Abstract
Background
Vitamin D insufficiency or deficiency is associated with an altered microbiota in older men. However, the relationship between the gut microbiota and 25-hydroxyvitamin D (25(OH)D) levels remains unknown in postmenopausal women. In this study, fecal microbiota profiles for 88 postmenopausal women in the high 25(OH)D (HVD) group (n = 44) and the low 25(OH)D (LVD) group (n = 44) were determined. An integrated 16S rRNA gene sequencing and liquid chromatography–mass spectrometry (LC–MS)-based metabolomics approach was applied to explore the association of serum 25(OH)D levels with the gut microbiota and fecal metabolic phenotype. Adjustments were made using several statistical models for potential confounding variables identified from the literature.
Results
The results demonstrated that the community diversity estimated by the Observe, Chao1 and ACE indexes was significantly lower in the LVD group than in the HVD group. Additionally, two kinds of characteristic differences in the microflora were analyzed in the HVD group, and ten kinds of characteristic differences in the microflora were analyzed in the LVD group. We observed that some bacteria belonging to the genera Bifidobacterium, Bacillus, F0332 and Gemella, were enriched in the LVD group, as were other genera, including Lachnoclostridium, UC5_1_2E3, Ruminococcus_gnavus_group and un_f_Lachnospiraceae. Christensenellaceae, Eggerthellaceae and Cloacibacillus were enriched in the HVD group. The L-pyroglutamic acid, inosine, and L-homocysteic acid levels were higher in the HVD group and were negatively correlated with the 1,2-benzenedicarboxylic acid and cholic acid metabolic levels.
Conclusions
These observations provide a better understanding of the relationships between serum 25(OH)D levels and the fecal microbiota and metabolites in postmenopausal women.
Similar content being viewed by others
Introduction
Vitamin D (VD) insufficiency or deficiency is a frequent comorbidity in Chinese women with postmenopausal osteoporosis (PMO) [1]. Osteoporosis is a chronic, systemic skeletal disease with increased susceptibility to brittle bones and fractures; 200 million people worldwide are affected, and 50% of postmenopausal women suffer an osteoporosis-related fracture during their lifetime [2, 3]. VD maintains calcium/phosphate homeostasis and is widely used to prevent and treat osteoporosis, and its concentration in the body, which is partly obtained from food sources, depends largely on 7-dehydrocholesterol (vitamin D3) being converted by ultraviolet irradiation in the skin [4]. In addition, VD deficiency is associated with nonskeletal diseases such as cardiovascular disease, cancer and diabetes [5,6,7]. The major sources of VD are sun exposure, diet, and supplements [8]. The problem, however, is that the ability to synthesize VD in the skin becomes decreasing with advancing age. Therefore, with increasing age, VD adequacy relies increasingly on dietary intake [9].
VD is metabolized by first undergoing hydroxylation by the enzyme 25-hydroxylase to form 25-hydroxyvitamin D3. In the kidneys, the enzyme 1α-hydroxylase (CYP27B1) forms an active form of VD, namely, 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) [10]. Eventually, 1,25(OH)2D3 is metabolized by the renal CYP24A1 enzyme in the kidney [11]. Clinically, serum 25-hydroxyvitamin D (25(OH)D) levels ≥ 20 ng/ml are considered adequate, while serum 25(OH)D levels < 20 ng/mL are defined as VD deficiency [12]. VD deficiency alters the microbiome and integrity of the gut epithelial barrier. Changes in serum levels of the 25(OH)D post-supplementation vary widely among individuals, with around 25% of people demonstrating no response [13,14,15,16]. The responder and non-responder groups differed with respect to changes in gut microbial composition post-supplementation [17].The trillions of microorganisms in the gastrointestinal tract are important factors in maintaining human health and are involved in inflammatory, metabolic, cardiovascular, autoimmune, neurological and psychiatric diseases [18]. The vitamin D receptor (VDR) is highly expressed on the intestinal epithelium and binds to 25(OH)D, which is a key player in intestinal homeostasis by regulating mucosal immunity [19].
VD also changes the microbiome, and manipulation of bacterial abundance or composition impacts disease manifestation [20]. Oral vitamin D3 supplementation influences the human gut microbiome of the upper gastrointestinal tract and is associated with reduced opportunistic pathogens and increased bacterial richness [21]. VD and the VDR adjust the innate immune response to the microbiome by controlling microbiota dysbiosis and maintaining tolerance in the gut [22]. Therefore, VD status is connected with the composition and function of the intestinal microflora.
To date, several studies have reported that the intestinal microbiota plays a key role in the pathogenesis of PMO and represents a new target for treating PMO [23]. Nonetheless, the evidence of an association between 25(OH)D levels and the gut microbiota in postmenopausal women remains inadequate. Our aim in the present study was to investigate whether intestinal microbiota features are associated with 25(OH)D levels using 16S rRNA gene sequencing and liquid chromatography–mass spectrometry (LC/MS)-based metabolomics to explore an alternative strategy to enhance calcium absorption as a strategy for osteoporosis prevention. It is of significant importance for preventing osteoporosis among the elderly or among people living in areas with little sunshine.
Materials and methods
Human participants
Ethical approval was granted by the Ethics Committee of Zhongshan Hospital of **amen University (No. 201808) before recruitment. Postmenopausal women were recruited from **amen, Fujian Province, China, between December 1, 2018, and February 1, 2019. Postmenopausal status was defined as at least one year since the last menstruation. Written informed consent was obtained from all the participants. We adopted the following exclusion criteria before fecal sample collection: (1) use of antibiotics or hormones within 3 months; (2) consumption of pro/pre/synbiotic products within two weeks; (3) use of medications (e.g., calcium, VD, calcitriol, alpha calcitriol, estrogen, glucocorticoids, diphosphonate, denosumab or teriparatide); and (4) presence of bone disease, hyperthyroidism, hypothyroidism, gastrointestinal disease, cancer, kidney disease, or mental illness or evidence of recent infections.
Determination of clinical parameters
The basic information of all the subjects, such as age, height and weight, was collected, and the body mass index (BMI) was calculated according to the data of the latter two items. Blood samples were collected from all the subjects in a fasting state and at similar time points in the morning. The levels of serum 25(OH)D, estradiol (E2), osteocalcin (OC), C-terminal telopeptide of type I collagen (CTX-I), procollagen type 1 n-terminal propeptide (P1NP), and parathyroid hormone (PTH) were measured with an automated Roche Osteoporosis Int electrochemiluminescence system (Roche Diagnostics GmbH, Germany). The inter- and intra-assay coefficients of variation (CVs) were 8.0% and 5.6% for 25(OH)D, 2.9% and 2.3% for E2, 4.0% and 2.9% for OC, 3.5% and 2.5% for CTX-1, 2.8% and 2.3% for P1NP, and 2.9% and 1.7% for PTH, respectively. The bone mineral densities (BMDs) of the lumbar spine (LS) (L1-4) and total hip joint (femoral neck (FN), trochanter, and intertrochanteric region) were measured with a daily calibrated Hologic 4500 A dual-energy X-ray absorptiometry scanner (Lunar Expert 1313, Lunar Corp, USA).
Sequencing and bioinformatics
Fecal samples were collected in sterile plastic cups, frozen, and stored at − 80 °C within 1 h until further processing [24]. Fecal microbial DNA was extracted using a QIAamp DNA Stool Mini Kit (Qiagen, Hilden, Germany). PCR amplification was carried out using an ABI 2720 Thermal Cycler (Thermo Fisher Scientific, USA). We used Multiskan™ GO spectrophotometry (Thermo Fisher Scientific, USA) to quantify bacterial genomic DNA as the template for amplification of the V3-V4 hypervariable region of the 16S rRNA gene in three replicate reactions with forward (Illumina adapter sequence 5′-CCTACGGGNBGCASCAG-3′) and reverse (Illumina adapter sequence 5′-GGACTACNVGGGTWTCTAAT-3′) primers. Replicate PCR products were pooled and purified with Agencourt AMPure XP magnetic beads (Beckman Coulter, USA). A TopTaq DNA Polymerase kit (Transgen, China) was used. The purity and concentration of sample DNA were assessed using a NanoDrop 2000 Spectrophotometer (Thermo Fisher Scientific, USA). Paired-end sequencing was performed by Treatgut Biotechnology Co., Ltd. with a HiSeq 2500 (Illumina, San Diego, CA, USA) with PE 250 bp reagents.
After sequencing, raw paired-end reads were assembled using FLASH [25] with the default parameters. Primers were removed using cutadapt, and clean tags were obtained by removing the lower reads using cutadapt [26]. To assign de novo operational taxonomic units (OTUs), we removed chimeric sequences and clustered sequences with 97% similarity and used Usearch (V10.0.240) [27] for the study. The representative sequences of OTUs were aligned to the SILVA132 database for taxonomic classification by RDP Classifier [28] and aggregated to various taxonomic levels.
Fecal metabolite extraction
At least fifty milligrams of sample was placed in an EP tube, and then 1000 μL of extraction liquid containing an internal target (V methanol:V acetonitrile:V water = 2:2:1, which was kept at − 20 °C before extraction) was added. The samples were homogenized in a bead mill for 4 min at 45 Hz and ultrasonicated for 5 min (incubated in ice water). After homogenization 3 times, the samples were incubated for 1 h at − 20 °C to precipitate proteins. The samples were centrifuged at 12,000 rpm for 15 min at 4 °C. The supernatant (750 μL) was transferred to fresh EP tubes, and the extracts were dried in a vacuum concentrator without heating. Then, 100 μL of extraction liquid (V acetonitrile:V water = 1:1) was added for reconstitution. The samples were vortexed for 30 s, sonicated for 10 min (4 °C water bath), and centrifuged for 15 min at 12,000 rpm and 4 °C. The supernatant (60 μL) was transferred to a fresh 2 mL LC/MS glass vial, and 10 μL was collected from each sample and pooled as quality control (QC) samples. Sixty microliters of supernatant was used for ultra-high-performance liquid chromatography combined with quadrupole time-of-flight mass spectrometry (UHPLC-QTOF-MS) analysis.
LC–MS/MS analysis and annotation
LC–MS/MS analyses were performed using a UHPLC system (1290, Agilent Technologies) with a UPLC BEH Amide column (1.7 μm 2.1 × 100 mm, Waters) coupled to a TripleTOF 6600 (Q-TOF, AB Sciex) & QTOF 6550 (Agilent). The mobile phase consisted of 25 mM NH4OAc and 25 mM NH4OH in water (pH = 9.75) (A) and acetonitrile (B), which was applied in an elution gradient as follows: 0 min, 95% B; 7 min, 65% B; 9 min, 40% B; 9.1 min, 95% B; and 12 min, 95% B, which was delivered at 0.5 mL/min. The injection volume was 2 μL. A TripleTOF mass spectrometer was used due to its ability to acquire MS/MS spectra on an information-dependent basis (IDA) during an LC/MS experiment. In this mode, the acquisition software (Analyst TF 1.7, AB Sciex) continuously evaluates the full-scan survey MS data as it collects and triggers the acquisition of MS/MS spectra depending on preselected criteria. In each cycle, 12 precursor ions with intensities greater than 100 were chosen for fragmentation at a collision energy of 30 V (15 MS/MS events with a product ion accumulation time of 50 ms each). The ESI source conditions were set as follows: ion source gas 1 at 60 psi, ion source gas 2 at 60 psi, curtain gas at 35 psi, source temperature at 650 °C, and ion spray voltage floating at 5000 V or − 4000 V in positive or negative modes, respectively.
MS raw data files were converted to mzXML format using ProteoWizard [29] and processed with the R package XCMS (version 3.2). The preprocessing results generated a data matrix that consisted of the retention time (RT), mass-to-charge ratio (m/z) values, and peak intensity. The R package CAMERA was used for peak annotation after XCMS data processing [30].
Statistical analyses and visualization
The rarefaction curves constructed from the sequenced data has been basically stable, indicating that the sequenced data has benn basically stable at this sequencing depth (Additional file 1: Fig. S1). The alpha diversity indexes, bacterial richness (observed OTUs), Shannon, Simpson, ACE, Chao1 index and evenness (J) were calculated based on OTU tables of the study. Significance tests between the HVD and LVD groups were conducted with the Wilcoxon test method. Differences in community structure across samples (beta diversity) were visualized by principal coordinates analysis (PCoA) plots based on Bray–Curtis distance. Significance tests were determined using permutational multivariate analysis of variance (PERMANOVA) with 999 permutations in vegan [31]. Linear discriminant analysis effect size [32] was performed to identify taxa with differential abundance between the HVD and LVD groups. We further explored the correlation between different genera and fecal metabolites by Spearman’s rank test. To evaluate functional differences in the gut microbiomes of the HVD versus LVD groups, we performed PICRUSt [33] to calculate the microbial abundances, assign metabolic pathways to the gut microbiomes using KEGG and COG, and then test the differences between the two groups. All statistical and correlational analyses were conducted in R (v3.6.0) [34]. Figures were plotted mainly using ggplot2 (v3.0.0) [35].
Results
Characteristics and clinical and biochemical indexes of the participants
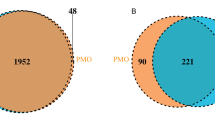
According to the inclusion and exclusion criteria, 18 patients were excluded because they used VD or calcium carbonate and vitamin D3 tablets (Caltrate D). Finally, samples and clinical information for 88 individuals were analyzed. Based on the 25(OH)D concentrations in the serum of the subjects, the 88 participants were divided into an HVD group with 25(OH)D levels ≥ 20 ng/ml (n = 44) and an LVD group with 25(OH)D levels < 20 ng/ml (n = 44) (Fig. 1). The patients’ clinical characteristics, including age, weight, BMI, E2, OC, P1NP, CTX-1, PTH, Z score, T score and BMD of the LS, FN and total hip were comparable between the HVD and LVD groups. There were no differences in age, weight, BMI, E2, CTX-1, PTH, LS Z score, LS T score, LS BMD, FN Z score, FN T score, FN BMD, total hip Z score, total hip T score or total hip BMD between the two groups (Table 1 and Additional file 5: Table S1). Notably, there was a significant difference in serum 25(OH)D levels between the two groups (p < 0.001). The OC and P1NP levels were generally lower in the HVD group than in the LVD group (p < 0.05, respectively) (Table 1).
Comparison of bacterial diversity between the HVD and LVD groups
The alpha diversity estimated by the Observe, Chao1 and ACE indexes was significantly lower in the LVD group than in the HVD group (p < 0.05, respectively). No statistically significant difference was observed between the HVD group and the LVD group in the J index, Simpson index or Shannon index (p > 0.05, respectively) (Fig. 2A). Analysis of similarities (ANOSIM) was used to compare the within- and between-group similarity through a distance measure to test the null hypothesis that the average rank similarity between samples within a group is the same as the average rank similarity between samples belonging to different groups. There were significant differences in colony distribution between the HVD group and the LVD group (Fig. 2B). PCoA was analyzed by using the distance matrix calculated from the species composition of the sample. The horizontal axis and the vertical axis represent the contribution rate of the first principal component (PCoA1) of 9.5% and the second principal component (PCoA2) of 8.02%, respectively (p = 0.015) (Fig. 2C). Venn diagram displaying the common and unique OTUs of the gut microbiota between the HVD and LVD groups. The size of the circle represents the number of OTUs. There were 760 common OTUs and 79 unique OTUs in the HVD group and 34 unique OTUs in the LVD group (Fig. 2D).
Decreased bacterial richness and diversity in the Low 25(OH)D group. A Comparison of bacterial alpha diversity indexes, including Observe, Chao1, ACE, Shannon, Simpson and J. B ANOSIM was used to analyze the significant differences in colony distribution between the High 25(OH)D group and the Low 25(OH)D group (R = 0.025, p = 0.046). C PCoA revealing the bacterial communities between the High 25(OH)D group and the Low 25(OH)D group on the PCoA1 vs. PCoA2 axis (p = 0.015). D Venn diagram showing the differences in bacterial community structures between samples from the High 25(OH)D group and the Low 25(OH)D group
Comparison of the bacterial community between the HVD and LVD groups
Linear discriminant analysis effect size can be used to analyze the differences between groups and identify different microbial species, which can be used to develop biomarkers and promote other studies. In the LVD group and the HVD group, the linear discriminant analysis (LDA) threshold was set as 3 to screen the characteristic flora of the corresponding groups. Two kinds of characteristic differences in microflora were analyzed in the HVD group, and 10 kinds of characteristic differences in microflora were analyzed in the LVD group (Fig. 3). We observed that Christensenellaceae, Eggerthellaceae and Cloacibacillus were enriched in the HVD group. G_Bifidobacterium, g_F0332, g_Gemella, g_Lachnoclostridium, g_UC5_1_2E3, g_Ruminococcus_gnavus_group and s_un_f_Lachnospiraceae were enriched in the LVD group (Fig. 3). The relative abundances of f_Family_XI, f_Bifidobacteriales, f_Bifidobacteriaceae, and o_Bacillales were significantly higher in the LVD group than in the HVD group. Furthermore, the relative abundances of f_Christensenellaceae, f_Eggerthellaceae, f_Defluviitaleaceae, and f_un_o_lzimaplasmatales were significantly higher in the HVD group than in the LVD group (Additional file 2: Fig. S2).
Discriminative taxa between the High 25(OH)D group and Low 25(OH)D group. LEfSe can be used to analyze the differences between groups and identify different microbial species, and this information can be used to develop biomarkers and promote other studies. In the above two syndrome types, the LDA threshold was set as 3 to screen the characteristic flora of the corresponding syndrome types
The results showed that the pathways associated with flavone and flavonol biosynthesis, pentose and glucoronate interconversions and cyanoamino acid metabolism were significantly enriched in the bacterial communities of the LVD group compared with those of the HVD group (Additional file 3: Fig. S3A). Furthermore, the 20 distinguished pathways were calculated to reveal the potential interaction mode (Additional file 3: Fig. S3B).
Fecal metabolism profiles in the HVD and LVD groups
To assess whether the profiles of fecal metabolites are associated with 25(OH)D levels, we performed metabolic sequencing of all stool samples. There were significant differences in fecal metabolites between the HVD group and the LVD group (Fig. 4A). Further stratified analysis by metabolite categories revealed that sucrose, l-pyroglutamic acid, inosine, l-homocysteic acid, oleanolic acid, N-acetyl-l-glutamate, glycocholic acid, trimethylamine N-oxide, nicotinamide, l-phenylalanine, and gamma-l-glutamyl-l-glutamic acid were significantly more abundant in the HVD group than in the LVD group. However, 1,2-benzenedicarboxylic acid, cholic acid, N-acetylcadaverine, histamine, and S-adenosylmethionine were significantly less abundant in the HVD group than in the LVD group (Fig. 4B).
Discriminative fecal metabolites between the High 25(OH)D group and Low 25(OH)D group. A Principal Component Analysis (PCA) revealing the inter-individual variability of the fecal metabolites between the High 25(OH)D group and the Low 25(OH)D group on the PC1 vs PC2 axis (p = 0.005). B The x-axis shows the logarithms (base 10) of the linear discriminant analysis (LDA). The y-axis shows the discriminative fecal metabolites
Association between featured bacterial taxa and metabolites
The relationships among the different bacteria, metabolites and clinical profiles were examined by correlation analysis (Spearman) to evaluate the relationship between the gut bacteria and clinical indexes and between the fecal metabolites and clinical indexes. We found that l-pyroglutamic acid, inosine, l-homocysteic acid, N-acetyl-l-glutamate, and l-phenylalanine correlated positively with 25(OH)D. 1,2-Benzenedicarboxylic acid, cholic acid, N-acetylcadaverine, gamma-l-glutamyl-l-glutamic acid, histamine, and S-adenosylmethionine were negatively correlated with 25(OH)D (Fig. 5A and B). There was a negative association between g__UC5_1_2E3, g__[Ruminococcus]_gnavus_group, g__Gemella and 25(OH)D. Conversely, there was a positive association between g__Ruminococcaceae_UCG−014, g__Cloacibacillus, g__Fournierella, g__un_f_Coriobacteriales_Incertae_Sedis, g__un_o_Chloroplast, g__Christensenellaceae_R−7_group, g__Defluviitaleaceae_UCG−011, g__Ruminococcaceae_UCG−010, g__un_o_DTU014, g__Hydrogenoanaerobacterium, g__un_f_Puniceicoccaceae, g__Lachnospiraceae_UCG−003, g__Ruminococcaceae_UCG−004, g__un_f_Burkholderiaceae, g__Ruminococcaceae_UCG−005, g__Ruminococcaceae_NK4A214_group, g__Subdoligranulum, g__Ruminococcus_1 and 25(OH)D (Fig. 5C). Furthermore, the correlations between distinguished bacteria and different fecal metabolites are shown in Additional file 4: Fig. S4.
The correlation heatmap among the discriminative genera, discriminative fecal metabolites and the clinical indexes. A, B Heatmap for Spearman correlation analysis between fecal metabolites and clinical variables at the family level. A Negative modes. B Positive modes. X-axis, clinical variables. Y-axis, fecal metabolites. C Heatmap for Spearman correlation analysis between intestinal flora and clinical variables at the family level. X-axis, clinical variables. Y-axis, family. Correlation significance, *denotes p < 0.05
Discussion
This study investigates associations among the gut microbiota, metabolites and serum 25(OH)D levels in postmenopausal women. Bacterial richness and diversity were decreased in postmenopausal women with low serum 25(OH)D (< 20 ng/ml). We also identified significant associations between different symbiotic gut microbial genera and serum 25(OH)D levels in this well-characterized cohort. Higher levels of sucrose, l-pyroglutamic acid, inosine, l-homocysteic acid, oleanolic acid, N-acetyl-l-glutamate, glycocholic acid, trimethylamine N-oxide, nicotinamide, l-phenylalanine, and gamma-l-glutamyl-l-glutamic acid were found in the HVD group. Furthermore, several distinguishing intestinal bacteria were associated with distinguishing metabolites related to serum 25(OH)D levels.
In a suburban area in China, over 60% of postmenopausal women had serum 25(OH)D levels over 20 ng/ml [36]. Low VD status is associated with greater bone turnover and bone loss. Women with hip fractures had significantly lower concentrations of serum 25(OH)D; higher concentrations of serum CTX-1, P1NP and OC; and lower FN and total hip BMDs [37]. Patients with nontraumatic osteonecrosis of the femoral head have been shown to have lower 25(OH)D levels and higher levels of P1NP, CTX-1 and OC [38]. In our study, postmenopausal females with lower levels of serum 25(OH)D also had higher OC, P1NP and CTX-1 levels. Due to estrogen deficiency, postmenopausal females have active bone turnover (bone resorption > bone formation) and increased bone loss [39].
Deep sequencing comparing pre- and postmenopausal women indicated that the gut microbiota displays different states before and after menopause. Compared to premenopausal women, postmenopausal women presented significantly lower richness and diversity [40]. Our results showed that postmenopausal women with high 25(OH)D levels had higher alpha diversity than the group with low 25(OH)D levels. A separate study showed inconsistent results: subjects with serum 25(OH)D concentrations below 50 nmol/L (n = 12) and subjects with serum 25(OH)D concentrations above 75 nmol/L (n = 10) had no significant differences in alpha diversity and beta diversity [41]. In contrast, another analysis of serum VD metabolites in 567 elderly men found that subjects with low 1,25(OH)2D levels had reduced bacterial richness and diversity [42]. These conflicting results may be related to the differences in the areas, sample sizes and populations.
There is evidence of a bidirectional interaction between VD and the gut microbiota in inflammation [17]. In rodents, VD regulates the composition and abundance of the gut microbiome, and 1,25(OH)2D3 or VDR deficiency leads to dysbiosis, increased inflammation, and higher susceptibility to intestinal injury [43]. In a meta-analysis of 8316 patients with inflammatory bowel disease (IBD), low levels of 25(OH)D were associated with increased odds of disease activity, mucosal inflammation, low quality of life, and clinical recurrence [44]. In this study, high serum 25(OH)D levels were positively correlated with Subdoligranulum, Ruminococcaceae and Cloacibacillus. At present, the direct relationship between these three bacterial groups and serum 25(OH)D levels in postmenopausal women is unknown. However, these three groups were found to comprise short-chain fatty acid (SCFA)-producing bacteria [45,46,47]. SCFAs play a key role in maintaining gut homeostasis and promoting organismal health by modulating dietary fiber and gut microbiota [48]. The abundance of Subdoligranulum has been shown to be significantly increased in patients with Crohn’s disease following one week of VD supplementation [49]. Research has shown that vitamin D3 positively correlates with hypertension-reduced bacterial genera, including Subdoligranulum and Ruminiclostridium [50].
Variations in the gut microbiota composition can interfere with VD status and biological functions [51]. Probiotic strains such as Lactobacillus rhamnosus and Lactobacillus plantarum increase VDR expression in both mouse and human intestinal epithelial cells [52]. In addition, probiotics L. reuteri and Bifidobacterium longum may increase BMD by promoting calcium absorption. Calcium absorption occurs throughout the intestine [53]. It has also been shown that the composition of the gut microbiota can influence the pH level of the gut [54], an important factor for nutrient absorption, especially calcium [55]. Moreover, some bacteria express enzymes involved in hydroxylation of steroids (i.e., Streptomyces griseolus with CYP105A1, Sebekia benihana with CYP-sb3a, Pseudonocardia autotrophica with Vdh) that are capable to hydroxylate and activate VD [56]. In humans, probiotics interfere with 25(OH)D levels and calcium intake and absorption and slightly decrease bone loss in elderly postmenopausal women to a magnitude quite similar to that observed with calcium and VD supplements [57]. In our study, Bifidobacterium and Gemella were negatively associated with low serum 25(OH)D levels. A study demonstrated that supplementation in 80 healthy VD-deficient women with a weekly oral dose of 50,000 IU vitamin D3 for 12 weeks significantly increased the diversity of the gut microbiota and the relative abundance of Bifidobacterium [17]. These findings indicate that several beneficial bacteria are associated with high serum 25(OH)D levels, but further studies are required to address questions on the potential detrimental impact and mechanisms of action in postmenopausal women. Moreover, VD supplementation may provide a benefit to the gut microbiota.
The findings of our study suggest that Ruminococcus_1 and un_f_Puniceicoccaceae were positively correlated with both 25(OH)D and oleanolic acid levels. It has been reported that oleanolic acid is a triterpenoid with reported bone antiresorption activities. Oleanolic acid significantly increases BMD, improves microarchitectural properties, reduces urinary Ca excretion, and increases 1,25(OH)2D3 and renal CYP27B1 mRNA expression in mature ovariectomized mice [58]. Clinical biochemical indicators and bone density were also reversed by oleanolic acid treatment. A total of 25 potential biomarkers were identified in a rat model of glucocorticoid-induced osteoporosis, and oleanolic acid has a regulatory effect on 17 of them related to some vital metabolic pathways, such as linoleic acid metabolism, valine, leucine and isoleucine biosynthesis, phenylalanine, tyrosine and tryptophan biosynthesis and cysteine and methionine metabolism [59].
We found that cholic acid was negatively correlated with 25(OH)D levels. Various bile acids may play key roles in the control of calcium absorption. It has been shown that ursodeoxycholic acid promotes calcium absorption, whereas deoxycholic acid inhibits calcium absorption [60]. Indirectly, the gut microbiota can hinder vitamin activity through secondary bile acids, mainly lithocholic acid, which compete with VD for VDR binding and activation. In addition, lithocholic acid interacts with the VDR to induce CYP24A1 mRNA expression in the ileum, which leads to calcitriol inactivation [61]. l-Homocysteine has been observed to be positively correlated with 25(OH)D levels. A study from a large community-based cohort of asymptomatic adults found an inverse relationship between 25(OH)D and serum homocysteine levels among those with 25(OH)D concentrations of 21 ng/mL or less. However, no statistical decrease in serum homocysteine was observed once the 25(OH)D concentration rose above 21 ng/ml [62]. 25(OH)D and serum homocysteine have been shown to be significantly negatively correlated in postmenopausal nonosteoporotic females but not in osteoporotic females [63]. In vitro studies of murine preosteoblastic cells have indicated that 1,25-dihydroxyvitamin D3 is involved in the direct regulation of cystathionine beta synthase, an important enzyme in homocysteine metabolism [64].
Phenylalanine is an essential amino acid that is the raw material for the synthesis of tyrosine. Fecal and serum metabolomics analyses suggested that tyrosine and tryptophan metabolism and valine, leucine, and isoleucine degradation were significantly linked to the identified microbiota biomarkers and osteoporosis, respectively [65]. Osteopenia is observed in some patients affected by phenylalanine hydroxylase-deficient phenylketonuria [66]. Adult phenylketonuria patients had poor VD status and exhibited a predominance of bone resorption over bone formation [67]. High serum phenylalanine levels led to lower VD levels and bone loss, but we found that the levels of 25(OH)D and the fecal metabolite l-phenylalanine were positively correlated. These findings appear to be inconsistent, but much of the relevant existing literature is based on rodent studies, a small number of specimens or various diseases. However, the relationship among l-phenylalanine, l-homocysteine and VD metabolism in postmenopausal women is a valuable research direction.
Epidemiological evidence has indicated a relationship between VD and depression with anxiety [68, 69]. It has been reported that l-pyroglutamic acid possesses anxiolytic activity [70]. l-Pyroglutamic acid showed a positive correlation with 25(OH)D levels in our study. VD supplementation can improve anxiety symptoms in depressive patients with low VD levels after 6 months of intervention [71]. Vitamin D3 (5.0 mg/kg) administration seems to attenuate the anxiety-like profile in long-term ovariectomized adult rats subjected to chronic unpredictable mild stress [72]. It is suggested that VD could potentially be used as a therapeutic agent to prevent anxiety in postmenopausal women, and the mechanism of action of l-pyroglutamic acid requires further exploration.
There are several limitations to this study. First, taxonomic classifications of intestinal bacteria were analyzed based on 16S rRNA amplicon profiling rather than comprehensive shotgun metagenomic sequencing data. Second, it was a cross-sectional study. We only analyzed the correlation between 25(OH)D levels and gut microbiota, and identification of a causal relationship between multiple factors was not possible. Third, all included subjects were recruited from **amen, a central city along the southeastern coast of China. There is a single source of subjects, and the topography, climatic characteristics, and dietary patterns are similar. The results of this analysis may only reflect the situation of postmenopausal women in this region, which is not representative. In the future, a multicenter study incorporating a larger sample size could be conducted to validate this result. In addition, more in-depth exploration studies are necessary to understand the underlying mechanism of the relationship between the gut microbiome and VD metabolism in postmenopausal women.
Conclusion
In summary, we identified the disordered profiles of the gut microbiota and fecal metabolomes in postmenopausal women with high or low serum 25(OH)D levels. The results were consistent in identifying associations between 25(OH)D levels and distinguishing bacteria and metabolites, and the relationships among them and the bone metabolic index were discussed. These differences provide strong evidence that specific genera within the gut represent a link between intestinal bacteria and serum 25(OH)D levels and could subsequently affect the health of postmenopausal women.
Availability of data and materials
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request. All sequence data is available from NCBIs Sequence Read Archive (SRA), BioProject ID: PRJNA814014.
References
Liao EY, Zhang ZL, **a WB, Lin H, Cheng Q, Wang L, Hao YQ, Chen DC, Tang H, Peng YD, et al. Calcifediol (25-hydroxyvitamin D) improvement and calcium-phosphate metabolism of alendronate sodium/vitamin D(3) combination in Chinese women with postmenopausal osteoporosis: a post hoc efficacy analysis and safety reappraisal. BMC Musculoskelet Disord. 2018;19:210.
Compston JE, McClung MR, Leslie WD. Osteoporosis. Lancet. 2019;393:364–76.
Cotts KG, Cifu AS. Treatment of osteoporosis. JAMA. 2018;319:1040–1.
Smith KW, Flinders B, Thompson PD, Cruickshank FL, Mackay CL, Heeren RMA, Cobice DF. Spatial localization of vitamin D metabolites in mouse kidney by mass spectrometry imaging. ACS Omega. 2020;5:13430–7.
Kim DH, Meza CA, Clarke H, Kim JS, Hickner RC. Vitamin D and endothelial function. Nutrients. 2020. https://doi.org/10.3390/nu12020575.
Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer. 2014;14:342–57.
Abdella NA, Mojiminiyi OA. Vitamin D-binding protein clearance ratio is significantly associated with glycemic status and diabetes complications in a predominantly vitamin D-deficient population. J Diabetes Res. 2018;2018:6239158.
Holick MF. Vitamin D: a D-lightful health perspective. Nutr Rev. 2008;66:S182-194.
Lips P, Hosking D, Lippuner K, Norquist JM, Wehren L, Maalouf G, Ragi-Eis S, Chandler J. The prevalence of vitamin D inadequacy amongst women with osteoporosis: an international epidemiological investigation. J Intern Med. 2006;260:245–54.
Shin JS, Choi MY, Longtine MS, Nelson DM. Vitamin D effects on pregnancy and the placenta. Placenta. 2010;31:1027–34.
Lyu H, Wang S, ** Y, Shen R, Chen J, Zhu C, Chen B, Wu C. Simultaneous determination of VD2, VD3, 25(OH) D2, and 25(OH) D3 in human plasma using electrospray LC-MS/MS as well as its application to evaluate VD plasma levels in depressive, schizophrenic patients and healthy individuals. Biomed Chromatogr. 2020;34: e4932.
Malabanan A, Veronikis IE, Holick MF. Redefining vitamin D insufficiency. Lancet. 1998;351:805–6.
Aloia JF, Patel M, DiMaano R, Li-Ng M, Talwar SA, Mikhail M, Pollack S, Yeh JK. Vitamin D intake to attain a desired serum 25-hydroxyvitamin D concentration. Am J Clin Nutr. 2008;87:1952–8.
Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77:204–10.
Talwar SA, Aloia JF, Pollack S, Yeh JK. Dose response to vitamin D supplementation among postmenopausal African American women. Am J Clin Nutr. 2007;86:1657–62.
Carlberg C, Haq A. The concept of the personal vitamin D response index. J Steroid Biochem Mol Biol. 2018;175:12–7.
Singh P, Rawat A, Alwakeel M, Sharif E, Al Khodor S. The potential role of vitamin D supplementation as a gut microbiota modifier in healthy individuals. Sci Rep. 2020;10:21641.
Lynch SV, Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med. 2016;375:2369–79.
Parker A, Fonseca S, Carding SR. Gut microbes and metabolites as modulators of blood-brain barrier integrity and brain health. Gut Microbes. 2020;11:135–57.
Yamamoto EA, Jørgensen TN. Relationships between vitamin D, gut microbiome, and systemic autoimmunity. Front Immunol. 2019;10:3141.
Bashir M, Prietl B, Tauschmann M, Mautner SI, Kump PK, Treiber G, Wurm P, Gorkiewicz G, Högenauer C, Pieber TR. Effects of high doses of vitamin D3 on mucosa-associated gut microbiome vary between regions of the human gastrointestinal tract. Eur J Nutr. 2016;55:1479–89.
Shang M, Sun J. Vitamin D/VDR, probiotics, and gastrointestinal diseases. Curr Med Chem. 2017;24:876–87.
Xu X, Jia X, Mo L, Liu C, Zheng L, Yuan Q, Zhou X. Intestinal microbiota: a potential target for the treatment of postmenopausal osteoporosis. Bone Res. 2017;5:17046.
Yang L, Hou K, Zhang B, Ouyang C, Lin A, Xu S, Ke D, Fang L, Chen Q, Wu J, et al. Preservation of the fecal samples at ambient temperature for microbiota analysis with a cost-effective and reliable stabilizer EffcGut. Sci Total Environ. 2020;741: 140423.
Magoč T, Salzberg SL. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27:2957–63.
Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011;17:3.
Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods. 2013;10:996–8.
Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73:5261–7.
Chambers MC, Maclean B, Burke R, Amodei D, Ruderman DL, Neumann S, Gatto L, Fischer B, Pratt B, Egertson J, et al. A cross-platform toolkit for mass spectrometry and proteomics. Nat Biotechnol. 2012;30:918–20.
Kuhl C, Tautenhahn R, Boettcher C, Larson TR. Neumann SJAC: CAMERA: an integrated strategy for compound spectra extraction and annotation of liquid chromatography/mass spectrometry data sets. Anal Chem. 2012;84:283–9.
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, OHara RB, Simpson GL, Solymos P, et al. vegan: Community Ecology Package. 2019.
Paulson JN, Stine OC, Bravo HC, Pop M. Differential abundance analysis for microbial marker-gene surveys. Nat Methods. 2013;10:1200–2.
Langille MGI, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, Clemente JC, Burkepile DE, Vega Thurber RL, Knight R, et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol. 2013;31:814–21.
Core R, Rdct R, Team R, Team RJC. A language and environment for statistical computing. 2015;1:12–21.
Wickham H, Chang W, Abstracts RJBo. ggplot2: create elegant data visualisations using the grammar of graphics. 2016.
Gao C, Qiao J, Li SS, Yu WJ, He JW, Fu WZ, Zhang ZL. The levels of bone turnover markers 25(OH)D and PTH and their relationship with bone mineral density in postmenopausal women in a suburban district in China. Osteoporos Int. 2017;28:211–8.
Fan J, Li N, Gong X, He L. Serum 25-hydroxyvitamin D, bone turnover markers and bone mineral density in postmenopausal women with hip fractures. Clin Chim Acta. 2018;477:135–40.
Shi Z, ** H, Ding Q, Ying J, Wang P-E, Tong P, He B. Bone turnover markers may predict the progression of osteonecrosis of the femoral head in aged males. Ann Transl Med. 2019;7:626–626.
Ma R, Wu M, Li Y, Wang J, Yang P, Chen Y, Wang W, Song J, Wang K. The use of bone turnover markers for monitoring the treatment of osteoporosis in postmenopausal females undergoing total knee arthroplasty: a prospective randomized study. J Orthop Surg Res. 2021;16:195–195.
Zhao H, Chen J, Li X, Sun Q, Qin P, Wang Q. Compositional and functional features of the female premenopausal and postmenopausal gut microbiota. FEBS Lett. 2019;593:2655–64.
Naderpoor N, Mousa A, Fernanda Gomez Arango L, Barrett HL, Dekker Nitert M, de Courten B. Effect of vitamin D supplementation on faecal microbiota: a randomised clinical trial. Nutrients. 2019. https://doi.org/10.3390/nu11122888.
Thomas RL, Jiang L, Adams JS, Xu ZZ, Shen J, Janssen S, Ackermann G, Vanderschueren D, Pauwels S, Knight R, et al. Vitamin D metabolites and the gut microbiome in older men. Nat Commun. 2020;11:5997.
Ooi JH, Li Y, Rogers CJ, Cantorna MT. Vitamin D regulates the gut microbiome and protects mice from dextran sodium sulfate-induced colitis. J Nutr. 2013;143:1679–86.
Gubatan J, Chou ND, Nielsen OH, Moss AC. Systematic review with meta-analysis: association of vitamin D status with clinical outcomes in adult patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2019;50:1146–58.
Van Hul M, Le Roy T, Prifti E, Dao MC, Paquot A, Zucker J-D, Delzenne NM, Muccioli G, Clément K, Cani PD. From correlation to causality: the case of Subdoligranulum. Gut Microb. 2020. https://doi.org/10.1080/19490976.2020.1849998.
D’Amato A, Di Cesare ML, Lucarini E, Man AL, Le Gall G, Branca JJV, Ghelardini C, Amedei A, Bertelli E, Regoli M, et al. Faecal microbiota transplant from aged donor mice affects spatial learning and memory via modulating hippocampal synaptic plasticity- and neurotransmission-related proteins in young recipients. Microbiome. 2020;8:140.
Kang Y, Feng D, Law HK-W, Qu W, Wu Y, Zhu G-H, Huang W-Y. Compositional alterations of gut microbiota in children with primary nephrotic syndrome after initial therapy. BMC Nephrol. 2019;20:434.
Sivaprakasam S, Prasad PD, Singh N. Benefits of short-chain fatty acids and their receptors in inflammation and carcinogenesis. Pharmacol Ther. 2016;164:144–51.
Schäffler H, Herlemann DP, Klinitzke P, Berlin P, Kreikemeyer B, Jaster R, Lamprecht G. Vitamin D administration leads to a shift of the intestinal bacterial composition in Crohn’s disease patients, but not in healthy controls. J Dig Dis. 2018;19:225–34.
Zuo K, Li J, Xu Q, Hu C, Gao Y, Chen M, Hu R, Liu Y, Chi H, Yin Q, et al. Dysbiotic gut microbes may contribute to hypertension by limiting vitamin D production. Clin Cardiol. 2019;42:710–9.
Stacchiotti V, Rezzi S, Eggersdorfer M, Galli F. Metabolic and functional interplay between gut microbiota and fat-soluble vitamins. Crit Rev Food Sci Nutr. 2021;61:3211–32.
Wu S, Yoon S, Zhang Y-G, Lu R, **a Y, Wan J, Petrof EO, Claud EC, Chen D, Sun J. Vitamin D receptor pathway is required for probiotic protection in colitis. Am J Physiol Gastrointest Liver Physiol. 2015;309:G341–9.
Chen YC, Greenbaum J, Shen H, Deng HW. Association between gut microbiota and bone health: potential mechanisms and prospective. J Clin Endocrinol Metab. 2017;102:3635–46.
Yang LC, Wu JB, Lu TJ, Lin WC. The prebiotic effect of Anoectochilus formosanus and its consequences on bone health. Br J Nutr. 2013;109:1779–88.
Palmer MF, Rolls BA. The absorption and secretion of calcium in the gastrointestinal tract of germ-free and conventional chicks. Br J Nutr. 1981;46:549–58.
Szaleniec M, Wojtkiewicz AM, Bernhardt R, Borowski T, Donova M. Bacterial steroid hydroxylases: enzyme classes, their functions and comparison of their catalytic mechanisms. Appl Microbiol Biotechnol. 2018;102:8153–71.
Rizzoli R, Biver E. Are probiotics the new calcium and vitamin D for bone health? Curr Osteoporos Rep. 2020;18:273–84.
Cao S, Dong XL, Ho MX, Yu WX, Wong KC, Yao XS, Wong MS. Oleanolic acid exerts osteoprotective effects and modulates vitamin D metabolism. Nutrients. 2018. https://doi.org/10.3390/nu10020247.
Xu Y, Chen S, Yu T, Qiao J, Sun G. High-throughput metabolomics investigates anti-osteoporosis activity of oleanolic acid via regulating metabolic networks using ultra-performance liquid chromatography coupled with mass spectrometry. Phytomedicine. 2018;51:68–76.
Rodríguez V, Rivoira M, Marchionatti A, Pérez A, de Talamoni NT. Ursodeoxycholic and deoxycholic acids: a good and a bad bile acid for intestinal calcium absorption. Arch Biochem Biophys. 2013;540:19–25.
Ishizawa M, Akagi D, Makishima M. Lithocholic acid is a vitamin D receptor ligand that acts preferentially in the ileum. Int J Mol Sci. 1975;2018:19.
Amer M, Qayyum R. The relationship between 25-hydroxyvitamin D and homocysteine in asymptomatic adults. J Clin Endocrinol Metab. 2014;99:633–8.
Tariq S, Tariq S, Lone KP. Interplay of vitamin D, vitamin B12, homocysteine and bone mineral density in postmenopausal females. Health Care Women Int. 2018;39:1340–9.
Kriebitzsch C, Verlinden L, Eelen G, van Schoor NM, Swart K, Lips P, Meyer MB, Pike JW, Boonen S, Carlberg C, et al. 1,25-dihydroxyvitamin D3 influences cellular homocysteine levels in murine preosteoblastic MC3T3-E1 cells by direct regulation of cystathionine β-synthase. J Bone Miner Res. 2011;26:2991–3000.
Ling C-W, Miao Z, **ao M-L, Zhou H, Jiang Z, Fu Y, **ong F, Zuo L-S-Y, Liu Y-P, Wu Y-Y, et al. The association of gut microbiota with osteoporosis is mediated by amino acid metabolism: multiomics in a large cohort. J Clin Endocrinol Metab. 2021;106:e3852–64.
Dobrowolski SF, Tourkova IL, Robinson LJ, Secunda C, Spridik K, Blair HC. A bone mineralization defect in the Pah(enu2) model of classical phenylketonuria involves compromised mesenchymal stem cell differentiation. Mol Genet Metab. 2018;125:193–9.
Okano Y, Nagasaka H. Optimal serum phenylalanine for adult patients with phenylketonuria. Mol Genet Metab. 2013;110:424–30.
Bertone-Johnson ER. Vitamin D and the occurrence of depression: causal association or circumstantial evidence? Nutr Rev. 2009;67:481–92.
de Koning EJ, Lips P, Penninx BWJH, Elders PJM, Heijboer AC, den Heijer M, Bet PM, van Marwijk HWJ, van Schoor NM. Vitamin D supplementation for the prevention of depression and poor physical function in older persons: the D-Vitaal study, a randomized clinical trial. Am J Clin Nutr. 2019;110:1119–30.
Beni M, Pellegrini-Giampietro DE, Moroni F. A new endogenous anxiolytic agent: l-pyroglutamic acid. Fundam Clin Pharmacol. 1988;2:77–82.
Zhu C, Zhang Y, Wang T, Lin Y, Yu J, **a Q, Zhu P, Zhu DM. Vitamin D supplementation improves anxiety but not depression symptoms in patients with vitamin D deficiency. Brain Behav. 2020;10: e01760.
Baranenko D, Fedotova J, Tol AJM. Vitamin D3 attenuates anxiety-like behavior in long-term ovariectomized rats with unpredictable mild stress. Biocell. 2019;43:299–311.
Acknowledgements
We are grateful to all the participants and investigators for their continued dedication to this study. We also thank all members of Treatgut Biotechnology Co., Ltd. (**amen, China) for participating in statistical and bioinformatics analysis about the manuscript. We would like to thank AJE (www.aje.com) for English language editing.
Funding
This study was funded by National Natural Science Foundation of China (82004433), Science Foundation of Fujian Province in China (2021J011327), Science Foundation of **amen City in China (3502Z20199022) and Key Clinical Specialty Discipline Construction Program of Fujian, P.R.C.
Author information
Authors and Affiliations
Contributions
Conception and design of the study: JQH, CXX, JC and JHG. Collection of data: JQH, LNH, QYZ, JHG, RMX and YYZ. Analysis and interpretation of data: BLC, MC, WXG, XJL, LL and YYZ. Drafting of the manuscript: JHG, LNH, QYZ, JQH, YYZ, MYG, GWW and BZZ. Critical revision of the manuscript for important intellectual content: JHG, JC and JQH. Administrative support and study supervision: CXX, JQH and JC. All authors revised the final draft. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Rarefaction curves in observed_species of all the samples. The rarefaction curves constructed from the sequenced data has been basically stable, indicating that the sequenced data has benn basically stable at this sequencing depth.
Additional file 2: Figure S2.
The taxonomic representation of statistically and biologically differences between High 25(OH)D group and Low 25(OH)D group. The color of discriminative taxa represents the taxa is more abundant in the corresponding group (High 25(OH)D group in blue, Low 25(OH)D group in orange).
Additional file 3: Figure S3.
Functional PICRUSt analysis among the LVD and HVD group. Kyoto Encyclopedia of Genes(A) and Genomes and cluster of orthologous group(B) was used to further investigate the mechanism of intestinal flora.
Additional file 4: Figure S4.
Heat map for spearman correlation analysis between fecal metabolites and discriminative genera at the family level. Only significant values (p < 0.05 after FDR adjustment) are shown. Orange and blue colors represent significant positive correlations and negative correlations. Darker color represents stronger correlations.
Additional file 5: Table S1.
Characteristics and clinical and biochemical indexes between HVD group (n=44) and LVD group (n=44).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Gong, J., He, L., Zou, Q. et al. Association of serum 25-hydroxyvitamin D (25(OH)D) levels with the gut microbiota and metabolites in postmenopausal women in China. Microb Cell Fact 21, 137 (2022). https://doi.org/10.1186/s12934-022-01858-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12934-022-01858-6