Abstract
Background
Although studies have demonstrated the value of the triglyceride–glucose (TyG) index for cardiovascular disease (CVD) and cardiovascular mortality, however, few studies have shown that the TyG index is associated with all-cause or CVD mortality in young patients with diabetes. This study aimed to investigate the association between the TyG index and all-cause and CVD mortality in young patients with diabetes in the United States.
Methods
Our study recruited 2440 young patients with diabetes from the National Health and Nutrition Examination Survey (NHANES) 2001–2018. Mortality outcomes were determined by linking to National Death Index (NDI) records up to December 31, 2019. Cox regression modeling was used to investigate the association between TyG index and mortality in young patients with diabetes. The nonlinear association between TyG index and mortality was analyzed using restricted cubic splines (RCS), and a two-segment Cox proportional risk model was constructed for both sides of the inflection point.
Results
During a median follow-up period of 8.2 years, 332 deaths from all causes and 82 deaths from cardiovascular disease were observed. Based on the RCS, the TyG index was found to have a U-shaped association with all-cause and CVD mortality in young patients with diabetes, with threshold values of 9.18 and 9.16, respectively. When the TyG index was below the threshold value (TyG index < 9.18 in all-cause mortality and < 9.16 in CVD mortality), its association with all-cause and CVD mortality was not significant. When the TyG index was above the threshold (TyG index ≥ 9.18 in all-cause mortality and ≥ 9.16 in CVD mortality), it showed a significant positive association with all-cause mortality and CVD mortality (HR 1.77, 95% CI 1.05–2.96 for all-cause mortality and HR 2.38, 95% CI 1.05–5.38 for CVD mortality).
Conclusion
Our results suggest a U-shaped association between TyG index and all-cause and CVD mortality among young patients with diabetes in the United States, with threshold values of 9.18 and 9.16 for CVD and all-cause mortality, respectively.
Similar content being viewed by others
Introduction
The incidence and mortality rates of cardiovascular diseases (CVDs) are on a steady rise, presenting a significant threat to both the global economy and human health. This surge in CVDs has emerged as a major public health challenge of global significance [1, 2]. Recent reports indicate a staggering increase in the prevalence of cardiovascular diseases worldwide, with cases soaring from 271 to 523 million over the past three decades [3]. Additionally, the number of cardiovascular-related deaths has surged by 6.5 million cases during this period [3]. Consequently, early identification of individuals with high cardiovascular risk factors is imperative to mitigate the prevalence and mortality rates associated with these conditions.
Insulin resistance is a state of pathophysiological impairment characterized by reduced sensitivity and response of the body to insulin, which ultimately leads to hyperglycemia [4, 5]. The hyperinsulin–hyperglycemic clamp is the gold standard for the assessment of IR, and its complexity leads to limited value in clinical application [6]. The homeostasis model assessment of insulin resistance (HOMA-IR), which uses fasting glucose and insulin levels indirectly to assess the degree of IR, is also limited in its widespread clinical use because of the high insulin measurement requirements and poor reproducibility [7]. In recent years, the triglyceride–glucose product index (TyG index), calculated from triglyceride and fasting glucose levels, has emerged as a potentially simple, convenient, and cost-effective alternative for detecting IR [8, 9]. Notably, the TyG index demonstrates superior predictability of IR compared to HOMA-IR, with an area under the curve (AUC) of 0.784 for the TyG index versus 0.728 for HOMA-IR [10]. The TyG index has demonstrated its capability to predict adverse clinical outcomes in patients with cardiovascular disease (CVD) [11,12,14], experiencing premature stroke [15], and encountering acute decompensated heart failure [16]. Notably, the prevalence of CVD among individuals with type 2 diabetes can be as high as 32.2% [17]. Given its role as an indicator of insulin resistance, the TyG index exhibits a close association with type 2 diabetes as well [18].
Given the limited scope of existing studies on the association between the TyG index and all-cause mortality and CVD mortality in young patients with diabetes, further validation is warranted. Therefore, this study aimed to provide epidemiologic evidence to demonstrate that the TyG index is an important marker of all-cause mortality and CVD mortality in young patients with diabetes.
Materials and methods
National Health and Nutrition Examination Survey (NHANES), a continuous survey, employs complex multistage probability sampling to select a representative group of Americans, assessing the health and nutritional status of both adults and children. The Centers for Disease Control and Prevention (CDC) is tasked with providing health statistics for the nation. The NHANES study plan received approval from the Ethics Review Committee of the National Center for Health Statistics (NCHS). All study participants provided written informed consent. The datasets generated and analyzed in this study are readily accessible on the website (https://www.cdc.gov/nchs/nhanes/index.html).
Study population
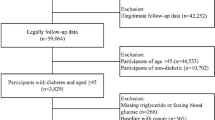
The data analyzed in this study spanned from the 2001 to 2018 NHANES database. Initially, our study enrolled 91,351 participants. Following the exclusion of individuals over 65 years of age and under 20 years of old (N = 53,039), and those with missing data on the TyG index (N = 21,714) and the follow-up data (N = 1), and those without diabetes (N = 14,157), the final analysis included 2440 participants (Fig. 1).
Assessment of TyG index
TyG served as the chosen exposure variable, and we calculated the TyG index using the formula Ln [triglycerides (mg/dl) * fasting glucose (mg/dl)/2]. The concentrations of triglycerides and fasting glucose underwent determination through an enzymatic assay performed on an automatic biochemistry analyzer. Specifically, the Roche Modular P and Roche Cobas 6000 chemistry analyzers were employed to measure serum triglyceride concentration. Additionally, fasting plasma glucose levels were evaluated through the hexokinase-mediated reaction, utilizing the Roche/Hitachi Cobas C 501 chemistry analyzer.
Assessment of mortality
To determine the mortality status in the follow-up population, we utilized the NHANES public-use linked mortality file, updated as of December 31, 2019. This file underwent linkage with the National Death Index (NDI) by the NCHS using a probability matching algorithm. Additionally, disease-specific deaths were identified using the International Statistical Classification of Diseases, 10th Revision (ICD-10). Cardiovascular deaths (including rheumatic heart disease, hypertensive heart disease, ischemic heart disease, acute myocardial infarction, pericardial disease, and acute myocarditis and heart failure) correspond to disease codes I00–I09, I11, I13, and I20–I51.
Assessment of covariates
Data on diverse demographic and health-related factors were collected through NHANES household interviews. This comprehensive information included details such as age, gender (female/male), race/ethnicity (Mexican American/Non-Hispanic Black/Non-Hispanic White/Others), education levels (less than 9th grade/9–11th grade/high school graduate/some college or AA degree/college graduate or above), the ratio of family income to poverty (< 1/1–4/ > 4), alcohol use (yes/no), smoking status (never/former/now), and disease status (diabetes/hypertension). Diabetes (DM) was defined as either treatment or medical diagnosis of hyperglycemia with hemoglobin A1c ≥ 6.5%, fasting blood glucose ≥ 126 mg/dl, or a 2-h blood glucose ≥ 200 mg/dl [19]. Hypertension was defined as the use of antihypertensive medications, a medical diagnosis of hypertension, or three consecutive measurements of systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg [20]. BMI was computed by dividing weight (in kilograms) by the square of height (in meters). Participants were classified as normal weight (< 25 kg/m2), overweight (25–29.9 kg/m2), or obese (≥ 30 kg/m2) based on their BMI. Total cholesterol (TC), triglyceride (TG), fasting glucose, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and glycosylated hemoglobin (HbA1c) were measured in the laboratory.
Statistical analysis
Statistical analyses adhered to the guidelines outlined by the CDC. Considering the intricate probability sample design and deliberate oversampling of specific populations in NHANES to ensure representativeness, sample weights were applied to amalgamate data from multiple survey cycles. Participants in the study were categorized into four groups based on quartiles (Q1–Q4) of the TyG index. Continuous variables were expressed as means ± standard deviations, while categorical variables were represented as percentages and corresponding 95% confidence intervals (CI). The weighted one-way ANOVA was employed for continuous variables, and the weighted chi-square test was utilized for categorical variables to evaluate differences in the descriptive analyses. To evaluate the hazard ratios (HR) and 95% CI for the association between the TyG index and the risk of all-cause mortality and CVD mortality, multivariate Cox proportional hazards regression models were developed. There were three models to control for confounding factors. Model 1 was unadjusted, Model 2 was adjusted for age, gender, and race. Model 3 was adjusted for age, gender, race, educational levels, PIR, BMI, hypertension, HDL-C, LDL-C, alcohol use, and smoking status. Multiple imputation was performed for covariates with missing values. The dose–response association between the TyG index and mortality was explored using restricted cubic spline (RCS) analysis. If the association exhibited nonlinearity, the threshold value was estimated by trying all possible values, choosing the threshold point with the highest likelihood. Subsequently, a two-piecewise Cox proportional risk model was employed on both sides of the inflection point to investigate the association between the TyG index and the risk of all-cause mortality and CVD mortality. For subgroup analysis of the association between the TyG index and the risk of all-cause mortality and CVD mortality, the data were stratified by gender (male/female), BMI (normal weight/overweight/obesity), hypertension (yes/no), smoking status (never/former/now) and alcohol use (yes/no). These stratified factors were also considered as potential effect modifiers. A significance level of two-tailed P < 0.05 was used to indicate statistical significance. All analyses were conducted using R version 4.3.2 (http://www.R-project.org, The R Foundation).
Results
Baseline characteristics of study participants
Table 1 presented the baseline characteristics of the study participants, stratified by quartiles of the TyG index. In total, 2440 participants were enrolled in our study, with an average age of 51.14 ± 0.28 years. Among them, 47.08% were female, and 52.92% were male. In addition, among these participants, 8.80% had no high school or higher education, 15.49% had poor household income, 63.13% were obese, 62.86% had hypertension, 72.54% were alcohol users, and 23.44% were current smokers. The average TyG index in the recruited subjects was 9.27 ± 0.02, and the TyG index range for quartiles 1–4 were 5.64–8.75, 8.75–9.18, 9.18–9.72, and 9.72–13.40, respectively. Statistically significant differences were in total cholesterol, triglycerides, fasting glucose, HDL-C, LDL-C, HbA1c%, BMI, alcohol consumption, and hypertension across the TyG index quartiles (all P < 0.05). Compared with the lowest TyG index quartile, individuals with increased TyG index group were more likely to have hypertension, elevated levels of total cholesterol, triglycerides, fasting glucose, LDL-C, HbA1c%, and BMI. Additionally, they exhibited a greater likelihood of being Mexican American and non-alcohol users. There was no statistically significant difference among quartiles in age, gender, educational levels, PIR, and smoking status (all P > 0.05).
Association of TyG index with all-cause mortality and CVD mortality
Table 2 illustrates the incidence of 332 all-cause deaths and 88 CVD-related deaths during a follow-up period of 8.2 years. Three Cox regression models were formulated to explore the independent association between the TyG index and all-cause mortality and CVD mortality risk. In Model 1, the TyG index was significantly positive with the risk of all-cause mortality (HR 1.28, 95% CI 1.08–1.42). This positive association persisted in the minimally adjusted model (HR 1.28, 95% CI 1.10–1.50). After full adjustment, the TyG index remained positively linked to the risk of all-cause mortality (HR 1.43, 95% CI 1.12–1.83). Similar results were shown when we categorized participants into quartiles by the TyG index. In Model 1 and Model 2, compared with those in the lowest quartile of the TyG index, individuals in the highest TyG index quartile had a higher risk of all-cause mortality (Model 1: 1.46, 95% CI1.02–2.09; Model 2: 1.53, 95% CI 1.05–2.24). In the fully adjusted Model 3, the HRs (95% CI) for all-cause mortality risk for quartile 2, quartile 3, and quartile 4 of the TyG index were 0.62 (0.35–1.11), 1.10 (0.67–1.81), 1.60 (1.01–2.54), respectively.
We observed a positive association between a higher TyG index and an elevated risk of CVD mortality. This association was statistically significant in both Model 1 (HR 1.19, 95% CI 1.01–1.39) and Model 2 (HR 1.35, 95% CI 1.05–1.74). Furthermore, this positive association between the TyG index and the risk of CVD mortality remained robust in Model 3 (HR 1.61, 95% CI 1.08–3.05). When the TyG index was categorized into quartiles, the multivariable-adjusted HRs (95% CI) from the lowest quartile to the highest quartile of the TyG index for CVD mortality was 1.00 (reference), 0.45 (0.16–1.23), 1.06 (1.03–1.09), 1.69 (1.22–4.27) respectively.
RCS analysis
In our multivariate Cox regression analysis, we identified a possible nonlinear association between the TyG index and all-cause mortality and CVD mortality. To further validate the association between the TyG index and the risk of all-cause mortality and CVD mortality, we employed RCS analysis. Our results demonstrated a U-shaped association between the TyG index and all-cause mortality and CVD mortality (Figs. 2 and 3). In addition to this, we further fitted the association between baseline TyG index and mortality using two segmented Cox proportional hazards regression models. Remarkably, we identified threshold values of 9.18 for all-cause mortality and 9.16 for CVD mortality (Table 3). Notably, when the TyG index was ≥ 9.18, we observed that a 1-unit increase in the TyG index was associated with a 77% increase in the risk of all-cause mortality (HR 1.77, 95% CI 1.05–2.96). When the TyG index was < 9.18, the risk of all-cause mortality was not significantly associated with changes in the TyG index. The risk of CVD mortality decreased at the lowest level when each unit of the TyG index increased to a critical value. And when the TyG index was ≥ 9.16, it showed a significant positive association with the risk of CVD mortality (HR 2.38, 95% CI 1.05–5.38).
Subgroup analysis
To further validate the high TyG index group (≥ 9.16 or 9.18) and the low TyG index group (< 9.16 or 9.18), we performed subgroup analyses (Tables 4 and 5). The analyses were stratified by gender, BMI, hypertension, smoking, and drinking status. Our results revealed a positive association between the TyG index and the risk of all-cause mortality among males (HR 2.19, 95% CI 1.14–3.83), individuals classified as overweight (HR 1.67, 95% CI 1.67–10.18), those with hypertension (HR 2.11, 95% CI 1.25–3.53), current smokers (HR 3.01, 95% CI 1.41–6.43), and alcohol consumers (HR 2.63, 95% CI 1.52–4.54) when the TyG index exceeded the threshold of 9.18. Notably, no significant interactions were observed between the TyG index and the stratification variables.
In contrast, when the TyG index was above the threshold of 9.16, we found a significant increase in CVD mortality among men (HR 2.54, 95% CI 1.14–5.64), hypertensive patients (HR 2.55, 95% CI 1.07–6.09), current smokers (HR 2.85, 95% CI 1.01–8.13), and nondrinkers (HR 3.69, 95% CI 1.23–11.01). Additionally, interaction tests indicated no significant differences among the various strata, illustrating the lack of a significant effect of the stratification variables on this positive association (all P for interaction > 0.05).
Discussion
In this current study involving 2440 young US patients with diabetes, we observed a U-shaped association between TyG index and all-cause and CVD mortality. In addition to this, threshold effect analysis revealed the inflection point of the TyG index with all-cause mortality (9.18) and CVD mortality (9.16). The results of subgroup analyses revealed no significant interactions observed between the TyG index and stratification variables. In conclusion, our study suggests that the TyG index is a valuable marker of risk of all-cause mortality and CVD mortality in young patients with diabetes, which may help to advance CVD prevention measures.
The TyG index has clear advantages over other indices as a valuable index for assessing the potential for IR [8]. Its association with atherosclerosis and coronary artery calcification underscores its close association with CVD [21, 22]. Previous studies have demonstrated the association between TyG index and the incidence and mortality of CVD disease in different populations. For instance, a study revealed a notable connection between elevated TyG indices and heightened incidences of coronary artery disease and myocardial infarction within the general population [12]. In patients with stable cardiovascular disease, there was a positive association between TyG index and the occurrence of future adverse cardiovascular events such as nonfatal myocardial infarction, stroke, and postdischarge revascularization [23]. Wu et al. identified the TyG index as a predictive factor for major adverse cardiovascular events among patients with premature coronary artery disease [24]. Moreover, an increased TyG index correlates with a higher risk of cardiovascular mortality among individuals deemed at elevated risk of CVD [13]. TyG index can also be a simple and convenient marker for early identification of future CVD disease in postmenopausal women [25]. In our study, we have identified a nonlinear association between the TyG index and the risk of CVD mortality. Specifically, we observed a positive association with CVD mortality when the TyG index exceeded 9.16. Similar to our findings, one study found a positive association between TyG index and CVD mortality when it exceeded 9.52 [26]. Zhao et al. also found a positive association with CVD mortality when TyG index exceeded 9 [27]. Furthermore, it is noteworthy that certain studies have indicated a reduced risk of CVD mortality when the baseline TyG index falls below a specific threshold [28]. In addition to this, studies have also found an increased incidence of diabetic nephropathy with either a TyG index below or above the threshold [29]. Low triglyceride levels have been identified as an adverse prognostic indicator for cardiovascular mortality in individuals with chronic heart failure [30]. Similarly, in patients experiencing stroke unrelated to cardiac embolism, decreased serum triglyceride levels emerged as an independent predictor of mortality following ischemic stroke [31]. Our results also found an association with an increased risk of all-cause mortality when the TyG index was greater than 9.18. This is consistent with the results of several previous studies. Sun et al. found that an elevated TyG index was associated with an increased risk of all-cause mortality in American men over 45 years of age [32]. Shen et al. also found that a higher TyG index was associated with an increased risk of all-cause mortality in diabetic patients with acute coronary syndromes [33]. There are also some studies that are not consistent with our results. Lopez-Jaramillo et al. did not find a significant association between TyG index and non-cardiovascular mortality and all-cause mortality [34]. No association between TyG index and all-cause mortality was also reported by Liu et al. [12]. These findings may vary depending on factors such as age, heterogeneity of the patient population, etc. Inconsistencies in the results of the various studies may also be due to differences in geographic location and economic status [35].
The mechanism by which TyG indices are associated with increased mortality is currently unknown, but IR may hold a key position between the association between TyG and mortality. IR is a pathophysiological disorder that manifests itself primarily in the form of decreased insulin sensitivity of muscle and liver tissues, resulting in a reduced bioavailability of insulin, a diminished capacity to uptake glucose, and ultimately hyperglycemia [36]. The hyperglycemic state further induces inflammation, as evidenced by elevated inflammatory cytokines such as tumor necrosis factor alpha (TNF-α), interleukin (IL)-6, and IL-8, the production of which further leads to smooth muscle cell proliferation and collagen deposition, ultimately leading to vascular senescence and vascular sclerosis [37,38,39]. IR also inhibits the expression of adiponectin, which has been reported to increase the risk of coronary artery disease in male patients with hypoadiponectinemia [40]. IR also promotes the production of fibrinogen activator inhibitor-1, which further contributes to thrombosis [41], in addition to elevated expression of adhesion-inducing factor and thromboxane A2-dependent tissue factor in platelets, which further promotes platelet activation [42]. IR-induced systemic dyslipidemia may also contribute to the development of atherosclerosis [43]. IR is also associated with enhanced oxidative stress, and excessive levels of oxidative stress are thought to be a key factor in stimulating smooth muscle cell migration and collagen deposition into damaged endothelial cell sites [44,45,46,47,48]. Inadequate bioavailability of nitric oxide in the endothelium further contributes to impaired endothelial function and greater endothelial cell damage [49, 50].
This study has several strengths. First, the study population for this study was drawn from a nationally representative NHANES population identified by a complex multistage probability sampling methodology and included 2440 young adults with diabetes, a relatively rich sample size. Second, our study evaluated the association between the TyG index for all-cause and CVD mortality in young patients with diabetes, enriching the research in this area. Third, this study used stratified and sensitivity analyses to further ensure the reliability and robustness of the findings.
This study also has some limitations that cannot be ignored. Firstly, our population in this study was predominantly young Americans, which may result in limited extrapolation of findings due to differences in ethnicity and life circumstances, and further validation of the generalizability of our main findings to populations of different ethnicities and broader age ranges is needed. Secondly, although we have adjusted for potential confounders, we have not been able to completely rule out all potential confounding bargains, and we also lacked an assessment of all metabolic indices associated with IR, e.g., due to a lack of fasting insulin levels. Thirdly, with only a singular baseline value for the TyG index, the impact of fluctuations in this index during follow-up on mortality risk remains ambiguous. Last but not least, because the NHANES design data did not clarify type 1 or type 2 diabetes in its study design, we could not further evaluate this association for subgroups stratified by type 1 and type 2 diabetes. Finally, our findings are observational, and the causal role of the TyG index on all-cause mortality and CVD mortality should be validated in further prospective intervention studies. Despite these limitations, our study contributes to the existing knowledge base, and future research with more extensive data collection could further elucidate these relationships.
Conclusion
Our findings suggest that the TyG index is an economical and valuable biomarker of the risk of all-cause mortality and CVD mortality in young patients with diabetes under 65 years of age in the United States. We also found a nonlinear association between the TyG index and all-cause and CVD mortality. Therefore, we advocate for the monitoring of the TyG index in young patients with diabetes as a potentially beneficial approach for assessing clinical prognosis and mortality risk in these patients.
Availability of data and materials
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/nhanes/index.htm.
References
Vaduganathan M, Mensah GA, Turco JV, Fuster V, Roth GA. The global burden of cardiovascular diseases and risk: a compass for future health. J Am Coll Cardiol. 2022;80(25):2361–71.
Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart Disease and Stroke Statistics-2020 update: a report from the American Heart Association. Circulation. 2020;141(9):e139–596.
Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76(25):2982–3021.
Lebovitz HE. Insulin resistance: definition and consequences. Exp Clin Endocrinol Diabetes. 2001;109(Suppl 2):S135-148.
Hill MA, Yang Y, Zhang L, Sun Z, Jia G, Parrish AR, et al. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabolism. 2021;119: 154766.
Wallace TM, Matthews DR. The assessment of insulin resistance in man. Diabet Med. 2002;19(7):527–34.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9.
Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6(4):299–304.
Kheirollahi A, Teimouri M, Karimi M, Vatannejad A, Moradi N, Borumandnia N, et al. Evaluation of lipid ratios and triglyceride–glucose index as risk markers of insulin resistance in Iranian polycystic ovary syndrome women. Lipids Health Dis. 2020;19(1):235.
Park HM, Lee HS, Lee YJ, Lee JH. The triglyceride–glucose index is a more powerful surrogate marker for predicting the prevalence and incidence of type 2 diabetes mellitus than the homeostatic model assessment of insulin resistance. Diabetes Res Clin Pract. 2021;180: 109042.
Rafiee H, Mohammadifard N, Nouri F, Alavi Tabatabaei G, Najafian J, Sadeghi M, et al. Association of triglyceride glucose index with cardiovascular events: insights from the Isfahan Cohort Study (ICS). Eur J Med Res. 2024;29(1):135.
Liu X, Tan Z, Huang Y, Zhao H, Liu M, Yu P, et al. Relationship between the triglyceride–glucose index and risk of cardiovascular diseases and mortality in the general population: a systematic review and meta-analysis. Cardiovasc Diabetol. 2022;21(1):124.
Cai XL, **ang YF, Chen XF, Lin XQ, Lin BT, Zhou GY, et al. Prognostic value of triglyceride glucose index in population at high cardiovascular disease risk. Cardiovasc Diabetol. 2023;22(1):198.
Yu H, Tao L, Li YG, Yang L, Liu D, Wang Y, et al. Association between triglyceride–glucose index trajectories and carotid atherosclerosis progression. Cardiovasc Diabetol. 2023;22(1):130.
Xu W, Zhao H, Han X, Liu J, Li H, Sun J, et al. Relationship between early-onset stroke and triglyceride–glucose index among young Chinese adults. Lipids Health Dis. 2023;22(1):3.
Huang R, Wang Z, Chen J, Bao X, Xu N, Guo S, et al. Prognostic value of triglyceride glucose (TyG) index in patients with acute decompensated heart failure. Cardiovasc Diabetol. 2022;21(1):88.
Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc Diabetol. 2018;17(1):83.
Chamroonkiadtikun P, Ananchaisarp T, Wanichanon W. The triglyceride–glucose index, a predictor of type 2 diabetes development: a retrospective cohort study. Prim Care Diabetes. 2020;14(2):161–7.
Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA. 2015;314(10):1021–9.
Fryar CD, Ostchega Y, Hales CM, Zhang G, Kruszon-Moran D. Hypertension prevalence and control among adults: United States, 2015–2016. NCHS Data Brief. 2017;289:1–8.
Liu F, Ling Q, **e S, Xu Y, Liu M, Hu Q, et al. Association between triglyceride glucose index and arterial stiffness and coronary artery calcification: a systematic review and exposure-effect meta-analysis. Cardiovasc Diabetol. 2023;22(1):111.
Lambrinoudaki I, Kazani MV, Armeni E, Georgiopoulos G, Tampakis K, Rizos D, et al. The TyG index as a marker of subclinical atherosclerosis and arterial stiffness in lean and overweight postmenopausal women. Heart Lung Circ. 2018;27(6):716–24.
** JL, Cao YX, Wu LG, You XD, Guo YL, Wu NQ, et al. Triglyceride glucose index for predicting cardiovascular outcomes in patients with coronary artery disease. J Thorac Dis. 2018;10(11):6137–46.
Wu Z, Liu L, Wang W, Cui H, Zhang Y, Xu J, et al. Triglyceride–glucose index in the prediction of adverse cardiovascular events in patients with premature coronary artery disease: a retrospective cohort study. Cardiovasc Diabetol. 2022;21(1):142.
Liu Q, Si F, Liu Z, Wu Y, Yu J. Association between triglyceride–glucose index and risk of cardiovascular disease among postmenopausal women. Cardiovasc Diabetol. 2023;22(1):21.
Liu XC, He GD, Lo K, Huang YQ, Feng YQ. The triglyceride–glucose index, an insulin resistance marker, was non-linear associated with all-cause and cardiovascular mortality in the general population. Front Cardiovasc Med. 2020;7: 628109.
Zhao M, **ao M, Tan Q, Lu F. Triglyceride glucose index as a predictor of mortality in middle-aged and elderly patients with type 2 diabetes in the US. Sci Rep. 2023;13(1):16478.
Zhang Q, **ao S, Jiao X, Shen Y. The triglyceride–glucose index is a predictor for cardiovascular and all-cause mortality in CVD patients with diabetes or pre-diabetes: evidence from NHANES 2001–2018. Cardiovasc Diabetol. 2023;22(1):279.
Shang J, Yu D, Cai Y, Wang Z, Zhao B, Zhao Z, et al. The triglyceride glucose index can predict newly diagnosed biopsy-proven diabetic nephropathy in type 2 diabetes: a nested case control study. Medicine (Baltimore). 2019;98(46): e17995.
Kozdag G, Ertas G, Emre E, Akay Y, Celikyurt U, Sahin T, et al. Low serum triglyceride levels as predictors of cardiac death in heart failure patients. Tex Heart Inst J. 2013;40(5):521–8.
Ryu WS, Lee SH, Kim CK, Kim BJ, Yoon BW. Effects of low serum triglyceride on stroke mortality: a prospective follow-up study. Atherosclerosis. 2010;212(1):299–304.
Sun M, Guo H, Wang Y, Ma D. Association of triglyceride glucose index with all-cause and cause-specific mortality among middle age and elderly US population. BMC Geriatr. 2022;22(1):461.
Shen J, Feng B, Fan L, Jiao Y, Li Y, Liu H, et al. Triglyceride glucose index predicts all-cause mortality in oldest-old patients with acute coronary syndrome and diabetes mellitus. BMC Geriatr. 2023;23(1):78.
Lopez-Jaramillo P, Gomez-Arbelaez D, Martinez-Bello D, Abat MEM, Alhabib KF, Avezum Á, et al. Association of the triglyceride glucose index as a measure of insulin resistance with mortality and cardiovascular disease in populations from five continents (PURE study): a prospective cohort study. Lancet Healthy Longev. 2023;4(1):e23–33.
Lopez-Jaramillo P, Lahera V, Lopez-Lopez J. Epidemic of cardiometabolic diseases: a Latin American point of view. Ther Adv Cardiovasc Dis. 2011;5(2):119–31.
Brown AE, Walker M. Genetics of insulin resistance and the metabolic syndrome. Curr Cardiol Rep. 2016;18(8):75.
Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuñiga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. 2018;17(1):122.
Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Investig. 2006;116(7):1793–801.
Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Investig. 2003;112(12):1821–30.
Kumada M, Kihara S, Sumitsuji S, Kawamoto T, Matsumoto S, Ouchi N, et al. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol. 2003;23(1):85–9.
Iwaki T, Urano T, Umemura K. PAI-1, progress in understanding the clinical problem and its aetiology. Br J Haematol. 2012;157(3):291–8.
Gerrits AJ, Koekman CA, van Haeften TW, Akkerman JW. Platelet tissue factor synthesis in type 2 diabetic patients is resistant to inhibition by insulin. Diabetes. 2010;59(6):1487–95.
Yang Q, Vijayakumar A, Kahn BB. Metabolites as regulators of insulin sensitivity and metabolism. Nat Rev Mol Cell Biol. 2018;19(10):654–72.
Jia G, DeMarco VG, Sowers JR. Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat Rev Endocrinol. 2016;12(3):144–53.
Leopold JA, Loscalzo J. Oxidative mechanisms and atherothrombotic cardiovascular disease. Drug Discov Today Ther Strateg. 2008;5(1):5–13.
Grant PJ, Cosentino F, Marx N. Diabetes and coronary artery disease: not just a risk factor. Heart. 2020;106(17):1357–64.
Zheng D, Liu J, Piao H, Zhu Z, Wei R, Liu K. ROS-triggered endothelial cell death mechanisms: focus on pyroptosis, parthanatos, and ferroptosis. Front Immunol. 2022;13:1039241.
Shu L, Yuan Z, Li F, Cai Z. Oxidative stress and valvular endothelial cells in aortic valve calcification. Biomed Pharmacother. 2023;163: 114775.
Molina MN, Ferder L, Manucha W. Emerging role of nitric oxide and heat shock proteins in insulin resistance. Curr Hypertens Rep. 2016;18(1):1.
Nishikawa T, Kukidome D, Sonoda K, Fujisawa K, Matsuhisa T, Motoshima H, et al. Impact of mitochondrial ROS production in the pathogenesis of insulin resistance. Diabetes Res Clin Pract. 2007;77(Suppl 1):S161-164.
Acknowledgements
The authors thank the staff and the participants of the NHANES study for their valuable contributions.
Funding
This study was funded by The First batch of key Disciplines on Public Health in Chongqing.
Author information
Authors and Affiliations
Contributions
DL and CL analyzed the data and wrote the primary manuscript. KX and LX reviewed and revised the manuscript. All the authors have approved the manuscript for publication.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The studies involving human participants were reviewed and approved by the NCHS Research Ethics Review Board (ERB). All participants provided written informed consent.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be recognized as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, C., Liang, D., **ao, K. et al. Association between the triglyceride–glucose index and all-cause and CVD mortality in the young population with diabetes. Cardiovasc Diabetol 23, 171 (2024). https://doi.org/10.1186/s12933-024-02269-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-024-02269-0