Abstract
Background
Leber’s hereditary optic neuropathy (LHON) is a maternally inherited eye disease due to mutations in mitochondrial DNA. However, there is no effective treatment for this disease. LHON-linked ND6 14484T > C (p.M64V) mutation caused complex I deficiency, diminished ATP production, increased production of reactive oxygen species (ROS), elevated apoptosis, and impaired mitophagy. Here, we investigated if the allotopic expression of human mitochondrial ND6 transgene corrected the mitochondrial dysfunctions due to LHON-associated m.14484T > C mutation.
Methods
Nucleus-versions of ND6 was generated by changing 6 non-universal codons with universal codons and added to mitochondrial targeting sequence of COX8. Stable transfectants were generated by transferring human ND6 cDNA expressed in a pCDH-puro vector into mutant cybrids carrying the m.14484T > C mutation and control cybrids. The effect of allotopic expression of ND6 on oxidative phosphorylation (OXPHOS) was evaluated using Blue Native gel electrophoresis and extracellular flux analyzer. Assessment of ROS production in cell lines was performed by flow cytometry with MitoSOX Red reagent. Analyses for apoptosis and mitophagy were undertaken via flow cytometry, TUNEL and immunofluorescence assays.
Results
The transfer of human ND6 into the cybrids carrying the m.14484T > C mutation raised the levels of ND6, ND1 and ND4L but did not change the levels of other mitochondrial proteins. The overexpression of ND6 led to 20~23% increases in the assembly and activity of complex I, and ~ 53% and ~ 33% increases in the levels of mitochondrial ATP and ΔΨm in the mutant cybrids bearing m.14484T > C mutation. Furthermore, mutant cybrids with overexpression of ND6 exhibited marked reductions in the levels of mitochondrial ROS. Strikingly, ND6 overexpression markedly inhibited the apoptosis process and restored impaired mitophagy in the cells carrying m.14484T > C mutation. However, overexpression of ND6 did not affect the ND6 level and mitochondrial functions in the wild-type cybrids, indicating that this ND6 level appeared to be the maximum threshold level to maintain the normal cell function.
Conclusion
We demonstrated that allotopic expression of nucleus-versions of ND6 restored complex I, apoptosis and mitophagy deficiencies caused by the m.14484T > C mutation. The restoration of m.14484T > C mutation-induced mitochondrial dysfunctions by overexpression of ND6 is a step toward therapeutic interventions for LHON and mitochondrial diseases.
Similar content being viewed by others
Introduction
Leber’s hereditary optic neuropathy (LHON) is the most common maternally inherited eye disease that presents with the loss of central vision in young adults, due to the degeneration of retinal ganglion cells and their axons [1,2,3,4,5,6]. The majority of LHON cases globally results from one of three mitochondrial DNA (mtDNA) mutations (ND1 3460G > A, ND4 11778G > A, and ND6 14484T > C), which affects the essential subunits of complex I (NADH: ubiquinone oxidoreductase) [7,8,9,10,11,12,13]. These mtDNA mutations resulted in the complex I deficiency, diminished ATP synthesis and an increasing generation of reactive oxygen species (ROS) [14,15,16,43]. The improvement of both OXPHOS and ΔΨm would reduce the production of ROS [36]. In this investigation, mutant cell lines with overexpression of ND6 exhibited marked reductions in the levels of mitochondrial ROS and three antioxidant enzymes, SOD2 in the mitochondrion and SOD1 and catalase in the cytosol catalase. The lower production of ROS can reduce a vicious cycle of oxidative stress in the mitochondria, thereby decreasing the damage of mitochondrial and cellular proteins, lipids and nuclear acids [50].
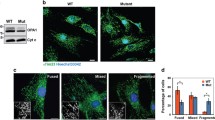
Mitochondrial dysfunctions caused by LHON-associated mtDNA mutations impaired the cell viability and affected the apoptotic process as well as mitophagy [19, 20, 45, 51,52,53]. In particular, mutant cybrids bearing the m.14484T > C mutation exhibited increasing ratio of Annexin V-positive cells and elevated releases of cytochrome c into cytosol than those in control cybrids [20]. In the present study, we demonstrated that the overexpression of ND6 suppressed the m.14484T > C mutation-induced the impairment of apoptosis. Lines of evidence from Annexin V/PI-based flow cytometry, TUNEL and immunocytostaining assays indicated much less apoptosis in the mutant cybrids with overexpression of ND6 than those in the parental mutant cybrids. These were further supported by decreasing levels in cytochrome c, and BAX which mediate cell death by apoptosis [54], raising expressions of Bcl-xL which has anti-apoptotic activity [55], and rescuing levels of apoptosis activated proteins: caspases 7, 9, and 3 in the mutant cybrids with overexpression of ND6, as compared with than those in the parental mutant cybrids [56, 57]. Notably, the levels of apoptosis in the mutant cybrids with overexpression of ND6 were comparable with those in the control cybrids. Furthermore, our previous study showed that the m.14484T > C mutation impaired the mitophagy [20]. In this study, we demonstrated that the overexpression of ND6 restored impaired mitophagy due to the m.14484T > C mutation by immunocytostaining and Western blot analyses. In particular, the average levels of LC3II/I + II, P62, PINK1 and Parkin in the mutant cybrids expressing nucleus-version of ND6 were significantly elevated, as compared with these in the parental mutant cybrids. These data indicated that overexpression of human ND6 reversed the abnormal cell apoptosis and mitophagy which caused by m.14484T > C mutation. Therefore, the overexpression of ND6 in mutant cells may reprogram energy metabolism and prevent the dysfunction or death of retinal ganglion cells due to the m.14484T > C mutation [58].
Conclusion
In this investigation, we demonstrated that allotopic expression of human ND6 restored complex I, apoptosis and mitophagy deficiencies caused by LHON-linked m.14484T > C mutation. The restoration of m.14484T > C mutation-induced mitochondrial dysfunctions by overexpression of ND6 is a step toward therapeutic interventions for LHON and other mitochondrial diseases.
Availability of data and materials
Representative experiments are shown in the figures and additional materials. For any additional information, please contact the corresponding author.
References
Wallace DC, Lott MT. Leber hereditary optic neuropathy: exemplar of an mtDNA disease. Handb Exp Pharmacol. 2017;240:339–76.
Yu-Wai-Man P, Griffiths PG, Chinnery PF. Mitochondrial optic neuropathies—disease mechanisms and therapeutic strategies. Prog Retin Eye Res. 2011;30:81–114.
Newman NJ. Hereditary optic neuropathies: from the mitochondria to the optic nerve. Am J Ophthalmol. 2005;140:517–23.
Yen MY, Wang AG, Wei YH. Leber’s hereditary optic neuropathy: a multifactorial disease. Prog Retin Eye Res. 2006;25:381–96.
Carelli V, La Morgia C, Valentino ML, Barboni P, Ross-Cisneros FN, Sadun AA. Retinal ganglion cell neurodegeneration in mitochondrial inherited disorders. Biochim Biophys Acta. 2009;1787:518–28.
Nie Z, Wang C, Chen J, Ji Y, Zhang H, Zhao F, Zhou X, Guan MX. Abnormal morphology and function in retinal ganglion cells derived from patients-specific iPSCs generated from individuals with Leber’s hereditary optic neuropathy. Hum Mol Genet. 2023;32:231–43.
Wallace DC, Singh G, Lott MT, Hodge JA, Schurr TG, Lezza AM, Elsas LJ, Nikoskelainen EK. Mitochondrial DNA mutation associated with Leber’s hereditary optic neuropathy. Science. 1988;242:1427–30.
Carelli V, La Morgia C, Yu-Wai-Man P. Mitochondrial optic neuropathies. Handb Clin Neurol. 2023;194:23–42.
Ruiz-Pesini E, Lott MT, Procaccio V, Poole JC, Brandon MC, Mishmar D, Yi C, Kreuziger J, Baldi P, Wallace DC. An enhanced mitomap with a global mtDNA mutational phylogeny. Nucleic Acids Res. 2007;35:D823-828.
Brown MD, Torroni A, Reckord CL, Wallace DC. Phylogenetic analysis of Leber’s hereditary optic neuropathy mitochondrial DNA’s indicates multiple independent occurrences of the common mutations. Hum Mut. 1995;6:311–25.
Jiang P, Liang M, Zhang J, Gao Y, He Z, Yu H, Zhao F, Ji Y, Liu X, Zhang M, Fu Q, Tong Y, Sun Y, Zhou X, Guan MX. Prevalence of mitochondrial ND4 mutations in 1281 Han Chinese subjects with Leber’s hereditary optic neuropathy. Invest Ophthalmol Vis Sci. 2015;56:4778–88.
Liang M, Jiang P, Li F, Zhang J, Ji Y, He Y, Xu M, Zhu J, Meng X, Zhao F, Tong Y, Liu X, Sun Y, Zhou X, Guan MX. Frequency and spectrum of mitochondrial ND6 mutations in 1218 Han Chinese subjects with Leber’s hereditary optic neuropathy. Invest Ophthalmol Vis Sci. 2014;55:1321–31.
Ji Y, Liang M, Zhang J, Zhu L, Zhang Z, Fu R, Liu X, Zhang M, Fu Q, Zhao F, Tong Y, Sun Y, Jiang P, Guan MX. Mitochondrial ND1 variants in 1281 Chinese subjects with Leber’s hereditary optic neuropathy. Invest Ophthalmol Vis Sci. 2016;57:2377–89.
Hofhaus G, Johns DR, Hurko O, Attardi G, Chomyn A. Respiration and growth defects in transmitochondrial cell lines carrying the 11778mutation associated with Leber’s hereditary optic neuropathy. J Biol Chem. 1996;271:13155–61.
Brown MD, Trounce IA, Jun AS, Allen JC, Wallace DC. Functional analysis of lymphoblast and cybrid mitochondria containing the 3460, 11778, or 14484 Leber’s hereditary optic neuropathy mitochondrial DNA mutation. J Biol Chem. 2000;275:39831–6.
Pello R, Martín MA, Carelli V, Nijtmans LG, Achilli A, Pala M, Torroni A, Gómez-Durán A, Ruiz-Pesini E, Martinuzzi A, Smeitink JA, Arenas J, Ugalde C. Mitochondrial DNA background modulates the assembly kinetics of OXPHOS complexes in a cellular model of mitochondrial disease. Hum Mol Genet. 2008;17:4001–11.
Ji Y, Zhang J, Lu Y, Yi Q, Chen M, **e S, Mao X, **ao Y, Meng F, Zhang M, Yang R, Guan MX. Complex I mutations synergize to worsen the phenotypic expression of Leber’s hereditary optic neuropathy. J Biol Chem. 2020;295:13224–38.
Jiang P, ** X, Peng Y, Wang M, Liu H, Liu X, Zhang Z, Ji Y, Zhang J, Liang M, Zhao F, Sun YH, Zhang M, Zhou X, Chen Y, Guan MX. The exome sequencing identified the mutation in YARS2 encoding the mitochondrial tyrosyl-tRNA synthetase as a nuclear modifier for the phenotypic manifestation of Leber’s hereditary optic neuropathy-associated mitochondrial DNA mutation. Hum Mol Genet. 2016;25:584–96.
Ji Y, Zhang J, Yu J, Wang Y, Lu Y, Liang M, Li Q, ** X, Wei Y, Meng F, Gao Y, Cang X, Tong Y, Liu X, Zhang M, et al. Contribution of mitochondrial ND1 3394T>C mutation to the phenotypic manifestation of Leber’s hereditary optic neuropathy. Hum Mol Genet. 2019;28:1515–29.
Liang M, Ji YC, Zhang LY, Wang X, Hu CF, Zhang JJ, Zhu YW, Mo JQ, Guan MX. Leber’s hereditary optic neuropathy-associated ND6 14484T > C mutation caused pleiotropic effects on the complex I, RNA homeostasis, apoptosis and mitophagy. Hum Mol Genet. 2022;31:3299–312.
Zhu J, Vinothkumar KR, Hirst J. Structure of mammalian respiratory complex I. Nature. 2016;536:354–8.
Chen JR, Chen C, Guan MX. Nuclear modifier YARS2 allele correction restored retinal ganglion cells-specific deficiencies in Leber’s hereditary optic neuropathy. Hum Mol Genet. 2023;32:1539–51.
Karanjia R, Chahal J, Ammar M, Sadun AA. Treatment of Leber’s hereditary optic neuropathy. Curr Pharm Des. 2017;23:624–8.
Schmiderer L, Yudovich D, Hjort M, Larsson J. Site-specific CRISPR-based mitochondrial DNA manipulation is limited by gRNA import. Sci Rep. 2022;12:18687.
Chen BS, Yu-Wai-Man P, Newman NJ. Developments in the treatment of Leber hereditary optic neuropathy. Curr Neurol Neurosci Rep. 2022;22:881–92.
Davila-Siliezar P, Carter M, Milea D, Lee AG. Leber hereditary optic neuropathy: new and emerging therapies. Curr Opin Ophthalmol. 2022;33:574–8.
Manfredi G, Fu J, Ojaimi J, Sadlock JE, Kwong JQ, Guy J, Schon EA. Rescue of a deficiency in ATP synthesis by transfer of MTATP6, a mitochondrial DNA-encoded gene, to the nucleus. Nature Genet. 2002;30:394–9.
Artika IM. Allotopic expression of mitochondrial genes: basic strategy and progress. Genes Dis. 2019;7:578–84.
Lewis CJ, Dixit B, Batiuk E, Hall CJ, O’Connor MS, Boominathan A. Codon optimization is an essential parameter for the efficient allotopic expression of mtDNA genes. Redox Biol. 2020;30: 101429.
Guy J, Qi XP, Pallotti F, Schon EA, Manfredi G, Carelli V, Martinuzzi A, Hauswirth WW, Lewin AS. Rescue of a mitochondrial deficiency causing Leber hereditary optic neuropathy. Ann Neurol. 2002;52:534–42.
Ellouze S, Augustin S, Bouaita A, Bonnet C, Simonutti M, Forster V, Picaud S, Sahel JA, Corral-Debrinski M. Optimized allotopic expression of the human mitochondrial ND4 prevents blindness in a rat model of mitochondrial dysfunction. Am J Hum Genet. 2008;83:373–87.
Bonnet C, Augustin S, Ellouze S, Bénit P, Bouaita A, Rustin P, Sahel JA, Corral-Debrinski M. The optimized allotopic expression of ND1 or ND4 genes restores respiratory chain complex I activity in fibroblasts harboring mutations in these genes. Biochim Biophys Acta. 2008;1783:1707–17.
Lam BL, Feuer WJ, Abukhalil F, Porciatti V, Hauswirth WW, Guy J. Leber hereditary optic neuropathy gene therapy clinical trial recruitment: year 1. Arch Ophthalmol. 2010;128:1129–35.
Rizzuto R, Nakase H, Darras B, Francke U, Fabrizi GM, Mengel T, Walsh F, Kadenbach B, Dimauro S, Schon EA. A gene specifying subunit VIII of human cytochrome c oxidase is localized to chromosome 11 and is expressed in both muscle and non-muscle tissues. J Biol Chem. 1989;264:10595–600.
Kaltimbacher V, Bonnet C, Lecoeuvre G, Forster V, Sahel JA, Corral-Debrinski M. mRNA localization to the mitochondrial surface allows the efficient translocation inside the organelle of a nuclear recoded ATP6 protein. RNA. 2006;12:1408–17.
Gong SS, Wang XQ, Meng FL, Cui LM, Yi QZ, Zhao Q, Cang XH, Cai ZY, Mo JQ, Liang Y, et al. Overexpression of mitochondrial histidyl-tRNA synthetase restores mitochondrial dysfunction caused by a deafness-associated tRNAHis mutation. J Biol Chem. 2020;295:940–54.
Yu J, Liang X, Ji Y, Ai C, Liu J, Zhu L, Nie Z, ** X, Wang C, Zhang J, Zhao F, Mei S, Zhao X, Zhou X, Zhang M, Wang M, Huang T, Jiang P, Guan MX. PRICKLE3 linked to ATPase biogenesis manifested Leber’s hereditary optic neuropathy. J Clin Invest. 2020;130:4935–46.
Zhou M, Xue L, Chen YR, Li HY, He QF, Wang BB, Meng FL, Wang M, Guan MX. A hypertension-associated mitochondrial DNA mutation introduces an m1G37 modification into tRNAMet, altering its structure and function. J Biol Chem. 2018;293:1425–38.
Ausenda C, Chomyn A. Purification of mitochondrial DNA from human cell cultures and placenta. Methods Enzymol. 1996;264:122–8.
Jha P, Wang X, Auwerx J. Analysis of mitochondrial respiratory chain super-complexes using blue native polyacrylamide gel electrophoresis (BN-PAGE). Curr Protoc Mouse Biol. 2016;6:1–14.
Jia Z, Meng F, Chen H, Zhu G, Li X, He Y, Zhang L, He X, Zhan H, Chen M, Ji Y, Wang M, Guan MX. Human TRUB1 is a highly conserved pseudouridine synthase responsible for the formation of Ψ55 in mitochondrial tRNAAsn, tRNAGln, tRNAGlu and tRNAPro. Nucleic Acids Res. 2022;50:9368–81.
Dranka BP, Benavides GA, Diers AR, Giordano S, Zelickson BR, Reily C, Zou L, Chatham JC, Hill BG, Zhang J, Landar A, Darley-Usmar VM. Assessing bioenergetic function in response to oxidative stress by metabolic profiling. Free Radic Biol Med. 2011;51:1621–35.
Reers M, Smiley ST, Mottola-Hartshorn C, Chen A, Lin M, Chen LB. Mitochondrial membrane potential monitored by JC-1 dye. Methods Enzymol. 1995;260:406–17.
Mahfouz R, Sharma R, Lackner J, Aziz N, Agarwal A. Evaluation of chemiluminescence and flow cytometry as tools in assessing production of hydrogen peroxide and superoxide anion in human spermatozoa. Fertil Steril. 2009;92:819–27.
Zhang J, Ji Y, Chen J, Xu M, Wang G, Ci X, Lin B, Mo JQ, Zhou X, Guan MX. Association between Leber’s Hereditary Optic Neuropathy and MT-ND1 3460G>A mutation-induced alterations in mitochondrial function, apoptosis, and mitophagy. Invest Ophthalmol Vis Sci. 2021;62:13.
Ji Y, Nie Z, Meng F, Hu C, Chen H, ** L, Chen M, Zhang M, Zhang J, Liang M, et al. Mechanistic insights into mitochondrial tRNAAla 3’ end metabolism deficiency. J Biol Chem. 2021;297: 100816.
Pickles S, Vigie P, Youle RJ. Mitophagy and quality control mechanisms in mitochondrial maintenance. Curr Biol. 2018;28:R170-185.
Dikic I. Proteasomal and autophagic degradation systems. Annu Rev Biochem. 2017;86:193–224.
Li R, Guan MX. Human mitochondrial leucyl-tRNA synthetase corrects mitochondrial dysfunctions due to the tRNALeu(UUR) A3243G mutation, associated with mitochondrial encephalomyopathy, lactic acidosis, and stroke-like symptoms and diabetes. Mol Cell Biol. 2010;30:2147–54.
Hayashi G, Cortopassi G. Oxidative stress in inherited mitochondrial diseases. Free Radic Biol Med. 2015;88:10–7.
Ghelli A, Zanna C, Porcelli AM, Schapira AHV, Martinuzzi A, Carelli V, Rugolo M. Leber’s hereditary optic neuropathy (LHON) pathogenic mutations induce mitochondrial-dependent apoptotic death in transmitochondrial cells incubated with galactose medium. J Biol Chem. 2003;278:4145–50.
Danielson SR, Wong A, Carelli V, Martinuzzi A, Schapira AH, Cortopassi GA. Cells bearing mutations causing Leber’s hereditary optic neuropathy are sensitized to Fas-Induced apoptosis. J Biol Chem. 2002;277:5810–5.
Danese A, Patergnani S, Maresca A, Peron C, Raimondi A, Caporali L, Marchi S, La Morgia C, Del Dotto V, Zanna C, et al. Pathological mitophagy disrupts mitochondrial homeostasis in Leber’s hereditary optic neuropathy. Cell Rep. 2022;40: 111124.
Westphal D, Dewson G, Czabotar PE, Kluck RM. Molecular biology of Bax and Bak activation and action. Biochim Biophys Acta. 2011;1813:521–31.
Punnoose EA, Leverson JD, Peale F, Boghaert ER, Belmont LD, Tan N, Young A, Mitten M, Ingalla E, Darbonne WC, Oleksijew A, Tapang P, Yue P, Oeh J, Lee L, et al. Expression profile of Bcl-2, Bcl-xl, and Mcl-1 predicts pharmacological response to the Bcl-2 selective antagonist venetoclax in multiple myeloma models. Mol Cancer Ther. 2016;15:1132–44.
Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol. 2008;9:231–41.
Wang C, Youle RJ. The role of mitochondria in apoptosis. Annu Rev Genet. 2009;43:95–118.
Wong-Riley MT. Energy metabolism of the visual system. Eye Brain. 2010;2:99–116.
Acknowledgements
This paper is dedicated to the memory of Dr. Yi Tong, former Professor of Fujian Medical University, a pioneer Chinese clinician in LHON. We are grateful to all patients and family members for their participation.
Funding
This work was supported by the Grants 2021YFC2700900 from National Key Research and Development Program of China (M.X.G), 31970557 (M.X.G) and 82171847(J.Y.) from the National Natural Science Foundation of China.
Author information
Authors and Affiliations
Contributions
MXG designed the experiments, monitored the project progression, data analysis and interpretation. JW, YJ, CA, JRC and DG performed the biochemical analyses, JW, JZ and JQM performed the data analysis. JW and YJ prepared the initial draft of the manuscript. MXG made the final version of the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
All authors have no proprietary or commercial interest in any of materials discussed in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, J., Ji, Y., Ai, C. et al. Optimized allotopic expression of mitochondrial ND6 transgene restored complex I and apoptosis deficiencies caused by LHON-linked ND6 14484T > C mutation. J Biomed Sci 30, 63 (2023). https://doi.org/10.1186/s12929-023-00951-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12929-023-00951-1