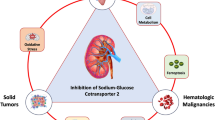
Abstract
Background
Heart failure (HF), chronic kidney disease (CKD), and type 2 diabetes mellitus (T2DM) are common and interrelated conditions, each with a significant burden of disease. HF and kidney disease progress through pathophysiologic pathways that culminate in end-stage disease, for which T2DM is a major risk factor. Intervention within these pathways can disrupt disease processes and improve patient outcomes. Sodium-glucose cotransporter-2 inhibitors (SGLT2is) have been investigated in patient populations with combinations of T2DM, CKD, and/or HF. However, until recently, the effect of these agents in patients with HF with preserved ejection fraction (HFpEF) was not well studied.
Main body
The aim of this review is to summarize key information regarding the interaction between HFpEF, CKD, and T2DM and discuss the role of SGLT2 inhibition in the management of patients with comorbid HFpEF and CKD, with or without T2DM. Literature was retrieved using Boolean searches for English-language articles in PubMed and Google Scholar and included terms related to SGLT2is, HFpEF, T2DM, and CKD. The reference lists from retrieved articles were also considered.
Conclusion
SGLT2is are efficacious and safe in treating HFpEF in patients with comorbid CKD with and without T2DM. The totality of evidence from clinical trials data suggests there are benefits in using SGLT2is across the spectrum of left ventricular ejection fractions, but there may be a potential for different renal effects in the different ejection fraction groups. Further analysis of these clinical trials has highlighted the need to obtain more accurate phenotypes for patients with HF and CKD to better determine which patients might respond to guideline-directed medical therapies, including SGLT2is.
Graphical Abstract
CI confidence interval, EF ejection fraction, eGFR estimated glomerular filtration rate, HF heart failure, HHF hospitalization for HF, HR hazard ratio, LVEF left ventricular ejection fraction, SGLT2i sodium-glucose cotransporter-2 inhibitor, UACR urine albumin-creatinine ratio. a Mean value, unless otherwise stated, b SGLT2i vs. placebo, c Data reanalyzed using more conventional endpoints (≥ 50% sustained decrease in eGFR, and including renal death) (UACR at baseline not stated in trial reports)

Similar content being viewed by others
Introduction
Heart failure (HF), chronic kidney disease (CKD), and type 2 diabetes mellitus (T2DM) are common and interrelated conditions, each conferring increased disease burden both individually and in combination [1]. Cardiovascular disease (CVD), particularly HF, and CKD each progress through a pathway of pathophysiologic steps, for which T2DM is a major risk factor [2]. Sodium-glucose cotransporter-2 inhibitors (SGLT2is) are used to treat hyperglycemia in patients with T2DM. Data from large, phase 3, randomized controlled trials (RCTs) have shown that SGLT2i therapy improved cardiovascular (CV) and kidney outcomes in patients with T2DM [3], and observed reductions in the risk of hospitalization for HF led to this drug class being evaluated in patients with HF, with or without T2DM. SGLT2is have been shown to reduce the development and progression of HF with reduced ejection fraction (HFrEF) [4, 5]; however, until recently, the effect of these agents in patients with HF with preserved ejection fraction (HFpEF) was not well studied. The aim of this review is to summarize key information regarding the interaction between HFpEF, CKD, and T2DM and discuss the role of SGLT2 inhibition in the management of patients with comorbid HFpEF and CKD, with or without T2DM.
Literature was retrieved from PubMed and Google Scholar databases using Boolean searches for terms related to SGLT2is, HFpEF, T2DM, and CKD (limits: English-language articles, humans). The reference lists from retrieved articles were also considered. Other relevant literature was obtained on the basis of the personal knowledge and experience of the authors. Additional data were obtained from the US National Institutes of Health website ClinicalTrials.gov and from websites pertaining to individual therapeutic agents of interest. The retrieved references were manually assessed by one reviewer and formed the basis for this narrative review.
Epidemiology of HF, CKD, and T2DM
HF epidemiology
HF is defined as a clinical syndrome with symptoms and/or signs caused by a structural and/or functional cardiac abnormality and corroborated by elevated natriuretic peptide levels and/or objective evidence of pulmonary or systemic congestion [6]. Left ventricular ejection fraction (LVEF) provides prognostic information for patients with HF and defines differing treatment groups [6,7,8]: HF with reduced EF (HFrEF; LVEF ≤ 40%), previously called “systolic HF”; HF with mildly reduced EF (HFmrEF; LVEF 41–49%); HF with preserved EF (HFpEF; LVEF ≥ 50%), previously called “diastolic HF”; and HF with improved EF (baseline LVEF ≤ 40%, followed by a ≥ 10-point increase from baseline and a second LVEF measurement > 40%) [6]. HF affects approximately 64 million adults globally (per 2017 data) [9]. This represents an almost doubling in HF cases over a 27-year period (1990–2017), with almost half of all cases coming from China and India [9]. In the United States (US), HF prevalence was 2.4% in 2012 and affected 5.7 million adults (aged ≥ 20 years) and is expected to rise to 3.0% by 2030, when it will affect > 8 million adults (aged ≥ 20 years) [10]. In the community setting, up to half of patients with HF have HFpEF [11,12,13], although this rate depends on diagnostic accuracy and an evolving clinical definition [12]. Factors contributing to the increased prevalence of HFpEF include an aging population and increased HFpEF-related risk factors (such as diabetes, hypertension, and obesity), as well as improved diagnosis and survival [12]. Trial-based analyses, mainly involving patients with chronic HF in ambulatory settings (i.e., outpatient care), report 1-year mortality rates for HFpEF of around 5%, whereas observational studies report rates of up to 30% using data primarily from inpatients with decompensated HFpEF [12]. The 5-year mortality rate in a community study of adults with HFpEF (aged > 45 years) was 10% for those with a mild degree of diastolic dysfunction, rising to 23% in moderate/severe disease [14]. A large meta-analysis of ambulatory patients with HF found that pooled survival rates were similar for HFpEF and HFrEF at 1 year (89% and 88%, respectively) and 5 years (70% and 63%, respectively) [15].
CKD epidemiology
CKD is defined as persistent albuminuria (albumin-creatinine ratio [ACR], ≥ 30 mg/g [≥ 3 mg/mmol]), persistently reduced estimated glomerular filtration rate (eGFR; < 60 mL/min per 1.73 m2), or both [16]. The Global Burden of Disease Study (2017 data) reported 698 million cases of all-stage CKD, giving a global prevalence of 9.1% [17], whereas an earlier systematic review and meta-analysis (100 studies; ~ 6,900,000 patients) reported a global prevalence of 13.4% [18]. In the US, the prevalence of CKD was 15% in 2021, equating to approximately 37 million American adults with CKD [19]. Changes in CKD prevalence over time have showed stabilization or even improvement (reviewed in [20]). The reasons for this are unclear, given the observed increases in CKD risk factors (such as T2DM and obesity), although hypertension prevalence has stabilized or decreased in many high- and middle-income countries due to improved detection and treatment [21]. The primary causes of CKD are T2DM (30–50% of cases), hypertension (~ 27%), and primary glomerulonephritis (~ 8%) [22]. The prognosis of CKD worsens with increasing Kidney Disease: Improving Global Outcomes (KDIGO) CKD category (based on GFR and albuminuria categories) [23, 24], but only a small proportion of individuals have severely decreased GFR (stage G4), kidney failure (G5), or severely increased albuminuria (A3) [23, 24]. In addition to the complications associated with CKD (such as anemia, mineral bone disease, end-stage kidney disease [ESKD], etc.), CKD is an important risk factor for CV morbidity and mortality, including coronary artery disease, HF, arrhythmias, and sudden CV death (reviewed in [25]). For individuals with CKD, the risk of develo** CVD is greater than that of develo** ESKD [25, 26].
T2DM epidemiology
T2DM accounts for up to 95% of all cases of diabetes, and is caused by a progressive loss of insulin secretion from pancreatic β-cells that becomes insufficient to compensate for insulin resistance, resulting in hyperglycemia [27]. T2DM can be diagnosed using various tests (e.g., fasting plasma glucose, glycated hemoglobin, etc.) and in a variety of clinical settings (e.g., incidental finding, asymptomatic/symptomatic screening, etc.) [27]. Traditional risk factors for T2DM include overweight and obesity, lack of physical activity, and unhealthy diet. Around 6% of the global population (~ 462 million people) are affected by T2DM, and T2DM accounted for more than 1 million deaths (Global Burden of Disease data from 2017) [28]. In the US, 35.4 million adults have T2DM (2019 data) [29]. In terms of disability-adjusted life years (DALYs), a measure of premature deaths and years lived with disability from a particular disease, T2DM causes the seventh highest burden of disease [28]. Global prevalence of T2DM has increased over the last 30 years and is forecasted to rise (cases per 100,000 people) from 6059 in 2017 to 7079 by 2030 [28]; furthermore, global trends modeled to 2025 show continued increases in T2DM incidence, age-standardized rates, deaths, and DALYs [30]. It is well established that diabetes-induced hyperglycemia is a causative factor in the development of microvascular disease (retinopathy, nephropathy, and neuropathy) and macrovascular disease (peripheral artery disease, coronary artery disease, and stroke) [31,32,33,34], via the activation of pathways that trigger cellular oxidative stress, release of inflammatory mediators, mitochondrial dysfunction, and the development of atherosclerosis (reviewed in [35]). Atherosclerotic CVD (ASCVD) is the leading cause of morbidity and mortality in patients with T2DM, and conditions that commonly coexist with T2DM (such as hypertension and dyslipidemia) are risk factors for ASCVD, as is T2DM itself [36].
The relationship between CKD and HF: cardiorenal syndrome
HF and CKD have a bidirectional relationship [36], and the presence of either condition is associated with a worse prognosis in the other. Patients with CKD have a three-fold increased risk of incident HF than those without CKD [37]. The presence of HF in patients with CKD is associated with increased risk of death, more frequent hospitalizations, and reduced health-related quality of life [37,38,39]. The coexistence of HF (with a reduced or preserved ejection fraction [EF]) and CKD can manifest as cardiorenal syndrome (CRS), although comparatively less is known about CRS in HFpEF than in HFrEF [84] and presented in Fig. 3A and B) [84]. Some of these groups may be expected to be more responsive to SGLT2i treatment than others [81], such as patient phenotypes related to obesity and metabolism/inflammation [85, 86]. In addition, other less common cardiac disorders may present with a HFpEF phenotype. These are broadly divided into conditions affecting the myocardium (such as inherited or acquired infiltrative, restrictive, inflammatory, or genetic cardiomyopathies) and those altering cardiac loading conditions (such as hypertension, congenital or acquired valvular and structural defects, rhythm abnormalities, etc.) [87]. Some of these presentations may not be responsive to SGLT2i treatment, particularly those caused by infiltrative diseases, such as cardiac amyloidosis [88]. Cardiac amyloidosis causes restrictive cardiomyopathy, of which the major clinical presentation is HFpEF, with symptoms caused by raised LV filling pressure secondary to increased stiffness and reduced elasticity of the heart tissue [89]. Kidney involvement may occur as part of the primary condition (e.g., systemic amyloidosis is a cause of type 5 CRS) or may be secondary to the ensuing cardiac disease. The importance of recognizing patients with previously undiagnosed cardiac amyloidosis in clinical trials for HFpEF was described recently, and this condition may contribute further to the heterogeneity of HFpEF populations and the failure to obtain positive results in some studies [90].

Reproduced from Heart 2022, volume 108, pages 1342–1350, Gevaert AB, Kataria R, Zannad F, Sauer AJ, Damman K, Sharma K, et al. Heart failure with preserved ejection fraction: recent concepts in diagnosis, mechanisms and management, Copyright 2022, with permission from BMJ Publishing Group Ltd
Evolution of pathophysiological understanding of HFpEF [84]. A Prevailing concept of HFpEF and HFrEF as separate diseases, HFpEF is caused by microvascular inflammation and HFrEF is caused by cardiomyocyte loss. B Emerging concept of heart failure as phenotypes overlap** across the spectrum of LV systolic function. There is a gradual change in underlying pathophysiology, mode of death, and response to HF therapies across the LVEF spectrum, with influences from genetics, sex, comorbidities, and lifestyle. C Personalized treatment of HFpEF. Different phenotypes (based on clinical, imaging, biomarker and/or transcriptomic data) are represented by red, green and blue colors. Personalized treatment: considering the phenotype-specific response to medical therapy, a targeted approach using specific drugs in specific phenotypes could lead to net clinical benefit for all patients. CV cardiovascular, GLS global longitudinal strain, HF heart failure, HFpEF heart failure with preserved ejection fraction, HFrEF heart failure with reduced ejection fraction, LV left ventricle, LVEF left ventricular ejection fraction, NO nitric oxide, ROS reactive oxygen species, SV stroke volume.
Factors related to the design of EMPEROR-Preserved may also be relevant to the observed effect of LVEF. For example, the inclusion of patients with LVEF > 40%, which encompasses HFmrEF (LVEF 41–49%), and the use of a variety of imaging techniques to measure LVEF up to 6 months before study entry, which increases variability and may underestimate potential changes over time [81]. However, the DELIVER trial design may refute this, as it also enrolled patients with LVEF > 40% and documented the EF over a longer period prior to trial enrollment (≤ 12 months), with assessment only via echocardiography or cardiac magnetic resonance imaging [58].
Secondary kidney outcomes in HFpEF vs. HFrEF
A further question is why the secondary kidney outcomes with HFpEF look different when compared with HFrEF. In a comparison of EMPEROR-Reduced and DAPA-HF [91], in which patients with HFrEF were enrolled, both trials reported similar and significant effects of SGLT2is in reducing the decline in the eGFR slope (mean slope of eGFR change vs. placebo: 1.73 and 1.78 mL/min/1.73 m2 per year for empagliflozin and dapagliflozin, respectively) [91]. However, the composite kidney outcome showed a statistical decrease in EMPEROR-Reduced (0.50; 95% CI 0.32–0.77) but not in DAPA-HF (0.71, 95% CI 0.44–1.16), possibly due to fewer kidney events in the latter because of a higher eGFR entry criterion (≥ 20 vs. ≥ 30 mL/min/1.73 m2, respectively) and differences in the eGFR decline defined in the composite kidney outcome (≥ 40% for EMPEROR-Reduced vs. ≥ 50% decline in DAPA-HF) [91]. A prespecified analysis of EMPEROR-Reduced, in which patients were categorized by the presence or absence of CKD at baseline (defined as eGFR < 60 mL/min/1.73 m2 or UACR > 300 mg/g), investigated the direct impact on kidney events via a prespecified composite kidney outcome (defined as a sustained profound decline in eGFR, chronic dialysis, or transplant) [92]. Empagliflozin reduced the slope of eGFR decline in patients with CKD (1.11 [95% CI 0.23–1.98] mL/min/1.73 m2 per year) and without CKD (2.41 [95% CI 1.49–3.32] mL/min/1.73 m2 per year), and the risk of the composite kidney outcome was similarly reduced in patients with and without CKD (HR: 0.53, 95% CI 0.31–0.91 vs. HR: 0.46, 95% CI 0.22–0.99, respectively) [92].
Despite these analyses, the reason why the kidney outcomes appear to be less impressive in patients with HFpEF largely remains unknown. The comparison of EMPEROR-Reduced and DAPA-HF presents relevant points concerning the level of eGFR at trial entry, number of kidney events, and differences in definitions, but it is equally possible that the difference in kidney outcomes is simply due to chance. Furthermore, it may be erroneous to postulate that HFrEF and HFpEF consistently behave differently with respect to kidney outcomes. Data from a post hoc analysis of renal outcomes in the Prospective Comparison of angiotensin receptor-neprilysin inhibitor (ARNI) with angiotensin receptor blocker (ARB) Global Outcomes in HF with Preserved Ejection Fraction trial (PARAGON-HF; NCT01920711) demonstrated a significant reduction in the prespecified kidney composite outcome (time to first occurrence of either ≥ 50% reduction in eGFR, ESKD, or death from renal causes) [93], even though the primary outcome (composite of total HF hospitalizations and CV death) was not achieved [43]. Conversely, the United Kingdom Heart and Renal Protection-III trial (UK HARP-III; ISRCTN11958993) showed no benefits in the kidney with ARNI use in patients with CKD only [94], although the results may have been affected by the trial design (patient characteristics, heterogeneous CKD etiologies, small study size, short follow-up duration, etc. [93]). These data highlight the need for obtaining a more accurate phenotype for patients with HF and CKD (carried out using methods other than cut-offs for eGFR and LVEF) to better determine which patients will respond to different guideline-directed medical therapies (as depicted in Fig. 3B).
Emerging therapies for HFpEF and CKD: finerenone
Although SGLT2is are undoubtedly valuable in the management of HF and CKD, other drugs are also important. Finerenone is one of the standards of care in patients with DKD [95, 96]. It is a selective, non-steroidal mineralocorticoid receptor (MR) antagonist (MRA) that blocks MR-mediated sodium reabsorption and MR overactivation and has demonstrated anti-inflammatory and anti-fibrotic effects in preclinical models of kidney and CV disease [97]. The complementary phase 3 RCTs, Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease (FIDELIO-DKD; NCT02540993 [98]) and Finerenone in Reducing Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease (FIGARO-DKD; NCT02545049 [99]) comprise the largest cardiorenal outcomes program in CKD in T2DM to date [100]. Patients with chronic symptomatic HFrEF were excluded from the FIDELIO and FIGARO trials. FIDELITY was a prespecified pooled analysis of efficacy and safety data from FIDELIO and FIGARO and allowed for evaluation across the range of CKD severity [96] (N = 13,026; broad spectrum of CKD and T2DM; all patients were treated with an optimized dose of angiotensin-converting enzyme inhibitor or ARB) [100]. Approximately 8% of all trial participants were noted to have HF at baseline. FIDELITY provided evidence of both CV and renal protection with finerenone compared with placebo. The analysis showed a 14% risk reduction in the composite CV outcome (consisting of CV death, non-fatal myocardial infarction [MI], non-fatal stroke, or hospitalization for HF) for finerenone vs. placebo (12.7% vs. 14.4%, respectively; HR: 0.86 [95% CI 0.78–0.95]; p = 0.0018) and 23% reduction in risk of the composite kidney outcome (consisting of sustained ≥ 57% decrease in eGFR from baseline over ≥ 4 weeks or renal death) for finerenone vs. placebo (5.5% vs. 7.1%; HR: 0.77 [95% CI 0.67–0.88]; p = 0.0002) [96, 100]. Hospitalization for HF was the primary contributor to the CV benefit observed in the FIDELITY analysis, with a 22% risk reduction (HR: 0.78, 95% CI 0.66–0.92; p = 0.0030) [100]. Per the US Food and Drug Administration, finerenone is now indicated to reduce the risk of sustained eGFR decline, ESKD, CV death, non-fatal MI, and hospitalization for HF in adults with CKD associated with T2DM [101].
Recommendations for management of HFpEF
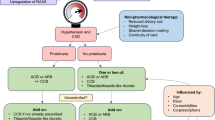
Joint guidelines from the American Heart Association/American College of Cardiology/Heart Failure Society of America (AHA/ACC/HFSA 2022) now include the use of SGLT2is for patients with HFpEF (Class of Recommendation 2a, evidence moderate; benefit > > risk) [8] due to their benefits in decreasing HF hospitalizations and CV mortality [61] (presented in Fig. 4). However, these recommendations were issued before the results of DELIVER were published, and will likely be updated when the new data are taken into consideration. MRAs and ARNIs may also be considered for decreasing hospitalizations in selected patients with HFpEF, particularly those at the lower end of the LVEF spectrum, per the AHA/ACC/HFSA guidelines (Class of Recommendation 2b, evidence weak; benefit ≥ risk) [8]. European Society of Cardiology (ESC) guidelines were published in September 2021 prior to the availability of data from recently completed trials with SGLT2is in HFpEF; thus, no recommendations regarding disease-modifying therapies are provided. However, the use of SGLT2is (dapagliflozin and empagliflozin) is recommended by the ESC in patients with HFrEF to reduce the risk of hospitalization for HF and death [7].

Reproduced from J Am Coll Cardiol. 2022, volume 79(17), pages e263–e421, Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines, Copyright (2022), with permission from The American Heart Association, Inc., The American College of Cardiology Foundation, and The Heart Failure Society of America
Recommendations for patients with preserved LVEF (≥ 50%), per AHA/ACC/HFSA 2022 [8]. Medication recommendations for HFpEF are displayed. ARB angiotensin receptor blocker, ARNI angiotensin receptor-neprilysin inhibitor, HF heart failure, HFpEF heart failure with preserved ejection fraction, LVEF left ventricular ejection fraction, MRA mineralocorticoid receptor antagonist, SGLT2i sodium-glucose cotransporter-2 inhibitor. *Greater benefit in patients with LVEF closer to 50%.
Limitations
There are several limitations associated with this work. Only two databases were used in the search strategy. As the retrieved references from the searches were only assessed by one reviewer, there is a possibility of selection bias.
Conclusions
SGLT2is have demonstrated efficacy and safety in treating HFpEF in patients with comorbid CKD, with and without T2DM. The efficacy of SGLT2is appears to be a class effect. Data from some clinical trials have led clinicians to question whether SGLT2is are effective across the spectrum of EFs in HF, and whether there may be a difference in kidney outcomes between patients with HFpEF vs. HFrEF. Further analysis of the individual trial designs and participant characteristics reveal potentially mitigating factors that may explain the relevant sets of ostensibly neutral results and highlights the need to obtain more accurate phenotypes for patients with HF and CKD (using more nuanced methods than cut-off values for eGFR and LVEF) to better determine which patients might respond to different guideline-directed medical therapies. Furthermore, due to their high risk of develo** HFpEF, patients with CKD may benefit from therapies such as SGLT2is, ARNis, ARBs, and MRAs even if they have not yet been diagnosed with HF.
Availability of data and materials
Data sharing is not applicable to this article, as no new data were created or analyzed in this report.
Abbreviations
- 6MWTD:
-
6-Minute walk test distance
- ACC:
-
American College of Cardiology
- ACR:
-
Albumin-creatinine ratio
- AHA:
-
American Heart Association
- ARB:
-
Angiotensin receptor blocker
- ARNI:
-
Angiotensin receptor-neprilysin inhibitor
- ASCVD:
-
Atherosclerotic cardiovascular disease
- CI:
-
Confidence interval
- CKD:
-
Chronic kidney disease
- CRS:
-
Cardiorenal syndrome
- CV:
-
Cardiovascular
- CVD:
-
Cardiovascular disease
- CSS:
-
Clinical Summary Score
- DALY:
-
Disability-adjusted life year
- DKD:
-
Diabetes kidney disease
- EF:
-
Ejection fraction
- eGFR:
-
Estimated glomerular filtration rate
- ESC:
-
European Society of Cardiology
- ESKD:
-
End-stage kidney disease
- GFR:
-
Glomerular filtration rate
- HF:
-
Heart failure
- HFmrEF:
-
Heart failure with mildly reduced ejection fraction
- HFpEF:
-
Heart failure with preserved ejection fraction
- HFrEF:
-
Heart failure with reduced ejection fraction
- HFSA:
-
Heart Failure Society of America
- HR:
-
Hazard ratio
- KCCQ:
-
Kansas City Cardiomyopathy Questionnaire
- LV:
-
Left ventricular
- LVEF:
-
Left ventricular ejection fraction
- MI:
-
Myocardial infarction
- MR:
-
Mineralocorticoid receptor
- MRA:
-
Mineralocorticoid receptor antagonist
- RCT:
-
Randomized controlled trial
- SGLT2:
-
Sodium-glucose cotransporter-2
- SGLT2i:
-
Sodium-glucose cotransporter-2 inhibitor
- T2DM:
-
Type 2 diabetes mellitus
- TSS:
-
Total Summary Score
- UACR:
-
Urine albumin-creatinine ratio
- US:
-
United States
References
Vijay K, Neuen BL, Lerma EV. Heart failure in patients with diabetes and chronic kidney disease: challenges and opportunities. Cardiorenal Med. 2022;12(1):1–10.
Fontes-Carvalho R, Santos-Ferreira D, Raz I, Marx N, Ruschitzka F, Cosentino F. Protective effects of SGLT-2 inhibitors across the cardiorenal continuum: two faces of the same coin. Eur J Prev Cardiol. 2022;29(9):1352–60.
Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393(10166):31–9.
Packer M, Butler J, Zannad F, Pocock SJ, Filippatos G, Ferreira JP, et al. Empagliflozin and major renal outcomes in heart failure. N Engl J Med. 2021;385(16):1531–3.
McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008.
Bozkurt B, Coats AJS, Tsutsui H, Abdelhamid CM, Adamopoulos S, Albert N, et al. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: Endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur J Heart Fail. 2021;23(3):352–80.
McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–726.
Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79(17):e263–421.
Bragazzi NL, Zhong W, Shu J, Abu Much A, Lotan D, Grupper A, et al. Burden of heart failure and underlying causes in 195 countries and territories from 1990 to 2017. Eur J Prev Cardiol. 2021;28(15):1682–90.
Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, et al. Heart disease and stroke statistics-2022 update: a report from the American Heart Association. Circulation. 2022;145(8):e153–639.
Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2017;14(10):591–602.
Teramoto K, Teng TK, Chandramouli C, Tromp J, Sakata Y, Lam CS. Epidemiology and clinical features of heart failure with preserved ejection fraction. Card Fail Rev. 2022;8:e27.
Savarese G, Becher PM, Lund LH, Seferovic P, Rosano GMC, Coats A. Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc Res. 2022;118(17):3272–87.
Redfield MM, Jacobsen SJ, Burnett JC Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289(2):194–202.
Jones NR, Roalfe AK, Adoki I, Hobbs FDR, Taylor CJ. Survival of patients with chronic heart failure in the community: a systematic review and meta-analysis. Eur J Heart Fail. 2019;21(11):1306–25.
Rossing P, Caramori ML, Chan JCN, Heerspink HJL, Hurst C, Khunti K, et al. Executive summary of the KDIGO 2022 clinical practice guideline for diabetes management in chronic kidney disease: an update based on rapidly emerging new evidence. Kidney Int. 2022;102(5):990–9.
GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395(10225):709–33.
Hill NR, Fatoba ST, Oke JL, Hirst JA, O’Callaghan CA, Lasserson DS, et al. Global prevalence of chronic kidney disease - a systematic review and meta-analysis. PLoS ONE. 2016;11(7):e0158765.
Centers for Disease Control and Prevention. Chronic kidney disease in the United States, 2021. 2021. https://www.cdc.gov/kidneydisease/publications-resources/CKD-national-facts.html. Accessed 16 Aug 2022.
Kovesdy CP. 2022 Epidemiology of chronic kidney disease: an update 2022. Kidney Int Suppl. 2011;12(1):7–11.
NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. 2021;398(10304):957–80.
Webster AC, Nagler EV, Morton RL, Masson P. Chronic kidney disease. Lancet. 2017;389(10075):1238–52.
KDIGO. Chapter 1: Definition and classification of CKD (In: KDIGO 2012 Clinical practice guideline for the evaluation and management of chronic kidney disease). Kidney Int Suppl 2013;3(1):19–62.
United States Renal Data System. 2022 USRDS annual data report: epidemiology of kidney disease in the United States. 2022. https://usrds-adr.niddk.nih.gov/2022?dkrd=/about-niddk/strategic-plans-reports/usrds/annual-data-report. Accessed 24 Mar 2023.
Jankowski J, Floege J, Fliser D, Böhm M, Marx N. Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation. 2021;143(11):1157–72.
Tonelli M, Wiebe N, Culleton B, House A, Rabbat C, Fok M, et al. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol. 2006;17(7):2034–47.
American Diabetes Association Professional Practice C. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S17–38.
Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, Al KJ. Epidemiology of type 2 diabetes - global burden of disease and forecasted trends. J Epidemiol Glob Health. 2020;10(1):107–11.
American Diabetes Association. Statistics about diabetes. 2022. https://diabetes.org/about-us/statistics/about-diabetes. Accessed 8 Dec 2022.
Lin X, Xu Y, Pan X, Xu J, Ding Y, Sun X, et al. Global, regional, and national burden and trend of diabetes in 195 countries and territories: an analysis from 1990 to 2025. Sci Rep. 2020;10(1):14790.
Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–12.
Selvin E, Marinopoulos S, Berkenblit G, Rami T, Brancati FL, Powe NR, et al. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med. 2004;141(6):421–31.
Zhang Y, Hu G, Yuan Z, Chen L. Glycosylated hemoglobin in relationship to cardiovascular outcomes and death in patients with type 2 diabetes: a systematic review and meta-analysis. PLoS ONE. 2012;7(8):e42551.
Erqou S, Lee CTC, Suffoletto M, Echouffo-Tcheugui JB, de Boer RA, van Melle JP, et al. Association between glycated haemoglobin and the risk of congestive heart failure in diabetes mellitus: systematic review and meta-analysis. Eur J Heart Fail. 2013;15(2):185–93.
Babel RA, Dandekar MP. A review on cellular and molecular mechanisms linked to the development of diabetes complications. Curr Diabetes Rev. 2021;17(4):457–73.
American Diabetes Association Professional Practice Committee. 10. Cardiovascular disease and risk management: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S144–74.
House AA, Wanner C, Sarnak MJ, Piña IL, McIntyre CW, Komenda P, et al. Heart failure in chronic kidney disease: conclusions from a Kidney Disease: improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2019;95(6):1304–17.
Kottgen A, Russell SD, Loehr LR, Crainiceanu CM, Rosamond WD, Chang PP, et al. Reduced kidney function as a risk factor for incident heart failure: the Atherosclerosis Risk in Communities (ARIC) study. J Am Soc Nephrol. 2007;18(4):1307–15.
Bansal N, Zelnick L, Bhat Z, Dobre M, He J, Lash J, et al. Burden and outcomes of heart failure hospitalizations in adults with chronic kidney disease. J Am Coll Cardiol. 2019;73(21):2691–700.
Porter AC, Lash JP, **e D, Pan Q, DeLuca J, Kanthety R, et al. Predictors and outcomes of health-related quality of life in adults with CKD. Clin J Am Soc Nephrol. 2016;11(7):1154–62.
Agrawal A, Naranjo M, Kanjanahattakij N, Rangaswami J, Gupta S. Cardiorenal syndrome in heart failure with preserved ejection fraction-an under-recognized clinical entity. Heart Fail Rev. 2019;24(4):421–37.
Joslin JR, Lioudaki E, Androulakis E. Interrelation between heart failure with preserved ejection fraction and renal impairment. Rev Cardiovasc Med. 2022;23(2):69.
van de Wouw J, Broekhuizen M, Sorop O, Joles JA, Verhaar MC, Duncker DJ, et al. Chronic kidney disease as a risk factor for heart failure with preserved ejection fraction: a focus on microcirculatory factors and therapeutic targets. Front Physiol. 2019;10:1108.
Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, et al. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381(17):1609–20.
Lejeune S, Roy C, Slimani A, Pasquet A, Vancraeynest D, Beauloye C, et al. Heart failure with preserved ejection fraction in Belgium: characteristics and outcome of a real-life cohort. Acta Cardiol. 2021;76(7):697–706.
Löfman I, Szummer K, Dahlström U, Jernberg T, Lund LH. Associations with and prognostic impact of chronic kidney disease in heart failure with preserved, mid-range, and reduced ejection fraction. Eur J Heart Fail. 2017;19(12):1606–14.
Fauchier L, Maisons V, Fauchier G, Herbert J, Angoulvant D, Ducluzeau PH, et al. Impact of type 2 diabetes on the incidence of cardiorenal syndromes and on subsequent clinical outcomes: a propensity-matched nationwide analysis. Eur Heart J. 2022;43(Suppl 2):1063.
Patel N, Yaqoob MM, Aksentijevic D. Cardiac metabolic remodelling in chronic kidney disease. Nat Rev Nephrol. 2022;18(8):524–37.
Ter Maaten JM, Damman K, Verhaar MC, Paulus WJ, Duncker DJ, Cheng C, et al. Connecting heart failure with preserved ejection fraction and renal dysfunction: the role of endothelial dysfunction and inflammation. Eur J Heart Fail. 2016;18(6):588–98.
Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62(4):263–71.
Ronco C, McCullough P, Anker SD, Anand I, Aspromonte N, Bagshaw SM, et al. Cardio-renal syndromes: report from the consensus conference of the acute dialysis quality initiative. Eur Heart J. 2010;31(6):703–11.
Kim JA, Wu L, Rodriguez M, Lentine KL, Virk HUH, Hachem KE, et al. Recent developments in the evaluation and management of cardiorenal syndrome: a comprehensive review. Curr Probl Cardiol. 2023;48(3): 101509.
Méndez AB, Azancot MA, Olivella A, Soler MJ. New aspects in cardiorenal syndrome and HFpEF. Clin Kidney J. 2022;15(10):1807–15.
Simmonds SJ, Cuijpers I, Heymans S, Jones EAV. Cellular and molecular differences between HFpEF and HFrEF: a step ahead in an improved pathological understanding. Cells. 2020;9(1):242.
Salvatore T, Galiero R, Caturano A, Rinaldi L, Di Martino A, Albanese G, et al. An overview of the cardiorenal protective mechanisms of SGLT2 inhibitors. Int J Mol Sci. 2022;23(7):3651.
Gao YM, Feng ST, Wen Y, Tang TT, Wang B, Liu BC. Cardiorenal protection of SGLT2 inhibitors-perspectives from metabolic reprogramming. EBioMedicine. 2022;83:104215.
Liu H, Sridhar VS, Boulet J, Dharia A, Khan A, Lawler PR, et al. Cardiorenal protection with SGLT2 inhibitors in patients with diabetes mellitus: from biomarkers to clinical outcomes in heart failure and diabetic kidney disease. Metabolism. 2022;126:154918.
Prandi FR, Barone L, Lecis D, Belli M, Sergi D, Milite M, et al. Biomolecular mechanisms of cardiorenal protection with sodium-glucose co-transporter 2 inhibitors. Biomolecules. 2022;12(10):1349.
Solomon SD, de Boer RA, DeMets D, Hernandez AF, Inzucchi SE, Kosiborod MN, et al. Dapagliflozin in heart failure with preserved and mildly reduced ejection fraction: rationale and design of the DELIVER trial. Eur J Heart Fail. 2021;23(7):1217–25.
Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022;387(12):1089–98.
Anker SD, Butler J, Filippatos GS, Jamal W, Salsali A, Schnee J, et al. Evaluation of the effects of sodium-glucose co-transporter 2 inhibition with empagliflozin on morbidity and mortality in patients with chronic heart failure and a preserved ejection fraction: rationale for and design of the EMPEROR-Preserved trial. Eur J Heart Fail. 2019;21(10):1279–87.
Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451–61.
McCausland FR, Claggett BL, Vaduganathan M, Desai AS, Jhund P, de Boer RA, et al. Dapagliflozin and kidney outcomes in patients with heart failure with mildly reduced or preserved ejection fraction: a prespecified analysis of the DELIVER randomized clinical trial. JAMA Cardiol. 2022;8(1):56–65.
Zoler ML. Empagliflozin win in EMPEROR-Preserved HF, but renal outcomes puzzle. In: Medscape Medical News. 2021. https://www.medscape.com/viewarticle/957997. Accessed 1 Nov 2022.
Khan MS, Bakris GL, Shahid I, Weir MR, Butler J. Potential role and limitations of estimated glomerular filtration rate slope assessment in cardiovascular trials: a review. JAMA Cardiol. 2022;7(5):549–55.
Tuttle KR, Rangaswami J. SGLT2 inhibitors as the bedrock of therapy for heart failure. Lancet. 2022;400(10354):711–3.
Packer M, Zannad F, Butler J, Filippatos G, Ferreira JP, Pocock SJ, et al. Influence of endpoint definitions on the effect of empagliflozin on major renal outcomes in the EMPEROR-Preserved trial. Eur J Heart Fail. 2021;23(10):1798–9.
Packer M, Butler J, Zannad F, Filippatos G, Ferreira JP, Pocock SJ, et al. Effect of empagliflozin on worsening heart failure events in patients with heart failure and preserved ejection fraction: EMPEROR-preserved trial. Circulation. 2021;144(16):1284–94.
Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413–24.
Packer M, Butler J, Filippatos G, Zannad F, Ferreira JP, Zeller C, et al. Design of a prospective patient-level pooled analysis of two parallel trials of empagliflozin in patients with established heart failure. Eur J Heart Fail. 2020;22(12):2393–8.
Vaduganathan M, Docherty KF, Claggett BL, Jhund PS, de Boer RA, Hernandez AF, et al. SGLT-2 inhibitors in patients with heart failure: a comprehensive meta-analysis of five randomised controlled trials. Lancet. 2022;400(10354):757–67.
Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2021;384(2):117–28.
Nassif ME, Windsor SL, Borlaug BA, Kitzman DW, Shah SJ, Tang F, et al. The SGLT2 inhibitor dapagliflozin in heart failure with preserved ejection fraction: a multicenter randomized trial. Nat Med. 2021;27(11):1954–60.
Abraham WT, Lindenfeld J, Ponikowski P, Agostoni P, Butler J, Desai AS, et al. Effect of empagliflozin on exercise ability and symptoms in heart failure patients with reduced and preserved ejection fraction, with and without type 2 diabetes. Eur Heart J. 2021;42(6):700–10.
Spertus JA, Birmingham MC, Nassif M, Damaraju CV, Abbate A, Butler J, et al. The SGLT2 inhibitor canagliflozin in heart failure: the CHIEF-HF remote, patient-centered randomized trial. Nat Med. 2022;28(4):809–13.
Voors AA, Angermann CE, Teerlink JR, Collins SP, Kosiborod M, Biegus J, et al. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: a multinational randomized trial. Nat Med. 2022;28(3):568–74.
Bhatt DL, Szarek M, Pitt B, Cannon CP, Leiter LA, McGuire DK, et al. Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med. 2021;384(2):129–39.
Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–306.
Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436–46.
Empa-Kidney Collaborative Group. Design, recruitment, and baseline characteristics of the EMPA-KIDNEY trial. Nephrol Dial Transplant. 2022;37(7):1317–29.
Empa-Kidney Collaborative Group. Empagliflozin in patients with chronic kidney disease. N Engl J Med. 2022;388(2):117–27.
Requena-Ibanez JA, Santos-Gallego CG, Zafar MU, Badimon JJ. SGLT2-inhibitors on HFpEF patients. Role of ejection fraction. Cardiovasc Drugs Ther. 2022. https://doi.org/10.1007/s10557-022-07371-7.
Shah SJ, Katz DH, Deo RC. Phenotypic spectrum of heart failure with preserved ejection fraction. Heart Fail Clin. 2014;10(3):407–18.
Murray EM, Greene SJ, Rao VN, Sun JL, Alhanti BA, Blumer V, et al. Machine learning to define phenotypes and outcomes of patients hospitalized for heart failure with preserved ejection fraction: findings from ASCEND-HF. Am Heart J. 2022;254:112–21.
Gevaert AB, Kataria R, Zannad F, Sauer AJ, Damman K, Sharma K, et al. Heart failure with preserved ejection fraction: recent concepts in diagnosis, mechanisms and management. Heart. 2022;108(17):1342–50.
Obokata M, Reddy YNV, Pislaru SV, Melenovsky V, Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation. 2017;136(1):6–19.
Packer M, Lam CSP, Lund LH, Maurer MS, Borlaug BA. Characterization of the inflammatory-metabolic phenotype of heart failure with a preserved ejection fraction: a hypothesis to explain influence of sex on the evolution and potential treatment of the disease. Eur J Heart Fail. 2020;22(9):1551–67.
Pieske B, Tschöpe C, de Boer RA, Fraser AG, Anker SD, Donal E, et al. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur J Heart Fail. 2020;22(3):391–412.
Cheng RK, Maurer MS. Recognition and Implications of undiagnosed cardiac amyloid patients in HFpEF trials. JACC Heart Fail. 2021;9(11):803–6.
Madan N, Kalra D. Clinical evaluation of infiltrative cardiomyopathies resulting in heart failure with preserved ejection fraction. Rev Cardiovasc Med. 2020;21(2):181–90.
Oghina S, Bougouin W, Bézard M, Kharoubi M, Komajda M, Cohen-Solal A, et al. The impact of patients with cardiac amyloidosis in HFpEF trials. JACC Heart Fail. 2021;9(3):169–78.
Verma S, McGuire DK, Kosiborod MN. Two tales: one story: EMPEROR-Reduced and DAPA-HF. Circulation. 2020;142(23):2201–4.
Zannad F, Ferreira JP, Pocock SJ, Zeller C, Anker SD, Butler J, et al. Cardiac and kidney benefits of empagliflozin in heart failure across the spectrum of kidney function: insights from EMPEROR-Reduced. Circulation. 2021;143(4):310–21.
McCausland FR, Lefkowitz MP, Claggett B, Anavekar NS, Senni M, Gori M, et al. Angiotensin-neprilysin inhibition and renal outcomes in heart failure with preserved ejection fraction. Circulation. 2020;142(13):1236–45.
Haynes R, Judge PK, Staplin N, Herrington WG, Storey BC, Bethel A, et al. Effects of sacubitril/valsartan versus irbesartan in patients with chronic kidney disease. Circulation. 2018;138(15):1505–14.
American Diabetes Association Professional Practice Committee. 11. Chronic kidney disease and risk management: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S175–84.
American Diabetes Association Professional Practice Committee. Addendum. 11. Chronic kidney disease and risk management: standards of medical care in diabetes-2022. Diabetes Care 2022;45(Suppl. 1): S175-S184. Diabetes Care. 2022;45(9):2182–4.
Agarwal R, Kolkhof P, Bakris G, Bauersachs J, Haller H, Wada T, et al. Steroidal and non-steroidal mineralocorticoid receptor antagonists in cardiorenal medicine. Eur Heart J. 2021;42(2):152–61.
Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P, et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020;383(23):2219–29.
Pitt B, Filippatos G, Agarwal R, Anker SD, Bakris GL, Rossing P, et al. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med. 2021;385(24):2252–63.
Agarwal R, Filippatos G, Pitt B, Anker SD, Rossing P, Joseph A, et al. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: the FIDELITY pooled analysis. Eur Heart J. 2022;43(6):474–84.
Bayer HealthCare Pharmaceuticals Inc. Kerendia (finerenone) [prescrbing information]. 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215341s001lbl.pdf. Accessed 1 Nov 2022.
Jhund PS, Solomon SD, Docherty KF, Heerspink HJL, Anand IS, Böhm M, et al. Efficacy of dapagliflozin on renal function and outcomes in patients with heart failure with reduced ejection fraction: results of DAPA-HF. Circulation. 2021;143(4):298–309.
Acknowledgements
The authors meet the criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE). Writing and editorial support was provided by Debra Brocksmith, MB ChB, PhD, of Elevate Scientific Solutions (Envision Pharma Group), which was contracted and funded by Boehringer Ingelheim Pharmaceuticals, Inc. (BIPI) and Lilly USA, LLC. BIPI was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations.
Funding
This work was supported by Boehringer Ingelheim Pharmaceuticals Inc. and Lilly, USA LLC. The authors received no direct compensation related to the development of the manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the concept for this manuscript, analysis, and interpretation of the literature and critical revision of the manuscript for important intellectual content. All authors read and approved the final manuscript and take public responsibility for this work.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
RJM reports research support and honoraria from Abbott, American Regent, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim/Eli Lilly and Company, Boston Scientific, Cytokinetics, Fast BioMedical, Gilead Sciences, Innolife, Medtronic, Merck, Novartis, Relypsa, Respicardia, Roche, Sanofi, Vifor, and Windtree Therapeutics and is Chair of the Council on the Kidney in Cardiovascular Disease, American Heart Association. SAB reports serving on the advisory board and speaker bureau for AstraZeneca, Boehringer Ingelheim/Eli Lilly and Company, and NovoNordisk and is on the on the advisory board for Abbott Diabetes Care. JR reports consultancy agreements with AstraZeneca, Boehringer Ingelheim/Eli Lilly and Company, and Edwards Lifesciences and serves on the medical advisory board of Procyrion Inc. (Aortix™)
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mentz, R.J., Brunton, S.A. & Rangaswami, J. Sodium-glucose cotransporter-2 inhibition for heart failure with preserved ejection fraction and chronic kidney disease with or without type 2 diabetes mellitus: a narrative review. Cardiovasc Diabetol 22, 316 (2023). https://doi.org/10.1186/s12933-023-02023-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-023-02023-y