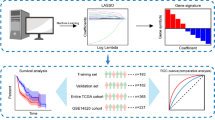
Abstract
Objective
Hepatocellular carcinoma (HCC) immunotherapy is a focus of current research. We established a model that can effectively predict the prognosis and efficacy of HCC immunotherapy by analyzing the immune genes of HCC.
Methods
Through the data mining of hepatocellular carcinoma in The Cancer Genome Atlas (TCGA), the immune genes with differences in tumor and normal tissues are screened, and then the univariate regression analysis is carried out to screen the immune genes with differences related to prognosis. The prognosis model of immune related genes is constructed by using the minimum absolute contraction and selection operator (lasso) Cox regression model in the TCGA training set data, The risk score of each sample was calculated, and the survival was compared with the Kaplan Meier curve and the receiver operating characteristic (ROC) curve to evaluate the predictive ability. Data sets from ICGC and TCGA were used to verify the reliability of signatures. The correlation between clinicopathological features, immune infiltration, immune escape and risk score was analyzed.
Results
Seven immune genes were finally determined as the prognostic model of liver cancer. According to these 7 genes, the samples were divided into the high and low risk groups, and the results suggested that the high-risk group had a poorer prognosis, lower risk of immune escape, and better immunotherapy effect. In addition, the expression of TP53 and MSI was positively correlated in the high-risk group. Consensus clustering was performed to identify two main molecular subtypes (named clusters 1 and 2) based on the signature. It was found that compared with cluster 1, better survival outcome was observed in cluster 2.
Conclusion
Signature construction and molecular subtype identification of immune-related genes could be used to predict the prognosis of HCC, which may provide a specific reference for the development of novel biomarkers for HCC immunotherapy.
Similar content being viewed by others
Introduction
Hepatocellular carcinoma (HCC) is currently the sixth most common tumor in the world and the fourth most common in terms of mortality [1]. HCC is expected to become the third leading cause of cancer-related deaths by 2030, according to epidemiology [2]. Despite known risk factors for HCC, including hepatitis B, alcoholism and cirrhosis, the incidence of HCC remains high worldwide. At present, there are many ways to treat hepatocellular carcinoma, among which surgical resection is the main method, and intervention, targeted drugs and immunotherapy are also important treatment methods. However, the therapeutic effect of hepatocellular carcinoma is still poor, and its recurrence rate and mortality rate have not been effectively controlled [3]. Only 30% of HCC patients are reported to be in the early stages suitable for radical surgery. In addition, the efficacy of chemotherapeutic agents and targeted agents for advanced HCC is still limited [4].
Although currently targeted drug therapy for unresectable HCC has been proven to be effective [5]. Such as sorafenib, lovastinib and the vascular endothelial growth factor (VEGF) inhibitor ramuzumab are widely used in clinical applications [6,7,8]. However, we found that all of these drugs had drug reactions of varying degrees, such as skin itching, gastrointestinal reactions, and elevated blood pressure [9]. In addition, long-term use of the body is easy to produce drug resistance, its treatment effect is not as expected [10]. In recent years, new therapeutic strategies such as tumor immunosuppressive therapy have extended patients' lives, and the combination of immune checkpoint inhibitors (ICIs) and VEGF inhibitors is currently positioned as the first-line treatment for advanced HCC.
Immunotherapy has opened a new era of tumor therapy, and immunocheckpoint inhibitors (ICIs), including programmed cell death 1(PD-1)/programmed cell death ligand 1(PD-L1) inhibitors, have become a breakthrough in tumor therapy. ICIs are typical immunotherapies that activate anti-tumor immunity by inhibiting negative regulatory receptors such as PD-1 and cytotoxic T lymphocyte antigen 4(CTLA4) [11].
At present, immunotherapy for hepatocellular carcinoma has received more and more attention. The occurrence and development of hepatocellular carcinoma is closely related to tumor microenvironment [12]. Tumor microenvironment is a dynamic system composed of tumor cells, complex cytokine environment, extracellular matrix and immune cell subsets [13].
In this study, we constructed and validated an immune-related prognostic model based on the TCGA-LIHC dataset and ICGC-LIRI-JP dataset. In addition, we explored the relationship between the constructed prognostic model and the clinical and pathological features of HCC patients. We analyzed the characteristics of tumor immune microenvironment, including tumor-infiltrating cell composition, immune escape, TP53 mutation rate, and tumor microenvironment. These findings may provide new insights into novel therapeutic targets for hepatocellular carcinoma.
Materials and methods
Data preparation and processing
From TCGA (https://portal.gdc.cancer.gov/) download the mRNA expression data and clinical data of LIHC. We obtained the data of 374 tumor specimens and 50 normal specimens. Excluding HCC samples with a survival time of less than 30 days, we finally obtained the data of 342 HCC patients. 342 TCGA tumor samples were randomly divided into two equal parts: training set (Set1) and verification set (set2). The total samples of TCGA were used as another verification set (set3). From ICGC (https://dcc.icgc.org/) download the data of the Japan Institute of liver cancer (ICGC-LIRI-JP), exclude patients with metastatic liver cancer and survival of less than 30 days, and finally 229 patients with HCC were included in the study. These sample data were used as an external validation set (Set4).
From the Gene List module of the Immunology Database and Analysis Portal (ImmPort) database, we downloaded complete gene names directly, totaling 1793 immune-related genes.
Differential expression analysis and prognostic gene screening
Based on the data of LIHC in TCGA, we analyzed the mRNA expression differences between 374 tumor samples and 50 normal samples. The "Limma" R package was used to screen out differential genes (DEGs) according to adjust P < 0.01 and |logFC|> 2. The differentially expressed immune genes (DEIGs) were obtained by the intersection of immune-related genes and DEGs of TCGA. Then DEIGs were obtained by univariate Cox analysis to explore the relationship between overall survival (OS) and gene expression level. When P value < 0.05, genes were considered to have significant prognostic potential.
Copy number variation and functional enrichment analysis
The "RCircos" R package was used to show the mutation locations of these prognostic DEIGs on 23 chromosomes. The gain or loss of these genes were visualized. The "org.hs.eg.db", "Enrichment plot" and "clusterProfiler" packages in R were used to analyze GO and KEGG enrichment of prognostic DEIGs in TCGA to explore potential molecular mechanisms and biological functions [14,15,16].
Establishment of immune risk scoring signature (irss) for prognosis
In the TCGA train set (set1), LASSO regression was adopted to process prognostic DEIGs to further identify differentially expressed genes with independent prognostic value. Multivariate cox regression analysis was then used to evaluate whether these genes could be used as independent prognostic predictors and finally determine the genes to construct the model. Next, the following formula was used to calculate the risk score for each patient:
The sample was divided into high-risk and low-risk groups based on the median value of the risk score.
Expression validation of immune-related signature genes
After obtaining informed consent from patients, we collected 20 pairs of HCC tissues and paraneoplastic tissues (from the Second Affiliated Hospital of Nanchang University), then cultured one normal hepatocyte line (7702) and four HCC cell lines (LM3, 97H, HepG2 and 7721), and after extracting RNA from the tissues and cells, q-PCR experiments were performed to verify the signature genes. The primer sequences of the signature genes are shown in Table 1.
Immunohistochemistry (IHC) experiments was used to detect protein expression differences, Relative optical density scores were used to compare the differences between the two groups.
Validation of the risk score with TCGA and ICGC datasets
According to the established IRSS scoring system, the risk score of each sample was calculated. Set1, set2 and set3 groups were divided into high risk group and low risk group respectively according to the median value of risk score. Then ROC curve and Kaplan–Meier curve were drawn to verify the prediction accuracy of the risk scoring model. Nomogram was used to assess the survival risk of HCC patients in TCGA, including gender, age, TNM stage, IRSS and other clinical information. Calibration curves (1, 2 and 3 years) were drawn to assess the accuracy of Nomogram predictions. To further verify the accuracy of this model, we compared it with published prediction models of HCC.
Genomic alterations analyses
To determine whether risk score levels were associated with specific genomic traits. Copy number variation (CNV) analysis was performed using the TCGA dataset.
Association between Microsatellite Instability (MSI) and constructed predictive models
The whole TCGA dataset was further analyzed based on the constructed prognostic model after removing the samples without microsatellite status information. Then, according to the microsatellite status information extracted from the phenotypic data, we first compared whether there was any difference in the expression level of MSI in the high and low risk groups, and then divided the total samples into MSI-high and MSI-low groups through the expression level of MSI in each sample, and compared whether there was a significant difference in OS between the two groups. Thus, the relationship between high and low risk groups and OS can be further determined.
Analysis of Tumor Mutation Burden (TMB) and immune escape
To further verify the relationship between the constructed model and tumor microenvironment and immunotherapy, correlation boxplot was constructed by Pearson correlation analysis to study the impact of risk score on TMB. TIDE scores were compared between the high and low risk groups to analyze whether there was a difference in the efficacy of immune checkpoint blocking treatment between the high and low risk groups.
Consensus clustering of prognostic genes
To investigate the functions of the seven selected prognostic DEIGs, we clustered the HCC into different groups with “ConsensusClusterPlus” (50 iterations, resample rate 80% and Pearson correlation). Kaplan–Meier survival curve was used to analyze the OS of each subtype, and the clinical data and gene expression levels between subgroups were compared and analyzed, as well as the immune cell infiltration and immune cell content between subgroups.
Statistical analysis
The data were analyzed by Excel software, and the visualization of DEGs was completed by "ggplot2", "Cairo" and "ggrepel" R packages. The difference in overall survival was calculated by Kaplan–Meier method, and the significant difference was determined by R. Spearman regression analysis by Cox. ROC curve of R package survival was drawn and visualized to calculate AUC [17]. Somatic mutation and CNV data were downloaded from TCGA database. Copy number changes associated with risk scores were analyzed using GISTIC 2. All data and statistical analysis were based on R software 4.1.2, and P < 0.05 was considered statistically significant.
Results
Prognostic DEIGs screening
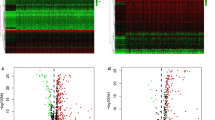
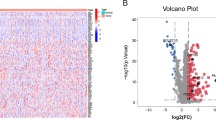
The overall process of the study is shown in Fig. 1. We first analyzed the differences between 374 tumor samples and 50 normal samples in TCGA. According to adjust P < 0.01 and |logFC|> 2, 2874 differential genes were screened out (Table S1), including 362 down-regulated genes and 2512 up-regulated genes. Visualization using heat and volcano maps (Fig. 2A, B). Then, 154 differential expression immune genes (DEIGs) were obtained through the intersection of immune-related genes (Fig. 2C). Univariate Cox proportional risk analysis was performed on DEIGs, and 28 DEIGs related to prognosis were obtained (Fig. 2D, Table S2).
Prognostic DEIGs Functional Enrichment and Genetic Alterations
GO and KEGG enrichment analysis was performed on 28 prognostic DEIGs. GO enrichment results showed that prognostic DEIGs were mainly enriched in Epithelial cell proliferation, gland development, regulation of cysteine-type endopeptidase activity, regulation of cysteine-type endopeptidase activity involved in apoptotic process, regulation of endopeptidase activity (Fig. 3A). KEGG results showed that prognostic DEIGs were enriched in Bladder cancer, Cytokine-cytokine receptor interaction, IL-17 signaling pathway, Melanoma, Neuroactive ligand-receptor interaction (Fig. 3B).
GO, KEGG function enrichment and CNV variation of prognosis-related DEIGs. (A) GO function enrichment of 28 prognosis-related DEIGs. (B) KEGG pathway enrichment of 28 prognosis-related DEIGs. (C) The location of CNV alteration of 28 prognosis-related DEIGs. (D) The CNV variation frequency of 28 prognosis-related DEIGs in LIHC
We also studied the copy number variation of 28 prognostic genes and summarized the CNV variation frequency of 28 prognostic DEIGs in TCGA-LIHC (Fig. 3C). Visualized the position of CNV variation on chromosomes (Fig. 3D).
Construction and prognostic value of IRSS
In order to calculate the risk score, patients with no survival information and survival time less than 30 days were excluded. Finally, 342 HCC patients were included in the study, and the total sample set (Sum) was randomly divided into the train set and the test set, with 171 patients in each group. Chi-square test was used to determine that there was no statistically significant difference in clinical characteristics among each group (Table 2). LASSO regression analysis was performed on 28 prognostic DEIGs in a training set containing 171 patients, and the model fitted best when the penalty index was 10 (Fig. 4A, B). Then, 7 prognostic genes were obtained through multivariate Cox regression analysis: GAL, NR0B1, MAPT, CCR3, GLP1R, BIRC5 and IL-17B (Table S3). Combined with the corresponding regression coefficients, the final IRSS is established:
Identification of seven optimal prognosis-related DEIGs. establishment of IRSS. (A) Ten-time cross-validation for tuning parameter selection in the lasso model. (B) lasso coefficient profiles. (C) The expression of 7 genes in TCGA train set. (D) Kaplan–Meier survival plots of high-risk and low-risk groups in TCGA train set. (E) The risk score and survival status of seven genes in patients. (F) Receiver operating characteristic curve (ROC) of risk score in the TCGA train set
The mRNA expression differences of seven genes in the train group were represented by heat maps (Fig. 4C). The risk score for each patient was calculated based on IRSS, and the sample was divided into high-risk and low-risk groups using the median. In the train set, the probability of OS was lower in the high-risk group than in the low-risk group (P < 0.05) (Fig. 4D). As the risk score increased, the survival time decreased and the number of patients in the state of death increased gradually (Fig. 4E). The accuracy of the model in predicting OS of HCC patients was evaluated by ROC curve, and the AUC values at 1, 2 and 3 years were 0.845, 0.823 and 0.808, respectively (Fig. 4F). The mRNA expression differences of 7 prognostic DEIGs in tumor and normal tissues in the TCGA data set (Figure S1).
Expression validation of Immune-related signature genes
The expression of the signature genes in cells (normal liver cells vs HCC cells) and tissues (HCC tissues vs paraneoplastic tissues) were verified by q-PCR assay (Fig. 5A-N). and we showed representative images of 7 genes, and then compared the expression differences between HCC tissues and paraneoplastic tissues using relative optical density scores (Fig. 6).
Validation of differential expression of 7 signature genes in cells and tissues by q-PCR. (A-G) Differential expression of 7 signature genes in 7702, LM3, 97H, HepG2 and 7721 cells. The results showed that the expression of BIRC5, CCR3, GAL, GLP1R, IL17B, MAPT and NR0B1 in HCC cells was significantly higher than that in normal liver cells. (H-N) Differential expression of 7 signature genes in HCC tissue and paraneoplastic tissue, the expression of BIRC5, CCR3, GAL, GLP1R, IL17B, MAPT and NR0B1 were significantly higher in HCC tissues
Validation of The Risk Score with TCGA and ICGC Datasets
In the test set, according to the risk score calculated by IRSS, the samples were divided into high and low risk groups using the median value. The analysis found that there was a significant difference in survival probability between the high and low risk groups (P < 0.05), and the high-risk group had a lower survival rate (Fig. 7A). The AUC values assessed by ROC curve at 1, 2 and 3 years were 0.748, 0.740 and 0.684, respectively (Fig. 7B). The mRNA expression differences of prognostic genes in the test set were represented by heat maps (Figure S2A).
Risk score validation. (A) Kaplan–Meier survival plots in TCGA test set. (B) ROC of risk score in the TCGA test set. (C) Kaplan–Meier survival plots in TCGA sum set. (D) ROC of risk score in the TCGA sum set. (E) Kaplan–Meier survival plots in ICGC validation set. (F) ROC of risk score in the ICGC validation set
In TCGA sum set, the total sample is also divided into high and low risk groups according to IRSS calculated risk score. Analysis showed that there was a significant difference in overall survival between the two groups (P < 0.05), and the survival rate was lower in the high-risk group (Fig. 7C). The AUC values of 1, 2 and 3 years were 0.798, 0.756 and 0.706, respectively (Fig. 7D). The mRNA expression differences of prognostic genes in TCGA sets were represented by heat maps (Figure S2B).
According to IRSS, ICGC data were divided into high risk group and low risk group, and the survival rate difference between the two groups was also statistically significant (P < 0.05) (Fig. 7E). The AUC values of 1, 2 and 3 years were 0.757, 0.759 and 0.772, respectively (Fig. 7F). After multiple validation, we find that the model has high robustness and accuracy. The mRNA expression differences of prognostic genes in the ICGC dataset were represented by heat maps (Figure S2C). Meanwhile, in order to further verify the reliability of the model, we compared it with four published prediction models of HCC [18,19,20,21,25].
Currently, systemic therapies including immune checkpoint inhibitors (ICIs), tyrosine kinase inhibitors (TKI), and monoclonal antibodies challenge the use of conventional therapies for HCC. Tumors often upregulate immune checkpoints to avoid detection and killing by the host immune system. Activation of checkpoint cascades, such as those controlled by PD-1 or CTLA-4, leads to tumor-specific T cell inactivation and immune evasion [26, 27]. Treatment with anti-PD-1, anti-PD-L1 or anti-CTLA-4 revitalizes T cells and allows the adaptive immune system to target tumor cells [28, 29]. Inhibitors of PD1, PDL1, and CTLA-4 are pillars of clinical practice or systemic therapies under development for HCC. Data from the CheckMate-040 Phase I/II trial, presented at the American Society of Clinical Oncology Annual Meeting 2019, The combination of Opdivo (PD-1 Antibody) and Yervoy (CTLA-4 Antibody) yielded an objective response rate of 31% and a response time of 17.5 months [30]. This result suggests that immunotherapy has a potential and surprising effect on HCC.
Tumor microenvironment (TME) of HCC refers to a complex and spatially structured mixture of liver parenchymal resident cells, tumor cells, immune cells and tumor-associated fibroblasts [31]. These populations interact dynamically through intercellular contact and the release or recognition of cytokines and other soluble factors. This complex cellular interaction has a significant impact on tumor immune evasion. TMB is often used as a proxy for the number of neoantigens because the probability of recognizing neoantigen-specific T lymphocytes is associated with TMB [32]. The frequency of these genetic changes for each type varies greatly between individual tumors and between tumor types [33]. TMB can be used to predict Immune checkpoint blockade (ICB) efficacy and has become a useful biomarker for multiple cancer types to identify patients who will benefit from immunotherapy [34].
GAL, NR0B1, MAPT, CCR3, GLP1R, BIRC5 and IL17B were the Prognostic DEIGs included in the prognostic signature. which can preliminarily predict the prognosis and immunotherapy effect of HCC patients by analyzing their relationship with tumor microenvironment, immune invasion and immunotherapy. It is helpful for the systemic treatment of HCC. Anti-GAL antibodies have been confirmed to play a role in the immunotherapy of pancreatic cancer [35]. NR0B1 has also been confirmed to be related to the occurrence and development of a variety of tumors, for example, the transformed phenotype of Ewing's sarcoma requires sustained NR0B1 expression [36]. Knockdown of NR0B1 can reduce the tumorigenic and anti-apoptotic potential of lung adenocarcinoma [37]. MAPT is a microtubule-related protein tau, which can inhibit the function of taxanes, and its high expression reduces the sensitivity to taxanes, which is of great significance in breast cancer research [38]. Studies have also confirmed that MAPT is often methylated, and hypermethylation is associated with poor prognosis in patients with stage II colorectal cancer [39]. Both CCR3 and IL17B have been found to be related to the role of tumor microenvironment in regulating tumor growth and metastasis, and may be new immunotherapy targets [40, 41]. Previous studies have shown that glucagon-like peptide-1 receptor (GLP1R) is essential for the regulation of glucose homeostasis, and in recent years, it has been found to be related to the occurrence and development of tumors [42, 43]. BIRC5 has been confirmed to be related to the occurrence of a variety of tumors, and its role in the progression of liver cancer has also been confirmed by studies [44]. However, there are few studies on its immunity to liver cancer, which is worth exploring.
In addition, by exploring tumor immune microenvironment and gene mutations, our study found that mutation rates of MSI and TP53 may also be independent prognostic indicators. The higher the risk score, the lower the survival rate. However, patients also had a lower immune escape frequency fraction, suggesting that the high-risk group may have a better effect on immunotherapy. The expression levels of PD-1 and CTLA-4 were higher in the high-risk group. Therefore, the treatment effect of anti-PD-1 and anti-CTLA-4 is better, which is expected to improve the prognosis of patients. Up to now, there are many prognostic models for HCC, but due to the complexity of HCC, no one model has been considered as the gold standard. Our study focused on the prognosis and immunoassay of hepatocellular carcinoma, combined with the current hot spot of immunotherapy, aiming to find effective immunotherapy targets for hepatocellular carcinoma. The disadvantages of this study are as follows: The clinical data in TCGA and ICGC databases did not specify in detail whether patients had received chemotherapy or radiotherapy, which may have certain influence on patient survival data and immunoassay results.
Conclusion
In summary, a robust immune-related prognosis model was constructed and tumor microenvironment and immune function were analyzed, providing potential targets for immunotherapy of HCC.
Availability of data and materials
The dataset supporting the conclusions of this article is included within the article and its supplementary file. TCGA-LIHC is available at https://portal.gdc.cancer.gov/; ICGC-LIRI-JP is available at https://dcc.icgc.org/releases/current/Projects/LINC-JP.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913–21.
McGlynn KA, Petrick JL, London WT. Global epidemiology of hepatocellular carcinoma: an emphasis on demographic and regional variability. Clin Liver Dis. 2015;19(2):223–38.
Xu F, ** T, Zhu Y, Dai C. Immune checkpoint therapy in liver cancer. J Exp Clin Cancer Res. 2018;37(1):110.
Ricke J, Klümpen HJ, Amthauer H, Bargellini I, Bartenstein P, de Toni EN, et al. Impact of combined selective internal radiation therapy and sorafenib on survival in advanced hepatocellular carcinoma. J Hepatol. 2019;71(6):1164–74.
Bruix J, Qin S, Merle P, Granito A, Huang Y-H, Bodoky G, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56–66.
Kudo M, Finn RS, Qin S, Han K-H, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–73.
Hinshaw DC, Shevde LA. The Tumor Microenvironment Innately Modulates Cancer Progression. Cancer Res. 2019;79(18):4557–66.
Greten TF, Lai CW, Li G, Staveley-O’Carroll KF. Targeted and Immune-Based Therapies for Hepatocellular Carcinoma. Gastroenterology. 2019;156(2):510–24.
Huang A, Yang X-R, Chung W-Y, Dennison AR, Zhou J. Targeted therapy for hepatocellular carcinoma. Signal Transduct Target Ther. 2020;5(1):146.
Tolba MF. Revolutionizing the landscape of colorectal cancer treatment: The potential role of immune checkpoint inhibitors. Int J Cancer. 2020;147(11):2996–3006.
Jiang Y, Han Q-J, Zhang J. Hepatocellular carcinoma: Mechanisms of progression and immunotherapy. World J Gastroenterol. 2019;25(25):3151–67.
Chew V, Lai L, Pan L, Lim CJ, Li J, Ong R, et al. Delineation of an immunosuppressive gradient in hepatocellular carcinoma using high-dimensional proteomic and transcriptomic analyses. Proc Natl Acad Sci U S A. 2017;114(29):E5900–9.
Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28(1):27–30.
Kanehisa M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 2019;28(11):1947–51.
Kanehisa M, Furumichi M, Sato Y, Ishiguro-Watanabe M, Tanabe M. KEGG: integrating viruses and cellular organisms. Nucleic Acids Res. 2021;49(D1):D545–51.
Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez J-C, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77.
Gu X, Guan J, Xu J, Zheng Q, Chen C, Yang Q, et al. Model based on five tumour immune microenvironment-related genes for predicting hepatocellular carcinoma immunotherapy outcomes. J Transl Med. 2021;19(1):26.
Ju M, Jiang L, Wei Q, Yu L, Chen L, Wang Y, et al. A Immune-Related Signature Associated with TME Can Serve as a Potential Biomarker for Survival and Sorafenib Resistance in Liver Cancer. Onco Targets Ther. 2021;14:5065–83.
Peng Y, Liu C, Li M, Li W, Zhang M, Jiang X, et al. Identification of a prognostic and therapeutic immune signature associated with hepatocellular carcinoma. Cancer Cell Int. 2021;21(1):98.
Wang Z, Pan L, Guo D, Luo X, Tang J, Yang W, et al. A novel five-gene signature predicts overall survival of patients with hepatocellular carcinoma. Cancer Med. 2021;10(11):3808–21.
**e J, Chen L, Sun Q, Li H, Wei W, Wu D, et al. An immune subtype-related prognostic signature of hepatocellular carcinoma based on single-cell sequencing analysis. Aging (Albany NY). 2022;14(7):3276–92.
Olivier M, Hollstein M, Hainaut P. TP53 mutations in human cancers: origins, consequences, and clinical use. Cold Spring Harb Perspect Biol. 2010;2(1):a001008.
Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42(5):1208–36.
Wu Y, Liu Z, Xu X. Molecular subty** of hepatocellular carcinoma: A step toward precision medicine. Cancer Commun (Lond). 2020;40(12):681–93.
Brown KE, Freeman GJ, Wherry EJ, Sharpe AH. Role of PD-1 in regulating acute infections. Curr Opin Immunol. 2010;22(3):397–401.
Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99(19):12293–7.
Li X, Shao C, Shi Y, Han W. Lessons learned from the blockade of immune checkpoints in cancer immunotherapy. J Hematol Oncol. 2018;11(1):31.
Rowshanravan B, Halliday N, Sansom DM. CTLA-4: a moving target in immunotherapy. Blood. 2018;131(1):58–67.
Del Pozo MY. 2019 ASCO Annual Meeting. Lancet Oncol. 2019;20(7):909–10.
Nakano S, Eso Y, Okada H, Takai A, Takahashi K, Seno H. Recent Advances in Immunotherapy for Hepatocellular Carcinoma. Cancers (Basel). 2020;12(4):775.
Chan TA, Yarchoan M, Jaffee E, Swanton C, Quezada SA, Stenzinger A, et al. Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann Oncol. 2019;30(1):44–56.
Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017;9(1):34.
Sugawara S, Lee JS, Kang JH, Kim HR, Inui N, Hida T, et al. Nivolumab with carboplatin, paclitaxel, and bevacizumab for first-line treatment of advanced nonsquamous non-small-cell lung cancer. Ann Oncol. 2021;32(9):1137–47.
Tanemura M, Miyoshi E, Nagano H, Eguchi H, Taniyama K, Kamiike W, et al. Role of α-gal epitope/anti-Gal antibody reaction in immunotherapy and its clinical application in pancreatic cancer. Cancer Sci. 2013;104(3):282–90.
Kinsey M, Smith R, Lessnick SL. NR0B1 is required for the oncogenic phenotype mediated by EWS/FLI in Ewing’s sarcoma. Mol Cancer Res. 2006;4(11):851–9.
Susaki Y, Inoue M, Minami M, Sawabata N, Shintani Y, Nakagiri T, et al. Inhibitory effect of PPARγ on NR0B1 in tumorigenesis of lung adenocarcinoma. Int J Oncol. 2012;41(4):1278–84.
Ikeda H, Taira N, Hara F, Fujita T, Yamamoto H, Soh J, et al. The estrogen receptor influences microtubule-associated protein tau (MAPT) expression and the selective estrogen receptor inhibitor fulvestrant downregulates MAPT and increases the sensitivity to taxane in breast cancer cells. Breast Cancer Res. 2010;12(3):R43.
Wang C, Liu Y, Guo W, Zhu X, Ahuja N, Fu T. promoter CpG island hypermethylation is associated with poor prognosis in patients with stage II colorectal cancer. Cancer Manag Res. 2019;11:7337–43.
Liu F, Wu H. CC Chemokine Receptors in Lung Adenocarcinoma: The Inflammation-Related Prognostic Biomarkers and Immunotherapeutic Targets. J Inflamm Res. 2021;14:267–85.
Fabre J, Giustiniani J, Garbar C, et al. Targeting the Tumor Microenvironment: The Protumor Effects of IL-17 Related to Cancer Type. Int J Mol Sci. 2016;17(9):1433.
Kanda R, Hiraike H, Wada-Hiraike O, Ichinose T, Nagasaka K, Sasajima Y, et al. Expression of the glucagon-like peptide-1 receptor and its role in regulating autophagy in endometrial cancer. BMC Cancer. 2018;18(1):657.
He L, Zhang S, Zhang X, Liu R, Guan H, Zhang H. Effects of insulin analogs and glucagon-like peptide-1 receptor agonists on proliferation and cellular energy metabolism in papillary thyroid cancer. Onco Targets Ther. 2017;10:5621–31.
Zhang M, Yan X, Wen P, Bai W, Zhang Q. CircANKRD52 Promotes the Tumorigenesis of Hepatocellular Carcinoma by Sponging miR-497-5p and Upregulating BIRC5 Expression. Cell Transplant. 2021;30:9636897211008874.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science Foundation of China (81760439).
Author information
Authors and Affiliations
Contributions
Liang Sun, Zhengyi Wu and Cairong Dong have contributed equally to this work. LS and ZW conceived this research, performed the data analysis and experimental verification, and wrote the manuscript. CD, HH and ZC performed bioinformatics analyses and assisted with analyzing other data. ZW and SY assisted in collecting tumor samples, XY helped to revise the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the Second Affiliated Hospital of Nanchang University. No personal information is involved, so informed consent is not required.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Table S1. Difference analysis between normal and tumor groups of LIHC.
Additional file 2:
Table S2. Univariate cox regression for DEIGs.
Additional file 3:
Table S3. The multivariate Cox regression analysis.
Additional file 4:
Figure S1. The expression of seven IRSS genes between normal and tumor.
Additional file 5:
Figure S2. Heat map results of seven IRSS genes expression levels.The expression of seven IRSS genes in the TCGA test set.The expression of seven IRSS genes in the TCGA sum set.The expression of seven IRSS genes in the ICGC validation set.
Additional file 6:
Figure S3. The differences in risk scores across clinical features.Age.Gender.Grade.Stage.T stage.N stage.M stage.
Additional file 7:
Figure S4. Identification of consensus clusters by prognostic genes.Consensus cluster matrix for k=2 in TCGA dataset.Relative change in area under CDF curve for k=2 to 10 in TCGA dataset.Consensus clustering cumulative distribution functionfor k=2 to 10 in TCGA dataset.Consensus cluster matrix for k=2 in ICGC dataset.Relative change in area under CDF curve for k=2 to 10 in ICGC dataset.Consensus clustering cumulative distribution functionfor k=2 to 10 in ICGC dataset.Contingency table showing the consistency between clustered groups and risk groups in TCGA.Contingency table showing the consistency between clustered groups and risk groups in ICGC.
Additional file 8:
Figure S5. Clustering analyses in TCGA and ICGC.PCA results for two groups of patients in TCGA.ggalluvial of two clusters in TCGA displayed the correlation between clusters, risk, and survival status.PCA results for two groups of patients in ICGC.ggalluvial of two clusters in ICGC displayed the correlation between clusters, risk, and survival status.
Additional file 9:
Figure S6. Correlation between immune cells and seven genes in TCGA. MAPT and NROB1 have no Significant correlation wirh immune cells in TCGA.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sun, L., Wu, Z., Dong, C. et al. Signature construction and molecular subtype identification based on immune-related genes for better prediction of prognosis in hepatocellular carcinoma. BMC Med Genomics 16, 130 (2023). https://doi.org/10.1186/s12920-023-01558-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12920-023-01558-z