Abstract
Background
The associations between short- and long-term exposure to ambient fine particulate matter with an aerodynamic diameter ≤ 2.5 µm (PM2.5) and allergic symptoms in middle-aged and elderly populations remain unclear, particularly in China, where most cities have severe air pollution.
Methods
Participants (n = 10,142; age = 40–75 years) were recruited from ten regions in China from 2018 to 2021 for the Predictive Value of Inflammatory Biomarkers and Forced Expiratory Volume in 1 s (FEV1) for Chronic Obstructive Pulmonary Disease (PIFCOPD) study. Short-term (lag0 and lag0–7 day) and long-term (1-, 3- and 5-year) PM2.5 concentrations at residences were extracted from the air pollutant database known as Tracking Air Pollution (TAP) in China. Multivariate logistic regression models were used to estimate associations for short- and long-term PM2.5 exposure concentrations and long-term exposure models were additionally adjusted for short-term deviations.
Results
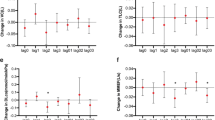
A 10 µg/m3 increase in PM2.5 on the day the allergic symptoms questionnaire was administered (lag0 day) was associated with higher odds of allergic nasal (1.09, 95% CI 1.05, 1.12) and eye symptoms (1.08, 95% CI 1.05, 1.11), worsening dyspnea caused by allergens (1.06, 95% CI 1.02, 1.10), and ≥ 2 allergic symptoms (1.07, 95% CI 1.03, 1.11), which was similar in the lag0–7 day concentrations. A 10 µg/m3 increase in the 1-year average PM2.5 concentration was associated with an increase of 23% for allergic nasal symptoms, 22% for eye symptoms, 20% for worsening dyspnea caused by allergens, and 21% for ≥ 2 allergic symptoms, similar to the 3- and 5-year average PM2.5 concentrations. These associations between long-term PM2.5 concentration and allergic symptoms were generally unchanged after adjustment for short-term deviations.
Conclusions
Short- and long-term exposure to ambient PM2.5 was associated with an increased risk of allergic nasal and eye symptoms, worsening dyspnea caused by allergens, and ≥ 2 allergic symptoms.
Trial registration
Clinical trial ID: NCT03532893 (29 Mar 2018).
Similar content being viewed by others
Background
Ambient fine particulate matter with an aerodynamic diameter ≤ 2.5 µm (PM2.5) is detrimental to public health [1, 2]. Over the past decades, there has been growing evidence that ambient PM2.5 exposure is a risk factor for develo** and exacerbating asthma and allergic diseases [1, 3,4,5,6,7,8]. The global population-weighted PM2.5 concentration increased from 39.7 µg/m3 in 1990 to 44.2 µg/m3 in 2015 [2]. There is growing concern that ambient PM2.5 may contribute to the prevalence of allergic diseases and symptoms.
With rapid economic development, urbanization, and industrialization in recent decades, China has become one of the most polluted countries worldwide [2]. PM2.5 concentrations increased from 1990 and peaked during 2011–2013 [9]. In the wake of the air pollution crisis, the State Council of China promulgated the toughest-ever Air Pollution Prevention and Control Action Plan (APPCAP) in 2013 [10]. China initiated reductions in anthropogenic PM2.5 emissions in 2013, and population-weighted PM2.5 concentrations rapidly decreased by 4.51 µg/m3/year from 2013 to 2016 [11]. However, as of the end of 2017, the entire Chinese population lived in areas with annual average PM2.5 concentrations exceeding 10 µg/m3 [12] (World Health Organization (WHO) interim target 4), and 81.1% lived in areas with concentrations above 35 µg/m3 [13] (Chinese grade I ambient air-quality standard) [9]. In contrast to other countries, China is currently transitioning from high to low air pollution levels, which provides an excellent opportunity to study the effects of air pollution on human health.
Short-term exposure to air pollution can exacerbate preexisting asthma [1], allergic diseases [4], and chronic obstructive pulmonary disease (COPD) [47]. Allergic respiratory symptoms can decrease with air quality improvement [48] and smoking cessation [49].
Our study found long-term PM2.5 exposure was more strongly associated with allergic symptoms than short-term PM2.5 exposure. The findings in our study could be attributable in part to differences between long- and short-term ambient PM2.5 exposure levels. Ambient PM2.5 exhibited the highest concentration in winter (90.08 µg/m3, 5-year average) and the lowest concentration in summer (44.99 µg/m3, 5-year average). Among the participants, 6325 (62%) were enrolled in the study in the summer, and only 373 (3.7%) were enrolled in the winter. The short-term (lag0 and lag0–7 day concentrations) ambient PM2.5 exposure levels were lower than the long-term (1-, 3- and 5-year average concentrations) in this study. Meanwhile, the questionnaire collected nonperiod-specified rather than recent allergic symptoms, which may be another important reason for the stronger associations for long-term than short-term PM2.5 exposure.
Short-term ambient PM2.5 concentration deviations might not significantly affect on long-term PM2.5 exposure models. Consistent with our results, a study of the LuftiBus cohort adjusting for short-term variations in nitrogen dioxide (NO2) and PM2.5 concentrations had little effect on the estimated associations between air pollution exposure and lung function parameters in long-term exposure models [50]. Other studies adjusted for previous single-day or moving average concentrations instead of short-term deviations and revealed that the conclusions of associations between lung function parameters and long-term air pollution exposure were not altered [51, 52]. Therefore, it might not be necessary to adjust for short-term air pollution concentrations, including short-term deviations, previous single-day or moving average concentrations, when estimating the effect of long-term ambient air pollution exposure.
Allergic diseases, which involve complex interactions of genetic, ethnic, environmental, and socioeconomic status or lifestyle risk factors, are primarily attributed to environmental factors such as indoor and outdoor air pollution, tobacco smoke exposure, and exposure to other pollutants [21, 37, 44, 53]. In addition to ambient PM2.5 exposure, we also found that allergic symptoms were positively associated with older age, higher education level, passive smoking, household cooking, occupational exposure and family history of asthma. Household cooking and tobacco smoke are major sources of indoor air pollution. Chinese cooking emits more PM2.5 than Western cooking [54]. For risk factors for allergic symptoms, previous studies have mainly focused on cooking fuel but not cooking itself [55]. In this study, a total of 58% (5896/10142) of the participants cooked frequently at home, only 4.9% (495/10142) had biomass exposure, and 84% (4969/5896) had kitchen ventilation. Household cooking itself, not cooking fuel, is a major risk factor for allergic symptoms. Despite smoking bans in public places in China, 9.6% of the participants in our study were still exposed to environmental tobacco smoke at home or in the workplace. We found that passive smoking was associated with increased allergic symptoms. Consistent with our results, a study based on the Respiratory Health in Northern Europe (RHINE) cohort revealed that passive smoking increased the risk of wheezing (1.26, 95% CI 1.02, 1.57) [56]. Family history of asthma is a well-known risk factor for asthma [53]. This study found that a history of asthma in close relatives is also a risk factor for nasal symptoms, eye symptoms, worsening dyspnea caused by allergens, and ≥ 2 allergic symptoms.
Several limitations of this study should be addressed. First, given the study design, it is challenging to provide causal inferences about the associations between PM2.5 exposure and allergic symptoms. Further intervention and prospective studies are needed to verify the causality of the association in this study. Second, allergic symptoms were assessed by self-report questionnaires, making the study prone to recall bias. Third, as an issue commonly reflected in other studies, PM2.5 exposure concentrations were only estimated at the residence due to a need for more information about work addresses or time-activity patterns. This might result in misclassification. Limited by data availability, information about other ambient pollutants was not available, and we could not distinguish between associations due to PM2.5 specifically or other correlated pollutants.
Conclusions
In conclusion, the findings from the PIFCOPD study showed that short- and long-term ambient PM2.5 exposure might have adverse effects on allergic symptoms among the middle-aged and elderly population in China, apart from other individual risk factors, including older age, higher education level, passive smoking, household cooking, occupational exposure and family history of asthma. Our findings contribute substantially to the evidence of adverse effects of ambient PM2.5 exposure on allergic symptoms in middle-aged and elderly populations. This study further supports for the urgent need to control air pollution to protect middle-aged and elderly adults.
Availability of data and materials
Requests for data should be directed to the corresponding author. Patient-level data will be made available within 5 years of publication. Requests will be assessed before being granted. Data will be anonymized and securely transferred. A data-sharing agreement might be required.
Abbreviations
- APPCAP:
-
Air Pollution Prevention and Control Action Plan
- BMI:
-
Body mass index
- CIs:
-
Confidence intervals
- COPD:
-
Chronic obstructive pulmonary disease
- CPH:
-
China Pulmonary Health
- ERA5:
-
The atmospheric reanalysis V5
- FEV1 :
-
Forced expiratory volume in 1 s
- IgE:
-
Immunoglobulin E
- IQR:
-
Interquartile range
- ORs:
-
Odds ratios
- PIFCOPD:
-
Predictive Value of Inflammatory Biomarkers and FEV1 for COPD
- PM2.5 :
-
Particulate matter with an aerodynamic diameter ≤ 2.5 µm
- SGRQ:
-
St George’s Respiratory Questionnaire
- TAP:
-
Tacking Air Pollution
- WHO:
-
World Health Organization
References
Guarnieri M, Balmes JR. Outdoor air pollution and asthma. Lancet. 2014;383:1581–92.
Cohen AJ, Brauer M, Burnett R, Anderson HR, Frostad J, Estep K, Balakrishnan K, Brunekreef B, Dandona L, Dandona R, et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet. 2017;389:1907–18.
D’Amato G. Effects of climatic changes and urban air pollution on the rising trends of respiratory allergy and asthma. Multidiscip Respir Med. 2011;6:28–37.
Mimura T, Ichinose T, Yamagami S, Fujishima H, Kamei Y, Goto M, Takada S, Matsubara M. Airborne particulate matter (PM2.5) and the prevalence of allergic conjunctivitis in Japan. Sci Total Environ. 2014;487:493–9.
Schultz AA, Schauer JJ, Malecki KM. Allergic disease associations with regional and localized estimates of air pollution. Environ Res. 2017;155:77–85.
Singh S, Sharma BB, Salvi S, Chhatwal J, Jain KC, Kumar L, Joshi MK, Pandramajal SB, Awasthi S, Bhave S, et al. Allergic rhinitis, rhinoconjunctivitis, and eczema: prevalence and associated factors in children. Clin Respir J. 2018;12:547–56.
Sugiyama T, Ueda K, Seposo XT, Nakashima A, Kinoshita M, Matsumoto H, Ikemori F, Honda A, Takano H, Michikawa T, Nitta H. Health effects of PM (2.5) sources on children’s allergic and respiratory symptoms in Fukuoka, Japan. Sci Total Environ. 2020;709:136023.
Zhang X, Morrison-Carpenter T, Holt JB, Callahan DB. Trends in adult current asthma prevalence and contributing risk factors in the United States by state: 2000–2009. BMC Public Health. 2013;13:1156.
Yin P, Brauer M, Cohen AJ, Wang H, Li J, Burnett RT, Stanaway JD, Causey K, Larson S, Godwin W, et al. The effect of air pollution on deaths, disease burden, and life expectancy across China and its provinces, 1990–2017: an analysis for the Global Burden of Disease Study 2017. Lancet Planet Health. 2020;4:e386–98.
Air pollution prevention and control action plan http://www.gov.cn/zwgk/2013-09/12/content_2486773.htm. Accessed 10 Oct 2013
Xue T, Zheng Y, Tong D, Zheng B, Li X, Zhu T, Zhang Q. Spatiotemporal continuous estimates of PM (2.5) concentrations in China, 2000–2016: a machine learning method with inputs from satellites, chemical transport model, and ground observations. Environ Int. 2019;123:345–57.
WHO global air quality guidelines: particulate matter (PM2.5 and PM10), ozone, nitrogen dioxide, sulfur. Geneva: World Health Organization https://www.who.int/publications/i/item/9789240034228. Accessed 22 Sept 2021
Ambient air quality standards https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/dqhjbh/dqhjzlbz/201203/t20120302_224165.shtml. Accessed 1 Jan 2016
Liang L, Cai Y, Barratt B, Lyu B, Chan Q, Hansell AL, **e W, Zhang D, Kelly FJ, Tong Z. Associations between daily air quality and hospitalisations for acute exacerbation of chronic obstructive pulmonary disease in Bei**g, 2013–17: an ecological analysis. Lancet Planet Health. 2019;3:e270–9.
Bowatte G, Lodge C, Lowe AJ, Erbas B, Perret J, Abramson MJ, Matheson M, Dharmage SC. The influence of childhood traffic-related air pollution exposure on asthma, allergy and sensitization: a systematic review and a meta-analysis of birth cohort studies. Allergy. 2015;70:245–56.
Anderson HR, Butland BK, van Donkelaar A, Brauer M, Strachan DP, Clayton T, van Dingenen R, Amann M, Brunekreef B, Cohen A, et al. Satellite-based estimates of ambient air pollution and global variations in childhood asthma prevalence. Environ Health Perspect. 2012;120:1333–9.
Fuertes E, Standl M, Cyrys J, Berdel D, von Berg A, Bauer CP, Krämer U, Sugiri D, Lehmann I, Koletzko S, et al. A longitudinal analysis of associations between traffic-related air pollution with asthma, allergies and sensitization in the GINIplus and LISAplus birth cohorts. PeerJ. 2013;1: e193.
Fan J, Li S, Fan C, Bai Z, Yang K. The impact of PM2.5 on asthma emergency department visits: a systematic review and meta-analysis. Environ Sci Pollut Res. 2016;23:843–50.
Nikasinovic L, Just J, Sahraoui F, Seta N, Grimfeld A, Momas I. Nasal inflammation and personal exposure to fine particles PM2.5 in asthmatic children. J Allergy Clin Immunol. 2006;117:1382–8.
9 out of 10 people worldwide breathe polluted air, but more countries are taking action https://www.who.int/news-room/detail/02-05-2018-9-out-of-10-people-worldwide-breathe-polluted-air-but-more-countries-are-taking-action. Accessed 2 May 2018
Huang K, Yang T, Xu J, Yang L, Zhao J, Zhang X, Bai C, Kang J, Ran P, Shen H, et al. Prevalence, risk factors, and management of asthma in China: a national cross-sectional study. Lancet. 2019;394:407–18.
Samarasekera U. Yaohui Zhao: dedicated to delivering healthy ageing in China. Lancet. 2022;400:1916.
Wang Y, Liao J, Zhong Y, Zhang C, Li X, Wang G. Predictive value of combining inflammatory biomarkers and rapid decline of FEV (1) for COPD in Chinese population: a prospective cohort study. Int J Chron Obstruct Pulmon Dis. 2019;14:2825–33.
Kilpeläinen M, Terho EO, Helenius H, Koskenvuo M. Validation of a new questionnaire on asthma, allergic rhinitis, and conjunctivitis in young adults. Allergy. 2001;56:377–84.
Tracking Air Pollution in China http://tapdata.org.cn/. Accessed 30 Dec 2021
Geng G, **ao Q, Liu S, Liu X, Cheng J, Zheng Y, Xue T, Tong D, Zheng B, Peng Y, et al. Tracking air pollution in China: near real-time PM (2.5) retrievals from multisource data fusion. Environ Sci Technol. 2021;55:12106–15.
Liu X, Zhao C, Shen X, ** T. Spatiotemporal variations and sources of PM (2.5) in the Central Plains Urban Agglomeration, China. Air Qual Atmos Health. 2022;15:1507–21.
Feng Y, Ning M, Lei Y, Sun Y, Liu W, Wang J. Defending blue sky in China: effectiveness of the “air pollution prevention and control action plan” on air quality improvements from 2013 to 2017. J Environ Manage. 2019;252: 109603.
Wüthrich B. Epidemiology of the allergic diseases: are they really on the increase? Int Arch Allergy Appl Immunol. 1989;90(Suppl 1):3–10.
Chen F, Lin Z, Chen R, Norback D, Liu C, Kan H, Deng Q, Huang C, Hu Y, Zou Z, et al. The effects of PM (2.5) on asthmatic and allergic diseases or symptoms in preschool children of six Chinese cities, based on China, Children, Homes and Health (CCHH) project. Environ Pollut. 2018;232:329–37.
Hong Z, Guo Z, Zhang R, Xu J, Dong W, Zhuang G, Deng C. Airborne fine particulate matter induces oxidative stress and inflammation in human nasal epithelial cells. Tohoku J Exp Med. 2016;239:117–25.
Wu P-C, Tsai J-C, Li F-C, Lung S-C, Su H-J. Increased levels of ambient fungal spores in Taiwan are associated with dust events from China. Atmos Environ. 2004;38:4879–86.
Lee S, Choi B, Yi S-M, Ko G. Characterization of microbial community during Asian dust events in Korea. Sci Total Environ. 2009;407:5308–14.
Ribeiro H, Guimarães F, Duque L, Noronha F, Abreu I. Characterisation of particulate matter on airborne pollen grains. Environ Pollut. 2015;206:7–16.
Phosri A, Ueda K, Tasmin S, Kishikawa R, Hayashi M, Hara K, Uehara Y, Phung VLH, Yasukouchi S, Konishi S, et al. Interactive effects of specific fine particulate matter compositions and airborne pollen on frequency of clinic visits for pollinosis in Fukuoka. Japan Environ Res. 2017;156:411–9.
Ormstad H. Suspended particulate matter in indoor air: relation to allergy. Tidsskr Nor Laegeforen. 2001;121:1344–50.
Higgins TS, Reh DD. Environmental pollutants and allergic rhinitis. Curr Opin Otolaryngol Head Neck Surg. 2012;20:209–14.
Chang CJ, Yang HH, Chang CA, Tsai HY. Relationship between air pollution and outpatient visits for nonspecific conjunctivitis. Invest Ophthalmol Vis Sci. 2012;53:429–33.
Wang YL, Gao W, Li Y, Wang YF. Concentration-dependent effects of PM (2.5) mass on expressions of adhesion molecules and inflammatory cytokines in nasal mucosa of rats with allergic rhinitis. Eur Arch Otorhinolaryngol. 2017;274:3221–9.
Fujishima H, Satake Y, Okada N, Kawashima S, Matsumoto K, Saito H. Effects of diesel exhaust particles on primary cultured healthy human conjunctival epithelium. Ann Allergy Asthma Immunol. 2013;110:39–43.
Eguiluz-Gracia I, Mathioudakis AG, Bartel S, Vijverberg SJH, Fuertes E, Comberiati P, Cai YS, Tomazic PV, Diamant Z, Vestbo J, et al. The need for clean air: the way air pollution and climate change affect allergic rhinitis and asthma. Allergy. 2020;75:2170–84.
Doiron D, de Hoogh K, Probst-Hensch N, Mbatchou S, Eeftens M, Cai Y, Schindler C, Fortier I, Hodgson S, Gaye A, et al. Residential air pollution and associations with wheeze and shortness of breath in adults: a combined analysis of cross-sectional data from Two Large European Cohorts. Environ Health Perspect. 2017;125: 097025.
Kyvsgaard JN, Krogsgaard Chawes BL, George Horner DL, Hesselberg LM, Melgaard ME, Jensen SK, Malby Schoos AM, Thorsen J, Tingskov Pedersen CE, Brustad N, et al. Risk factors and age-related patterns of asthma-like symptoms in early childhood. J Allergy Clin Immunol Pract. 2023. https://doi.org/10.1016/j.jaip.2023.02.031.
Hastert TA, Babey SH, Brown ER, Meng YY. Pets and smoking in the home associated with asthma symptoms and asthma-like breathing problems. Policy Brief UCLA Cent Health Policy Res. 2007;18:1–7.
Papageorgiou N, Gaga M, Marossis C, Reppas C, Avarlis P, Kyriakou M, Tsipra S, Zeibecoglou K, Tracopoulos G. Prevalence of asthma and asthma-like symptoms in Athens, Greece. Respir Med. 1997;91:83–8.
Zemp E, Elsasser S, Schindler C, Künzli N, Perruchoud AP, Domenighetti G, Medici T, Ackermann-Liebrich U, Leuenberger P, Monn C, et al. Long-term ambient air pollution and respiratory symptoms in adults (SAPALDIA study). The SAPALDIA Team. Am J Respir Crit Care Med. 1999;159:1257–66.
Stanek LW, Brown JS, Stanek J, Gift J, Costa DL. Air pollution toxicology–a brief review of the role of the science in sha** the current understanding of air pollution health risks. Toxicol Sci. 2011;120(Suppl 1):S8-27.
Laney AS, Cragin LA, Blevins LZ, Sumner AD, Cox-Ganser JM, Kreiss K, Moffatt SG, Lohff CJ. Sarcoidosis, asthma, and asthma-like symptoms among occupants of a historically water-damaged office building. Indoor Air. 2009;19:83–90.
Jarvis D, Newson R, Janson C, Corsico A, Heinrich J, Anto JM, Abramson MJ, Kirsten AM, Zock JP, Bono R, et al. Prevalence of asthma-like symptoms with ageing. Thorax. 2018;73:37–48.
Strassmann A, de Hoogh K, Röösli M, Haile SR, Turk A, Bopp M, Puhan MA. NO2 and PM2.5 exposures and lung function in swiss adults: estimated effects of short-term exposures and long-term exposures with and without adjustment for short-term deviations. Environ Health Perspect. 2021;129:17009.
Rice MB, Ljungman PL, Wilker EH, Dorans KS, Gold DR, Schwartz J, Koutrakis P, Washko GR, O’Connor GT, Mittleman MA. Long-term exposure to traffic emissions and fine particulate matter and lung function decline in the Framingham heart study. Am J Respir Crit Care Med. 2015;191:656–64.
Adar SD, Kaufman JD, Diez-Roux AV, Hoffman EA, D’Souza J, Stukovsky KH, Rich SS, Rotter JI, Guo X, Raffel LJ, et al. Air pollution and percent emphysema identified by computed tomography in the Multi-Ethnic study of Atherosclerosis. Environ Health Perspect. 2015;123:144–51.
Burke W, Fesinmeyer M, Reed K, Hampson L, Carlsten C. Family history as a predictor of asthma risk. Am J Prev Med. 2003;24:160–9.
Jung CC, Su HJ. Chemical and stable isotopic characteristics of PM (2.5) emitted from Chinese cooking. Environ Pollut. 2020;267:115577.
Wong GW, Brunekreef B, Ellwood P, Anderson HR, Asher MI, Crane J, Lai CK. Cooking fuels and prevalence of asthma: a global analysis of phase three of the International Study of Asthma and Allergies in Childhood (ISAAC). Lancet Respir Med. 2013;1:386–94.
Wang J, Janson C, Jogi R, Forsberg B, Gislason T, Holm M, Torén K, Malinovschi A, Sigsgaard T, Schlünssen V, et al. A prospective study on the role of smoking, environmental tobacco smoke, indoor painting and living in old or new buildings on asthma, rhinitis and respiratory symptoms. Environ Res. 2021;192: 110269.
Acknowledgements
We thank the participants of Predictive Value of Inflammatory Biomarkers and FEV1 for COPD. For continuous support, assistance, and cooperation, we thank Yunxia Wang, Zhu Tian, Meng Wu, ** District ** Liao have contributed equally to this work
Authors and Affiliations
Contributions
GW, SW, and JL conceived and designed the study. SW and JL developed the protocol, analyzed the data, and drafted the manuscript. TX and HL provided the PM2.5 air pollutant database. GW contributed to the interpretation of data and the final approval of publication. The other authors collected data and revised the manuscript. All authors approved the final version before submission.