, thus indicating the predominance of the anti-apoptotic isoform [26, 30]. This variation in the isoform has a significant impact on Mcl-1 function and even on its clinical presentation [30, 45]. The effects of Mcl-1 on different oncogenic cascades have been evaluated in interference studies. Mcl-1 siRNA inhibited cell growth and induced apoptosis by inhibiting the FAK–MAPK pathway in OSCC [32]. Mithramycin inhibits Mcl-1 and RNAi regulates Bax to induce apoptosis in oral cancer cell lines [33]. These results suggest that Mcl-1 is affected and regulated by a variety of protein kinases, transcription factors, miRNA, etc. The molecular interactions and associations of Mcl-1 in oral cancers are summarized in Table 3, whereas and the protein–protein interactions (PPIs) between the identified biomarkers are presented in Fig. 3.
STRING protein–protein interaction (PPI) analyses. PPI network connectivity for proteins identified following the review. Nodes represent the proteins required for interaction. Edges represent the associations between the proteins. The STRING web resource (http://www.stringdb.org) was used in the prediction of the PPI (Protein–Protein Interaction) network whereby an interaction score of > 0.900 denoted a significant interactive relationship
Therapeutic strategy targeting Mcl-1
Various compounds that can result in apoptosis can reduce the expression level of Mcl-1 by inhibiting its translation or increasing its rate of degradation. These compounds have been found to have an effect on the levels of Mcl-1 when used alone or in combination with other agents. Therefore, the key factors that inhibit Mcl-1 can be used as potential treatment strategies in the treatment of oral cancer.
Synthetic compounds
Several direct and indirect approaches to inhibit the activity of Mcl-1 have been used. Although small molecule inhibitors that directly target Mcl-1 by interrupting the PPIs have been developed, no drugs that can directly target this protein have been used in the treatment of oral cancer to date. Alternatively, some synthetic or natural compounds were found to target Mcl-1 indirectly as a part of their mechanism of action.
A Bcl-2 inhibitor, obatoclax, was found to induce apoptosis in head and neck SCC in an Mcl-1-dependent manner [46]. ABT-737 repressed cellular Mcl-1 by upregulating Noxa [47]. TW-37 was reported to sensitize cryptotanshinone-mediated apoptosis in OSCC cells by suppressing STAT3–Mcl-1 signaling [48]. Furthermore, the proteasome inhibitor MG132 induced the accumulation of Bik, which can activate Bak sequestered by Mcl-1, to sensitize the TRAIL-mediated apoptosis [49]. Several kinase inhibitors have been shown to downregulate Mcl-1 in oral cancer; e.g., the aurora-A kinase inhibitor, alisertib, degraded Mcl-1 in HPV E7-expressing head and neck SCC cells [50]. Similarly, the multikinase inhibitor sorafenib induced apoptosis in mucoepidermod carcinoma cells through the STAT3/Mcl-1/t-Bid signaling pathway [51]. EGFR inhibitors induced apoptosis in head and neck SCC by downregulating Mcl-1 expression [52, 53]. Mithramycin A reduced the expression of Mcl-1 in oral cancer cells, leading to an increase in Bax protein, followed by its translocation into the mitochondria and oligomerization [33]. An HDAC inhibitor, panobinostat, suppressed Sp1 and downregulated Mcl-1 levels [54]. An inhibitor of the splicing factor 3B1, meayamycin B, reportedly to inhibited SF3B, leading to a reduction in the anti-apoptotic Mcl-1 L isoform and the generation of the pro-apoptotic Mcl1-S by switching the splicing pattern of the Mcl-1 pre-mRNA [55]. YM155 inhibited Mcl-1 through lysosomal-dependent degradation to induce apoptosis in head and neck SCC cell lines [56]. Aspirin downregulated the Mcl-1 protein, followed by a significant reduction in ERK-1/2 and Akt phosphorylation and significant increase in IκB-α phosphorylation, thus resulting in the activation of NF-κB [57]. The immunosuppressant FTY720 downregulated Akt/NF-κB signaling through a Mcl-1-dependent mechanism [58]. Propofol induced apoptosis via a significant reduction in Mcl-1 and an increase in phospho-Mcl-1 (Ser 159) thereby indicating its effect on the stability of Mcl-1 protein [59]. Biochemical synthetic products such as glucosamine hydrochloride and the anti-malaria semi-synthetic dihydroartemisinin demonstrated a reduction in Mcl-1 in OSCC cell lines [60,61,62,63]. Several combination treatments affected the function of Mcl-1;e.g., a combination of fenretinide and ABT263 induced Mcl-1 degradation [64]. Co-treatment with C6 ceramide significantly augmented PKC412-induced lethality by downregulating Mcl-1 in head and neck cell lines and animal models [65]. These results suggest that synthetic compounds targeting Mcl-1 is a promising therapeutic strategy for the treatment of oral cavity cancers.
The combination of thioridazine and carboplatin induced apoptosis by downregulating c-FLIP and Mcl-1 [66], indicating that Mcl-1 can be used as a molecular target of combination therapy in oral cancer. Clinical studies on Mcl-1 inhibitors are under way, and anticancer effects have been identified in several cancers other than those of the oral cavity [31]. Venetoclax and others drugs are under clinical trials for the treatment of acute myeloid leukemia and other hematological malignancies [24]. Table 4 summarizes various synthetic agents used to target Mcl-1.
Natural compounds
Many natural compounds are known to affect STAT3, which is known as one of the major upstream molecules of Mcl-1 in oral cancers [67]. Epigallocatechin gallate abrogated the interleukin-6-induced phosphorylation of STAT3 and downregulated its target gene products [68]. Licochalcone C inhibited the JAK2/STAT3 pathway, and downregulated Bcl-2 and Mcl-1 [69]. Nitidine chloride decreased the Mcl-1 protein by inhibiting the STAT3 pathway [70]. Additionally, bitter melon extract inhibited the c-Met signaling pathway and reduced the downstream signaling molecules such as phospho-STAT3 (Tyr705) and Mcl-1 [71]. These findings suggest that the STAT3/Mcl-1 signaling axis is a promising molecular mechanism that can be used in the treatment of oral cancers.
Various phytochemicals may mimic the effects of BH-3 proteins. Guggulsterone phytosterol targets 14-3-3 zeta to initiate apoptosis through the intrinsic mitochondrial pathway by the dephosphorylation of p-Bad and suppression of the expression level of Mcl-1 in OSCC cells [72]. Furano-1,2-naphthoquinone upregulated Bax and Bad and downregulated Mcl-1 in Ca9.22 cells [73]. Convallaria keiskei reduced the expression level of Mcl-1, leading to a truncated Bid-induced mitochondrial apoptosis in salivary gland cancer cell lines [74]. Lycorine hydrochloride induced the mitochondria-mediated apoptosis pathway through the downregulation of Mcl-1 [75]. Treatment with Juniperus squamata induced a mitotic catastrophe, leading to apoptosis via Mcl-1 reduction in OSCC cell lines [76].
Extracts from various plants were found to target Sp1, which combines with a specific DNA sequence and is overexpressed in many cancers [77]. Sp1, a transcription factor that binds to the Mcl-1 promoter region [78], has already been tested and found to play important physiological roles, such as in apoptosis, by targeting Mcl-1 in cancer [54, 79]. Honokiol inhibited Sp1 and reduced Mcl-1 and survivin leading to the induction of apoptosis in OSCC cells [80]. Manumycin A inhibited Mcl-1 by downregulating Sp1 [81]. Sanguisorba officinalis [82] and C. officinale Makino, C. bursapastoris [83], and Dianthus chinensis and Acalypha australis [84] were found to reduce Mcl-1 via Sp1 and induce apoptosis in oral cancer cell lines.
ROS production results in a reduction in the mitochondrial transmembrane potential which leads to mitochondria-dependent apoptosis in human cancer cells [85]. ROS has been implicated in the activation of various cellular signaling pathways and transcription factors [86]. Phenethyl isothiocyanate induced G2/M cell cycle arrest and apoptosis by inducing ROS production and reducing Mcl-1 expression [87]. Benzyl isothiocyanate led to a reduction in Mcl-1 followed by the development of mitochondria-mediated apoptosis in oral cancer [88]. Cardiac glycosides induced apoptosis by lowering Mcl-1 levels in OSCC cell lines [89]. Wogonin was noted to selectively kill cisplatin-resistant head and neck SCC cells by targeting Nrf2, which was then accompanied by the downregulation of Mcl-1 [90]. Cyclocommunol downregulated the phosphorylation/expression of Akt/mTOR and Mcl-1 leading to the generation of ROS [91]. Taken together, the most commonly observed mechanism of action of these natural compounds in the regulation of Mcl-1 was through the inhibition of STAT3 or Sp1. Table 5 presents an overview of the effects of the natural compounds on Mcl-1.
Conclusions
In this paper, we attempted to review the expression, function, molecular mechanism and pathway, and therapeutic approach of Mcl-1 in oral cavity cancers. Mcl-1 is frequently amplified and upregulated in cancerous lesions of oral cavity and affects the clinical progression and survival of patients with oral cancer. Various transcription factors and protein kinases affect Mcl-1 activity, which further facilitates cancer progression. These findings indicate its significant role in oral carcinogenesis. This review also successfully summarized the agents, both synthetic and natural, that have an inhibitory effect on Mcl-1 in oral cancer. To the best of our knowledge, this review is the first specific summary suggesting that Mcl-1 is a promising molecular target for the treatment of oral cancer. Although the development of direct Mcl-1 inhibitors remains challenging, this review will help researchers and clinicians to identify the avenues that can be investigated to provide better disease prediction and therapeutic planning of oral cancers expressing Mcl-1 in the future.
Availability of data and materials
Not applicable.
Abbreviations
- MeSH:
-
Medical subject headings
- PPI:
-
Protein–protein interaction
- SCC:
-
Squamous cell carcinomas
- SGT:
-
Salivary gland tumors
- TNBC:
-
Triple negative breast cancer
References
Siegel RL, Miller KD, Jemal A, Cancer statistics. 2020. CA Cancer J Clin. 2020;70:7–30.
Montero PH, Patel SG. Cancer of the oral cavity. Surg Oncol Clin N Am. 2015;24:491–508.
Pons Vicente O, Almendros Marqués N, Berini Aytés L. Gay Escoda C Minor salivary gland tumors: a clinicopathological study of 18 cases. Med Oral Patol Oral Cir Bucal. 2008;13:E582-8.
Wetzel SL, Wollenberg J. Oral potentially malignant disorders. Dent Clin North Am. 2020;64:25–37.
Wang X, Luo Y, Li M, Yan H, Sun M, Fan T. Management of salivary gland carcinomas—a review. Oncotarget. 2017;8:3946–56.
Kozopas KM, Yang T, Buchan HL, Zhou P, Craig RW. MCL1, a gene expressed in programmed myeloid cell differentiation, has sequence similarity to BCL2. Proc Natl Acad Sci U S A. 1993;90:3516–20.
Slomp A, Moesbergen LM, Gong JN, Cuenca M, von dem Borne PA, Sonneveld P, Huang DCS, Minnema MC. Peperzak V Multiple myeloma with 1q21 amplification is highly sensitive to MCL-1 targeting. Blood Adv. 2019;3:4202–14.
Rinkenberger JL, Horning S, Klocke B, Roth K, Korsmeyer SJ. Mcl-1 deficiency results in peri-implantation embryonic lethality. Genes Dev. 2000;14:23–7.
Perciavalle RM, Opferman JT. Delving deeper: MCL-1’s contributions to normal and cancer biology. Trends Cell Biol. 2013;23:22–9.
Levine B, Sinha S, Kroemer G. Bcl-2 family members: dual regulators of apoptosis and autophagy. Autophagy. 2008;4:600–6.
Willis SN, Chen L, Dewson G, Wei A, Naik E, Fletcher JI, Adams JM, Huang DC. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005;19:1294–305.
Li HN, Shan CG, Fan CZ, Wu SN, Lai MY, Cai LB, Zhu D, Wang FF, Chen YD, Shi YQ, Liu KH, Zhang X, Bao H, Wu X, Wang XN, Shao Y, Li Z. Genomic profiling identified novel prognostic biomarker in Chinese glioma patients. J Clin Oncol. 2020;38:607429.
Senichkin VV, Streletskaia AY, Gorbunova AS, Zhivotovsky B, Kopeina GS. Saga of Mcl-1: regulation from transcription to degradation. Cell Death & Differentiation. 2020;27:405–19.
Senichkin VV, Streletskaia AY, Gorbunova AS, Zhivotovsky B, Kopeina GS. Saga of Mcl-1: regulation from transcription to degradation. Cell Death Differ. 2020;27:405–19.
Wei AH, Roberts AW, Spencer A, Rosenberg AS, Siegel D, Walter RB, Caenepeel S, Hughes P, McIver Z, Mezzi K, Morrow PK. Stein A Targeting MCL-1 in hematologic malignancies: rationale and progress. Blood Rev. 2020;44:100672.
Campbell KJ, Dhayade S, Ferrari N, Sims AH, Johnson E, Mason SM, Dickson A, Ryan KM, Kalna G, Edwards J, Tait SWG. Blyth K MCL-1 is a prognostic indicator and drug target in breast cancer. Cell Death Dis. 2018;9:19.
Wein L, Loi S. Mechanisms of resistance of chemotherapy in early-stage triple negative breast cancer (TNBC). Breast. 2017;34:27–30.
Nakano T, Go T, Nakashima N, Liu D, Yokomise H. Overexpression of Antiapoptotic MCL-1 Predicts Worse Overall Survival of Patients With Non-small Cell Lung Cancer. Anticancer Res. 2020;40:1007–14.
Lee WS, Park YL, Kim N, Oh HH, Son DJ, Kim MY, Oak CY, Chung CY, Park HC, Kim JS, Myung DS, Cho SB. Joo YE Myeloid cell leukemia-1 regulates the cell growth and predicts prognosis in gastric cancer. Int J Oncol. 2015;46:2154–62.
Bolomsky A, Vogler M, Kose MC, Heckman CA, Ehx G, Ludwig H, Caers J. MCL-1 inhibitors, fast-lane development of a new class of anti-cancer agents. J Hemat Oncol. 2020;13:173.
Tron AE, Belmonte MA, Adam A, Aquila BM, Boise LH, Chiarparin E, Cidado J, Embrey KJ, Gangl E, Gibbons FD, Gregory GP, Hargreaves D, Hendricks JA, Johannes JW, Johnstone RW, Kazmirski SL, Kettle JG, Lamb ML, Matulis SM, Nooka AK, Packer MJ, Peng B, Rawlins PB, Robbins DW, Schuller AG, Su N, Yang W, Ye Q, Zheng X, Secrist JP, Clark EA, Wilson DM, Fawell SE. Hird AW Discovery of Mcl-1-specific inhibitor AZD5991 and preclinical activity in multiple myeloma and acute myeloid leukemia. Nat Commun. 2018;9:5341.
Kawiak A, Domachowska A, Krolicka A, Smolarska M, Lojkowska E. 3-Chloroplumbagin induces cell death in breast cancer cells through MAPK-mediated Mcl-1 inhibition. Front Pharmacol. 2019;10:784.
Mukherjee N, Skees J, Todd KJ, West DA, Lambert KA, Robinson WA, Amato CM, Couts KL, Van Gulick R, MacBeth M, Nassar K, Tan AC, Zhai Z, Fujita M, Bagby SM, Dart CR, Lambert JR, Norris DA. Shellman YG MCL1 inhibitors S63845/MIK665 plus Navitoclax synergistically kill difficult-to-treat melanoma cells. Cell Death Dis. 2020;11:443.
Ramsey HE, Fischer MA, Lee T, Gorska AE, Arrate MP, Fuller L, Boyd KL, Strickland SA, Sensintaffar J, Hogdal LJ, Ayers GD, Olejniczak ET, Fesik SW. Savona MR A Novel MCL1 inhibitor combined with venetoclax rescues venetoclax-resistant acute myelogenous leukemia. Cancer Discov. 2018;8:1566–81.
Ribeiro IP, Marques F, Barroso L, Rodrigues J, Caramelo F, Melo JB. Carreira IM Genomic profile of oral squamous cell carcinomas with an adjacent leukoplakia or with an erythroleukoplakia that evolved after the treatment of primary tumor: a report of two cases. Mol Med Rep. 2017;16:6780–6.
Mallick S, Patil R, Gyanchandani R, Pawar S, Palve V, Kannan S, Pathak KA, Choudhary M. Teni TR Human oral cancers have altered expression of Bcl-2 family members and increased expression of the anti-apoptotic splice variant of Mcl-1. J Pathol. 2009;217:398–407.
Shin JA, Seo JM, Oh S, Cho SD, Lee KE. Myeloid cell leukemia-1 is a molecular indicator for malignant transformation of oral lichen planus. Oncol Lett. 2016;11:1603–7.
Sulkshane P, Pawar SN, Waghole R, Pawar SS, Rajput P, Uthale A, Oak S, Kalkar P, Wani H, Patil R, Nair S, Rane P, Teni T. Elevated USP9X drives early-to-late-stage oral tumorigenesis via stabilisation of anti-apoptotic MCL-1 protein and impacts outcome in oral cancers. Brit J Cancer. 2021;125:547–60.
Yu CH, Chen HM, Lin HP, Chiang CP. Expression of Bak and Bak/Mcl-1 ratio can predict photodynamic therapy outcome for oral verrucous hyperplasia and leukoplakia. J Oral Pathol Med. 2013;42:257–62.
Palve V, Mallick S, Ghaisas G, Kannan S, Teni T. Overexpression of Mcl-1L splice variant is associated with poor prognosis and chemoresistance in oral cancers. PLoS ONE. 2014;9:e111927.
Wang HL, Guo M, Wei HD, Chen YH. Targeting MCL-1 in cancer: current status and perspectives. J Hematol Oncol. 2021;14:67.
Nagata M, Wada K, Nakajima A, Nakajima N, Kusayama M, Masuda T, Iida S, Okura M, Kogo M. Kamisaki Y role of myeloid cell leukemia-1 in cell growth of squamous cell carcinoma. J Pharmacol Sci. 2009;110:344–53.
Shin JA, Jung JY, Ryu MH, Safe S, Cho SD. Mithramycin A inhibits myeloid cell leukemia-1 to induce apoptosis in oral squamous cell carcinomas and tumor xenograft through activation of Bax and oligomerization. Mol Pharmacol. 2013;83:33–41.
Heiduschka G, Erovic BM, Pammer J, Kotowski U, Kaider A, Ch Grasl M, Thurnher D. Mcl-1 expression is up-regulated in malignancies of the parotid gland. Dis Markers. 2011;30:229–33.
Ross JS, Wang K, Rand JV, Sheehan CE, Jennings TA, Al-Rohil RN, Otto GA, Curran JC, Palmer G, Downing SR, Yelensky R, Lipson D, Balasubramanian S, Garcia L, Mahoney K, Ali SM, Miller VA, Stephens PJ. Comprehensive genomic profiling of relapsed and metastatic adenoid cystic carcinomas by next-generation sequencing reveals potential new routes to targeted therapies. Am J Surg Pathol. 2014;38:235–8.
Mallick S, Agarwal J, Kannan S, Pawar S, Kane S. Teni T PCNA and anti-apoptotic Mcl-1 proteins predict disease-free survival in oral cancer patients treated with definitive radiotherapy. Oral Oncol. 2010;46:688–93.
Sitailo LA, Jerome-Morais A, Denning MF. Mcl-1 functions as major epidermal survival protein required for proper keratinocyte differentiation. J Invest Dermatol. 2009;129:1351–60.
Maji S, Shriwas O, Samal SK, Priyadarshini M, Rath R, Panda S, Das Majumdar SK, Muduly DK, Dash R. STAT3- and GSK3β-mediated Mcl-1 regulation modulates TPF resistance in oral squamous cell carcinoma. Carcinogenesis. 2019;40:173–83.
Heimer S, Knoll G, Neubert P, Hammer KP, Wagner S, Bauer RJ, Jantsch J, Ehrenschwender M. Hypertonicity counteracts MCL-1 and renders BCL-XL a synthetic lethal target in head and neck cancer. FEBS J. 2021;288:1822–38.
Slomp A, Peperzak V. Role and regulation of pro-survival BCL-2 proteins in multiple myeloma. Front Oncol. 2018;8:533.
Xu L-H, Zhao F, Yang W-W, Chen C-W, Du Z-H, Fu M, Ge X-Y, Li S-L.MYB promotes the growth and metastasis of salivary adenoid cystic carcinoma. Int J Oncol. 2019;54:1579–90.
He L, Torres-Lockhart K, Forster N, Ramakrishnan S, Greninger P, Garnett MJ, McDermott U, Rothenberg SM, Benes CH, Ellisen LW. Mcl-1 and FBW7 control a dominant survival pathway underlying HDAC and Bcl-2 inhibitor synergy in squamous cell carcinoma. Cancer Discov. 2013;3:324–37.
Ge C, Dong J, Chu Y, Cao S, Zhang J, Wei J. LncRNA FGD5-AS1 promotes tumor growth by regulating MCL1 via sponging miR-153-3p in oral cancer. Aging. 2020;12:14355–64.
Wang DY. Promotive effects of HOXA10 antisense RNA on the stemness of oral squamous cell carcinoma stem cells through a microRNA-29a/MCL-1/phosphatidyl inositol 3-kinase/protein kinase B axis. Arch Oral Biol. 2021;126:105114
Palve VC, Teni TR. Association of anti-apoptotic Mcl-1L isoform expression with radioresistance of oral squamous carcinoma cells. Radiat Oncol. 2012;7:135.
Yazbeck VY, Li C, Grandis JR, Zang Y, Johnson DE. Single-agent obatoclax (GX15-070) potently induces apoptosis and pro-survival autophagy in head and neck squamous cell carcinoma cells. Oral Oncol. 2014;50:120–7.
Gilormini M, Malesys C, Armandy E, Manas P, Guy JB, Magne N, Rodriguez-Lafrasse C, Ardail D. Preferential targeting of cancer stem cells in the radiosensitizing effect of ABT-737 on HNSCC. Oncotarget. 2016;7:16731–44.
Yang IH, Hong SH, Jung M, Ahn CH, Yoon HJ, Hong SD, Cho SD, Shin JA. Cryptotanshinone chemosensitivity potentiation by TW-37 in human oral cancer cell lines by targeting STAT3-Mcl-1 signaling. Cancer Cell Int. 2020;20:405.
Sung E-S, Park K-J, Choi H-J, Kim C-H, Kim Y-S. The proteasome inhibitor MG132 potentiates TRAIL receptor agonist-induced apoptosis by stabilizing tBid and Bik in human head and neck squamous cell carcinoma cells. Exp Cell Res. 2012;318:1564–76.
Shaikh MH, Idris A, Johnson NW, Fallaha S, Clarke DTW, Martin D, Morgan IM, Gabrielli B, McMillan NAJ. Aurora kinases are a novel therapeutic target for HPV-positive head and neck cancers. Oral Oncol. 2018;86:105–12.
Yu HJ, Shin JA, Jung JY, Nam JS, Hong IS, Cho NP, Cho SD. Inhibition of myeloid cell leukemia-1: Association with sorafenib-induced apoptosis in human mucoepidermoid carcinoma cells and tumor xenograft. Head Neck. 2015;37:1326–35.
Barzegar M, Ma S, Zhang C, Chen X, Gu Y, Shang C, Jiang X, Yang J, Nathan CA, Yang S, Huang S. SKLB188 inhibits the growth of head and neck squamous cell carcinoma by suppressing EGFR signalling. Br J Cancer. 2017;117:1154–63.
Liu X, Lv Z, Zou J, Liu X, Ma J, Wang J, Sa N, **g P, Xu W. Afatinib down-regulates MCL-1 expression through the PERK-eIF2alpha-ATF4 axis and leads to apoptosis in head and neck squamous cell carcinoma. Am J Cancer Res. 2016;6:1708–19.
Jeon YJ, Ko SM, Cho JH, Chae JI, Shim JH. The HDAC inhibitor, panobinostat, induces apoptosis by suppressing the expresssion of specificity protein 1 in oral squamous cell carcinoma. Int J Mol Med. 2013;32:860–6.
Gao Y, Trivedi S, Ferris RL, Koide K. Regulation of HPV16 E6 and MCL1 by SF3B1 inhibitor in head and neck cancer cells. Sci Rep. 2014;4:6098.
Sachita K, Yu HJ, Yun JW, Lee JS, Cho SD. YM155 induces apoptosis through downregulation of specificity protein 1 and myeloid cell leukemia-1 in human oral cancer cell lines. J Oral Pathol Med. 2015;44:785–91.
Park I-S, Jo J-R, Hong H, Nam K-Y, Kim J-B, Hwang S-H, Choi M-S, Ryu N-H, Jang H-J, Lee S-H, Kim C-S, Kwon T-G, Park G-Y, Park J-W, Jang B-C. Aspirin induces apoptosis in YD-8 human oral squamous carcinoma cells through activation of caspases, down-regulation of Mcl-1, and inactivation of ERK-1/2 and AKT. Toxicol In Vitro. 2010;24:713–20.
Bai LY, Chiu CF, Chiu SJ, Chu PC, Weng JR. FTY720 induces autophagy-associated apoptosis in human oral squamous carcinoma cells, in part, through a reactive oxygen species/Mcl-1-dependent mechanism. Sci Rep. 2017;7:5600.
Gao C, Ren C, Liu Z, Zhang L, Tang R, Li X. GAS5, a FoxO1-actived long noncoding RNA, promotes propofol-induced oral squamous cell carcinoma apoptosis by regulating the miR-1297-GSK3beta axis. Artif Cells Nanomed Biotechnol. 2019;47:3985–93.
Chien C-M, Lin K-L, Su J-C, Chuang P-W, Tseng C-H, Chen Y-L, Chang L-S, Lin S-R. Naphtho[1,2-b]furan-4,5-dione induces apoptosis of oral squamous cell carcinoma: Involvement of EGF receptor/PI3K/Akt signaling pathway. Eur J Pharmacol. 2010;636:52–8.
Jia L, Song Q, Zhou C, Li X, Pi L, Ma X, Li H, Lu X, Shen Y. Dihydroartemisinin as a putative STAT3 inhibitor, suppresses the growth of head and neck squamous cell carcinoma by targeting Jak2/STAT3 signaling. PLoS ONE. 2016;11:e0147157.
Jung C-W, Jo J-R, Lee S-H, Park Y-K, Jung N-K, Song D-K, Bae J, Nam K-Y, Ha J-S, Park I-S, Park G-Y, Jang B-C, Park J-W. Anti-cancer properties of glucosamine-hydrochloride in YD-8 human oral cancer cells: Induction of the caspase-dependent apoptosis and down-regulation of HIF-1α. Toxicol In Vitro. 2012;26:42–50.
Lee H-E, Choi E-S, Shin J-A, Lee S-O, Park K-S, Cho N-P, Cho S-D. Fucoidan induces caspase-dependent apoptosis in MC3 human mucoepidermoid carcinoma cells. Exp Ther Med 2014;7:228–32.
Britt EL, Raman S, Leek K, Sheehy CH, Kim SW, Harada H. Combination of fenretinide and ABT-263 induces apoptosis through NOXA for head and neck squamous cell carcinoma treatment. PLoS ONE. 2019;14:e0219398.
Zhu Y, Wang C, Zhou Y, Ma N, Zhou J. C6 ceramide motivates the anticancer sensibility induced by PKC412 in preclinical head and neck squamous cell carcinoma models. J Cell Physiol. 2018;233:9437–46.
Seo SU, Cho HK, Min KJ, Woo SM, Kim S, Park JW, Kim SH, Choi YH, Keum YS, Hyun JW, Park HH, Lee SH, Kim DE. Kwon TK Thioridazine enhances sensitivity to carboplatin in human head and neck cancer cells through downregulation of c-FLIP and Mcl-1 expression. Cell Death Dis. 2017;8:e2599.
Geiger JL, Grandis JR, Bauman JE. The STAT3 pathway as a therapeutic target in head and neck cancer: Barriers and innovations. Oral Oncol. 2016;56:84–92.
Lin HY, Hou SC, Chen SC, Kao MC, Yu CC, Funayama S, Ho CT, Way TD. (-)-Epigallocatechin gallate induces Fas/CD95-mediated apoptosis through inhibiting constitutive and IL-6-induced JAK/STAT3 signaling in head and neck squamous cell carcinoma cells. J Agric Food Chem. 2012;60:2480–9.
Oh HN, Seo JH, Lee MH, Kim C, Kim E, Yoon G, Cho SS, Cho YS, Choi HW, Shim JH, Chae JI. Licochalcone C induced apoptosis in human oral squamous cell carcinoma cells by regulation of the JAK2/STAT3 signaling pathway. J Cell Biochem. 2018;119:10118–30.
Yang IH, Jung W, Kim LH, Shin JA, Cho NP, Hong SD, Hong KO, Cho SD. Nitidine chloride represses Mcl-1 protein via lysosomal degradation in oral squamous cell carcinoma. J Oral Pathol Med. 2018;47:823–9.
Rajamoorthi A, Shrivastava S, Steele R, Nerurkar P, Gonzalez JG, Crawford S, Varvares M, Ray RB. Bitter melon reduces head and neck squamous cell carcinoma growth by targeting c-Met signaling. PLoS ONE. 2013;8:e78006.
Macha MA, Matta A, Chauhan SS, Siu KWM, Ralhan R. 14-3-3 zeta is a molecular target in guggulsterone induced apoptosis in head and neck cancer cells. Bmc Cancer. 2010;10:655.
Lin KL, Chien CM, Tseng CH, Chen YL, Chang LS, Lin SR. Furano-1,2-Naphthoquinone Inhibits Src and PI3K/Akt signaling pathways in Ca9-22 human oral squamous carcinoma cells. Integr Cancer Ther. 2014;13:Np18-28.
Lee HE, Nam JS, Shin JA, Hong IS, Yang IH, You MJ, Cho SD. Convallaria keiskei as a novel therapeutic alternative for salivary gland cancer treatment by targeting myeloid cell leukemia-1. Head Neck-J Sci Spec. 2016;38:E761-E70.
Li MH, Liao X, Li C, Wang TT, Sun YS, Yang K, Jiang PW, Shi ST, Zhang WX, Zhang K, Li C, Yang P. Lycorine hydrochloride induces reactive oxygen species-mediated apoptosis via the mitochondrial apoptotic pathway and the JNK signaling pathway in the oral squamous cell carcinoma HSC-3 cell line. Oncol Lett. 2021;21:236.
Jung M, Han DJ, Ahn CH, Hong KO, Choi YS, Kim JS, Yoon HJ, Hong SD, Shin JA. Cho SD In vitro induction of mitotic catastrophe as a therapeutic approach for oral cancer using the ethanolic extract of Juniperus squamata. Oncol Rep. 2021;45:103.
Dupuis-Maurin V, Brinza L, Baguet J, Plantamura E, Schicklin S, Chambion S, Macari C, Tomkowiak M, Deniaud E, Leverrier Y, Marvel J, Michallet MC. Overexpression of the transcription factor Sp1 activates the OAS-RNAse L-RIG-I pathway. PLoS ONE. 2015;10:e0118551.
Pietrzak M, Puzianowska-Kuznicka M. p53-dependent repression of the human MCL-1 gene encoding an anti-apoptotic member of the BCL-2 family: the role of Sp1 and of basic transcription factor binding sites in the MCL-1 promoter. Biol Chem. 2008;389:383–93.
Choi KH, Shim JH, Huong LD, Cho NP, Cho SD. Inhibition of myeloid cell leukemia-1 by tolfenamic acid induces apoptosis in mucoepidermoid carcinoma. Oral Dis. 2011;17:469–75.
Kim DW, Ko SM, Jeon YJ, Noh YW, Choi NJ, Cho SD, Moon HS, Cho YS, Shin JC, Park SM, Seo KS, Choi JY, Chae JI, Shim JH. Anti-proliferative effect of honokiol in oral squamous cancer through the regulation of specificity protein 1. Int J Oncol. 2013;43:1103–10.
Cho JJ, Chae JI, Kim KH, Cho JH, Jeon YJ, Oh HN, Yoon G, Yoon DY, Cho YS, Cho SS, Shim JH. Manumycin A from a new Streptomyces strain induces endoplasmic reticulum stress-mediated cell death through specificity protein 1 signaling in human oral squamous cell carcinoma. Int J Oncol. 2015;47:1954–62.
Shin JA, Kim JS, Kwon KH, Nam JS, Jung JY, Cho NP, Cho SD. Apoptotic effect of hot water extract of Sanguisorba officinalis L. in human oral cancer cells. Oncol Lett. 2012;4:489–94.
Lee KE, Shin JA, Hong IS, Cho NP, Cho SD. Effect of methanol extracts of Cnidium officinale Makino and Capsella bursa-pastoris on the apoptosis of HSC-2 human oral cancer cells. Exp Ther Med. 2013;5:789–92.
Shin JA, Kim JJ, Choi ES, Shim JH, Ryu MH, Kwon KH, Park HM, Seo JY, Lee SY, Lim DW, Cho NP, Cho SD. In vitro apoptotic effects of methanol extracts of Dianthus chinensis and Acalypha australis L. targeting specificity protein 1 in human oral cancer cells. Head Neck. 2013;35:992–8.
Liou GY, Storz P. Reactive oxygen species in cancer. Free Radic Res. 2010;44:479–96.
Zhang JX, Wang XL, Vikash V, Ye Q, Wu DD, Liu YL, Dong WG. ROS and ROS-mediated cellular signaling. Oxid Med Cell Longev. 2016;2016.
Yeh Y-T, Yeh H, Su S-H, Lin J-S, Lee K-J, Shyu H-W, Chen Z-F, Huang S-Y, Su S-J. Phenethyl isothiocyanate induces DNA damage-associated G2/M arrest and subsequent apoptosis in oral cancer cells with varying p53 mutations. Free Radic Biol Med. 2014;74:1–13.
Yeh Y-T, Hsu Y-N, Huang S-Y, Lin J-S, Chen Z-F, Chow N-H, Su S-H, Shyu H-W, Lin C-C, Huang W-T, Yeh H, Chih Y-C, Huang Y-H, Su S-J. Benzyl isothiocyanate promotes apoptosis of oral cancer cells via an acute redox stress-mediated DNA damage response. Food Chem Toxicol. 2016;97:336–45.
Weng JR, Bai LY, Chiu SJ, Chiu CF, Lin WY, Hu JL, Shieh TM. Divaricoside exerts antitumor effects, in part, by modulating Mcl-1 in human oral squamous cell carcinoma cells. Comput Struct Biotechnol J. 2019;17:151–9.
Kim EH, Jang H, Shin D, Baek SH, Roh JL. Targeting Nrf2 with wogonin overcomes cisplatin resistance in head and neck cancer. Apoptosis. 2016;21:1265–78.
Weng J-R, Bai L-Y, Ko H-H, Tsai Y-T. Cyclocommunol induces apoptosis in human oral squamous cell carcinoma partially through a Mcl-1-dependent mechanism. Phytomedicine. 2018;39:25–32.
Hotz MA, Bosq J, Zbaeren P, Reed J, Schwab G, Krajewski S, Brousset P, Borner MM. Spontaneous apoptosis and the expression of p53 and Bcl-2 family proteins in locally advanced head and neck cancer. Arch Otolaryngol Head Neck Surg. 1999;125:417–22.
Erovic BM, Pelzmann M, Grasl M, Pammer J, Kornek G, Brannath W, Selzer E, Thurnher D. Mcl-1, vascular endothelial growth factor-R2, and 14-3-3sigma expression might predict primary response against radiotherapy and chemotherapy in patients with locally advanced squamous cell carcinomas of the head and neck. Clin Cancer Res. 2005;11:8632–6.
Majchrzak E, Szybiak B, Wegner A, Pienkowski P, Pazdrowski J, Luczewski L, Sowka M, Golusinski P, Malicki J, Golusinski W. Oral cavity and oropharyngeal squamous cell carcinoma in young adults: a review of the literature. Radiol Oncol. 2014;48:1–10.
Capkova M, Sachova J, Strnad H, Kolar M, Hroudova M, Chovanec M, Cada Z, Steffl M, Valach J, Kastner J, Vlcek C, Smetana K, Jr., Plzak J. Microarray analysis of serum mRNA in patients with head and neck squamous cell carcinoma at whole-genome scale. Biomed Res Int. 2014;2014:408683.
Zhou Z, Sturgis EM, Liu Z, Wang LE, Wei Q, Li G. Genetic variants of NOXA and MCL1 modify the risk of HPV16-associated squamous cell carcinoma of the head and neck. BMC Cancer. 2012;12:159.
Maji S, Samal SK, Pattanaik L, Panda S, Quinn BA, Das SK, Sarkar D, Pellecchia M, Fisher PB, Dash R. Mcl-1 is an important therapeutic target for oral squamous cell carcinomas. Oncotarget. 2015;6:16623–37.
Zang Y, Thomas SM, Chan ET, Kirk CJ, Freilino ML, DeLancey HM, Grandis JR, Li C, Johnson DE, Carfilzomib and ONX 0912 inhibit cell survival and tumor growth of head and neck cancer and their activities are enhanced by suppression of Mcl-1 or autophagy. Clin Cancer Res. 2012;18:5639–49.
Tanaka N, Patel AA, Wang J, Frederick MJ, Kalu NN, Zhao M, Fitzgerald AL, **e TX, Silver NL, Caulin C, Zhou G, Skinner HD, Johnson FM, Myers JN, Osman AA. Wee-1 kinase inhibition sensitizes high-risk HPV + HNSCC to apoptosis accompanied by downregulation of MCl-1 and XIAP antiapoptotic proteins. Clin Cancer Res. 2015;21:4831–44.
Skoda C, Erovic BM, Wachek V, Vormittag L, Wrba F, Martinek H, Heiduschka G, Kloimstein P, Selzer E, Thurnher D. Down-regulation of Mcl-1 with antisense technology alters the effect of various cytotoxic agents used in treatment of squamous cell carcinoma of the head and neck. Oncol Rep. 2008;19:1499–503.
Roh J-L, Kim EH, Jang H, Shin D. Aspirin plus sorafenib potentiates cisplatin cytotoxicity in resistant head and neck cancer cells through xCT inhibition. Free Radic Biol Med. 2017;104:1–9.
Hsieh MJ, Hsieh YH, Lin CW, Chen MK, Yang SF, Chiou HL. Transcriptional regulation of Mcl-1 plays an important role of cellular protective effector of vincristine-triggered autophagy in oral cancer cells. Expert Opin Ther Targets. 2015;19:455–70.
Seo SU, Kim TH, Kim DE, Min KJ, Kwon TK. NOX4-mediated ROS production induces apoptotic cell death via down-regulation of c-FLIP and Mcl-1 expression in combined treatment with thioridazine and curcumin. Redox Biol. 2017;13:608–22.
Shih YL, Hung FM, Lee CH, Yeh MY, Lee MH, Lu HF, Chen YL, Liu JY, Chung JG. Fisetin induces apoptosis of HSC3 human oral cancer cells through endoplasmic reticulum stress and dysfunction of mitochondria-mediated signaling pathways. Vivo. 2017;31:1103–14.
Won DH, Chung SH, Shin JA, Hong KO, Yang IH, Yun JW, Cho SD. Induction of sestrin 2 is associated with fisetin-mediated apoptosis in human head and neck cancer cell lines. J Clin Biochem Nutr. 2019;64:97–105.
Cho JJ, Chae J-I, Yoon G, Kim KH, Cho JH, Cho S-S, Cho YS, Shim J-H. Licochalcone A, a natural chalconoid isolated from Glycyrrhiza inflata root, induces apoptosis via Sp1 and Sp1 regulatory proteins in oral squamous cell carcinoma. Int J Oncol. 2014;45:667–74.
Oh H, Yoon G, Shin JC, Park SM, Cho SS, Cho JH, Lee MH, Liu K, Cho YS, Chae JI, Shim JH. Licochalcone B induces apoptosis of human oral squamous cell carcinoma through the extrinsic- and intrinsic-signaling pathways. Int J Oncol. 2016;48:1749–57.
Hsieh MJ, Lin CW, Chiou HL, Yang SF, Chen MK. Dehydroandrographolide, an iNOS inhibitor, extracted from Andrographis paniculata (Burm.f.) Nees, induces autophagy in human oral cancer cells. Oncotarget. 2015;6:30831–49.
Han JM, Hong KO, Yang IH, Ahn CH, ** B, Lee W, Jung YC, Kim KA, Shin JA, Cho SD, Hong SD. Oridonin induces the apoptosis of mucoepidermoid carcinoma cell lines in a myeloid cell leukemia–1–dependent manner. Int J Oncol. 2020;57:377–85.
Sachita K, Kim Y, Yu HJ, Cho SD, Lee JS. In vitro assessment of the anticancer potential of evodiamine in human oral cancer cell lines. Phytother Res. 2015;29:1145–51.
Yu H-J, Park C, Kim S-J, Cho N-P, Cho S-D. Signal transducer and activators of transcription 3 regulates cryptotanshinone-induced apoptosis in human mucoepidermoid carcinoma cells. Pharmacognosy Mag. 2014;10:622.
Ramu AK, Ali D, Alarifi S, Syed Abuthakir MH, Ahmed Abdul BA. Reserpine inhibits DNA repair, cell proliferation, invasion and induces apoptosis in oral carcinogenesis via modulation of TGF-β signaling. Life Sci. 2021;264:118730.
Weng JR, Lin WY, Bai LY, Hu JL, Feng CH. Antitumor activity of the cardiac glycoside alphaldiginoside by modulating Mcl-1 in human oral squamous cell carcinoma cells. Int J Mol Sci. 2020;21:7947.
Chien CM, Lin KL, Su JC, Chang LS, Lin SR. Inactivation of epidermal growth factor receptor and downstream pathways in oral squamous cell carcinoma Ca9-22 cells by cardiotoxin III from Naja naja atra. J Nat Prod. 2009;72:1735–40.
Acknowledgements
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science ICT & Future Planning [Grant Number 2019R1A2C1085896] and the Ministry of Education [Grant Number 2020R1I1A1A01070547].
Funding
None.
Author information
Authors and Affiliations
Contributions
SJC and NS performed the literature review, conducted the data extraction, and wrote the draft. JAS and SDH conducted the literature review and edited the manuscript. SDC designed this research and wrote the final draft of the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare the absence of any competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Choi, SJ., Swarup, N., Shin, JA. et al. Myeloid cell leukemia-1 expression in cancers of the oral cavity: a sco** review. Cancer Cell Int 22, 182 (2022). https://doi.org/10.1186/s12935-022-02603-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12935-022-02603-0