Abstract
Background
Intrahepatic cholangiocarcinoma (ICC) is a malignant tumor, which poses a serious threat to human health. Histone 3 lysine 9 trimethylation (H3K9me3) is a post-translational modification involved in regulating a broad range of biological processes and has been considered as potential therapeutic target in types of cancer. However, there is limited research on investigating profiles of histone modification H3K9me3 in ICC patients.
Methods
In this study, we applied the ChIP-seq technique to investigate the effect of H3K9me3 on ICC. Anti-H3K9me3 antibody was used for ChIP-seq in ICC (RBE cell lines) and HIBEpic (normal cell lines). MACS2 (peak-calling tools) was then used to identify the peaks recorded in RBE and HIBEpic cell lines. Gene expression, mutation and clinical data were downloaded from TCGA and cBioPortal databases.
Results
H3K9me3 exhibited abnormal methylation and influenced the process of abnormal gene expression in patients suffering from ICC. The Wnt/β-Catenin signaling pathway (also known as simply the WNT signaling pathway) was enriched in H3K9me3-regulated genes.
Conclusions
We are the first to report that H3K9me3 may play an important role in the progression of ICC. It promotes the understanding of epigenetic molecular mechanisms for ICC.
Similar content being viewed by others
Background
Cholangiocarcinoma (CCA) is a life-threatening malignancy. Based on the anatomical location, CCA can be classified as intrahepatic cholangiocarcinoma (ICC), perihilar cholangiocarcinoma (PCCA), or distal cholangiocarcinoma (DCCA). The incidence and case-fatality rates are increasing worldwide [1,2,3]. ICC is one of the most common malignant tumor arising from the liver, and it makes up about 10% of all CCA. The poor outcome can be attributed to the insidious and aggressive nature of ICC[4, 5]. Effective treatment methods are urgently needed to be developed [6]. It is believed that the development and progression of ICC are multifactorial and multistep pathological processes involving oncogene activation, inactivation of tumor suppressor genes, tumor metastasis, apoptosis and cell cycle dysregulation, and tumor genetics and epigenetic alterations [3]. It has been reported that multi-omics data analysis was performed and the results revealed that most of the genetic variations observed in patients suffering from ICC could be attributed to copy number variations (CNV) [7, 21]. Integrative Genomics Viewer (IGV) was used to visualize the peak abundance tracks [22]. The raw data obtained using the ChIP-seq technique has been deposited in the Sequence Read Archive (SRA) database (https://www.ncbi.nlm.nih.gov/sra) under the accession number SRP333541 (https://www.ncbi.nlm.nih.gov/Traces/study/?acc=SRP333541). To validate our results, other RNA-Seq data for cholangiocarcinoma cell lines were downloaded from the SRA database https://www.ncbi.nlm.nih.gov/Traces/study/?acc=SRP229534&o=acc_s%3Aa).
TCGA download and analysis
Data on gene expression (level 3), mutation, and clinical data of patients were downloaded from the TCGA (https://tcga-data.nci.nih.gov/) and cBioPortal (https://www.cbioportal.org/) databases [23]. EdgeR (an R package) was used to identify various expressed genes (The significant differently expressed genes was defined as the ones with Benjamini–Hochberg FDR < 0.01 based on all the tests, and |log2(Fold change)|> 2)[24]. The clinical data analysis method was used to analyze the data obtained from cholangiocarcinoma patients. R v3.5.1 was used to perform the analyses. We used the survival data of patients from TCGA database and the overall Kaplan–Meier (KM) survival analysis in each subtype was performed using the “survfit” and “survdiff” functions in the “survival” package [25]. We used GeneMANIA (http://genemania.org) to generate hypotheses on gene function and gene intersection for functional assays [26].
Results
Difference in the histone H3K9me3 modifications observed in cholangiocarcinoma and normal cell lines
We used the ChIP-seq data to analyze histone H3K9me3 present in the RBE and control cell lines. The results revealed that the ChIP peaks of histone H3K9me3 modifications in the ICC cell lines was lesser than that in normal cell lines (Fig. 1A). We also found the number of H3K9me3 peaks of the ICC cell line (RBE) was lesser than that in the normal cell line in a genome-wide scale (Fig. 1B). We annotated the location of identified peaks corresponding to H3K9me3 in the ICC and normal cell lines (Fig. 1C). The number of peaks recorded for RBE was higher by 22,280 when compared to the number of peaks recorded for the normal cell lines (Fig. 1D), suggesting the total number of H3K9me3 peaks decreased in ICC. To further verify our analysis results, we tested H3K9me3 by western blotting to recognize H3K9me3 states, and found the overall H3K9me3 level decreased in ICC patients (Figs. 1E, F, Additional file 2: Fig. S1).
H3K9me3 profiles in RBE and HIBEpic cells. A Heatmaps depicting H3K9me3 ChIP-seq peaks enriched at regions spanning TSS sites (± 2 kb) in RBE cells (Right) and HIBEpic cell (Left). B Tracks displaying the read coverage of H3K9me3 ChIP-seq normalized by inputs at a whole-genome scale. C Distribution of H3K9me3 peaks recorded for RBE and HIBEpic cells. D Different H3K9me3 peaks recorded for RBE and HIBEpic represented as Venn diagrams. E, F H3K9me3 level validated by western blot
Analysis of the data obtained from the TCGA database by studying the aberrant expression of cholangiocarcinoma genes
To identify H3K9me3-regulated genes, we identified 5628 differentially expressed genes (Benjamini–Hochberg FDR < 0.01 based on all the tests, and |log2(Fold change)|> 2) by analyzing the RNA-seq data of ICC in TCGA database (Fig. 2A). We observed that most of the differentially expressed genes were highly expressed in ICC tissue. DNA copy number gains occurred more frequently than copy number losses (Fig. 2B, C). These results agreed well with previously reported results that DNA copy number gains occurred more frequently than copy number losses [27]. We analyzed the intersection of the differentially expressed genes and the H3K9me3 histone modifications (specifically absent in the ICC cells) (Fig. 2D). We observed that 886 differentially expressed genes may be potentially regulated by H3K9me3 in ICC (Fig. 2D).
Different expressed genes in ICC. A Heatmap and hierarchical clustering tree of differentially expressed genes in CHOL. Rows indicate genes showing significant differences in expression between the normal and cancer tissue of CHOL. B Oncoprint showing copy number variation in CHOL. C Oncoprint showing the genetic alterations in CHOL. D Overlap of H3K9me3 and differentially expressed genes represented as Venn diagrams. E–F western blotting with antibodies validated to recognize H3K9me3 states
The relationship of H3K9me3 site and differentially expressed genes in cholangiocarcinoma (CHOL)
We further explored the biological functions of these 886 differentially expressed genes regulated by H3K9me3. We used gene oncology (GO) analysis and KEGG analysis methods to analyze these differentially expressed genes that were regulated by H3K9me3[28]. We found these overlapped genes were enriched in the WNT pathway, Complement and coagulation cascades, Insulin secretion, Calcium signaling pathway, Axon guidance, PI3K-AKT signaling pathway (Fig. 3A). Therefore, we assumed that WNT pathway is involved in H3K9me3 regulated genes in cholangiocarcinoma. These overlapped genes were enriched in cellular component morphogenesis, regulation of ion transport and trans-synaptic signaling (Fig. 3B). These enriched GO terms are highly related with WNT pathway.
Clinical features of differentially expressed genes regulated by H3K9me3 in the WNT pathway
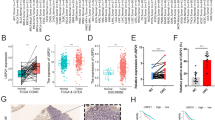
To further investigate the association between WNT pathway and H3K9me3-regulated genes. We constructed a network from the differentially expressed H3K9me3-regulated genes that functioned via the WNT pathway by analyzing the data presented in GeneMANIA (Fig. 4A). We identified the core genes (WNT2B and WNT10A) presented in the network. As shown in Fig. 4A, we displayed the WNT-related genes changed in H3K9me3 and gene expression. We observed that the extent of H3K9me3 in WNT2B and WNT10A of the RBE cell line was higher than that in the HIBEpic cell line (Fig. 4B, C). The expression levels of WNT2B and WNT10A in ICC tissue were higher than that in normal tissue (Fig. 4D, E), it is consistent with the result of the extent of H3K9me3 in WNT2B and WNT10A of the RBE cell line. Then, we analyzed the impact of the core gene WNT2B on the prognosis of patients. WNT2B gene also had an effect on the prognosis of ICC patients from TCGA (Fig. 4F).
Role of WNT pathway in ICC A Gene–gene interaction network among the WNT pathway members. Each node represents a gene, and the inter-node connection lines represent the types of gene–gene interactions. B, C Integrative Genomics Viewer (IGV) browser shows the tracks of the H3K9me3 peaks in WNT2B and WNT10A. D, E Boxplot for gene expression level for WNT2B and WNT10A in CHOL. F Overall survival curve of WNT2B expression level in ICC patients
Validation of H3K9me3 involved in WNT pathway in ICC using RNAseq data
H3K9me3 was reported to be mainly regulated by SUV39H1, SUV39H2, SETDB1, SETDB2, LSD1 and KDM4B [29, 30]. To further validate our main conclusions that H3K9me3 was involved in the WNT pathway in ICC, we explored the correlations between these H3K9me3’ regulators and core WNT pathway genes—WNT2B and WNT10A using RNA-seq data of ICC cell line and normal cell line. As we expected, the expression levels of WNT2B and WNT10A are associated with the expression level of H3K9me3 regulators (Fig. 5). This is an evidence that H3K9me3 is involved in WNT pathway in ICC.
Discussion
It has been reported that H3K9me3 significantly influenced the progression of several types of cancers and it could regulate the expression of various cancer-related genes [31,32,33,34,35]. The role of H3K9me3 in regulating genes in cholangiocarcinoma is still unclear. Under conditions of aberrant DNA methylation, H3K9me3 can promote the process of gene silencing [34]. Several histone lysine regulators, such as SUV39H1, SUV39H2, SETDB1, SETDB2, LSD1, EZH2 and KDM4B, significantly influence the process of oncogenesis [29, 36,37,38]. SUV39H1 and EZH2 has emerged as a drug target and results from studies conducted on SUV39H1 and EZH2 have helped in the development of several potential therapeutic strategies that can be followed to treat cancer [30]. In our study, those regulators were also identified as significantly change gene (Additional file 3: Table S2), indicating H3K9me3 regulators were potential therapeutic target in ICC.
Numerous researchers believed that the initiation of cholangiocarcinoma could be attributed to WNT signaling [39,40,41]. It is known that WNT signaling can result in cholangiocarcinoma. The initiation occurs when downstream target genes are activated. Researchers have now identified the involvement of the WNT signaling pathway in the differentiation of normal cholangiocytes. Boulter et al. has revealed that the WNT ligands (WNT7B and WNT10A) and β-catenin were highly expressed in the tumor tissues. The interaction between CTBP1 and β-catenin promoted the expression of the WNT target genes [42]. The interactions could be blocked by ICG-001, which was used to study the cholangiocarcinoma model. It was found that ICG-001 significantly hindered the expression of the WNT target genes in cholangiocarcinoma tissues [42]. Under these conditions, a significant reduction in the number of tumors was observed. Therefore, WNT could be considered as a potential therapeutic target in ICC.
Conclusions
Recently, lots of efforts have been made to develop small-molecule drugs including Porcupine (PORCN) Inhibitor,Tankyrase Inhibitors,TCF/β-Catenin Complex Inhibitors,CBP/β-Catenin Inhibitors,BCL9/β-Catenin Inhibitors,Natural Compounds Target Wnt Signaling Pathway,Challenges to Inhibiting the Wnt/β-Catenin Pathway,to inhibit the WNT pathway for the treatment of many types of cancers[43]. Here, we found that H3K9me3 was involved in regulating gene expression in the WNT pathway in ICC. The simultaneous activity of the EZH2 and WNT inhibitors can potentially help treat ICC. However, further cell biology experimental of the effect of EZH2 and WNT inhibitors might be helpful for arriving at concrete conclusions. Besides, future preclinical experiments that use the mouse and organoids as models would helpful for transformation of our discovery to ICC therapeutic.
Availability of data and materials
ChIP-seq data generated or analyzed during this study are available in the SRA repository (https://www.ncbi.nlm.nih.gov/Traces/study/?acc=SRP333541).
References
Sia D, Tovar V, Moeini A, Llovet JM. Intrahepatic cholangiocarcinoma: pathogenesis and rationale for molecular therapies. Oncogene. 2013;32(41):4861–70.
Sungwan P, Lert-Itthiporn W, Silsirivanit A, Klinhom-On N, Okada S, Wongkham S, Seubwai W. Bioinformatics analysis identified CDC20 as a potential drug target for cholangiocarcinoma. PeerJ. 2021;9:e11067.
Sarcognato S, Sacchi D, Fassan M, Fabris L, Cadamuro M, Zanus G, Cataldo I, Capelli P, Baciorri F, Cacciatore M, et al. Cholangiocarcinoma. Pathologica. 2021;113(3):158–69.
Zhang C, Xu J, Ye J, Zhang X. Prognostic value of HHLA2 expression in solid tumors: a meta-analysis based on the Chinese population. Medicine (Baltimore). 2021;100(30):e26789.
Shi T, Morishita A, Kobara H, Masaki T. The role of microRNAs in cholangiocarcinoma. Int J Mol Sci. 2021;22(14):7627.
Yamamura M, Sato Y, Takahashi K, Sasaki M, Harada K. The cyclindependent kinase pathway involving CDK1 is a potential therapeutic target for cholangiocarcinoma. Oncol Rep. 2020;43(1):306–17.
Kang MJ, Kim J, Jang JY, Park T, Lee KB, Kim SW. 22q11-q13 as a hot spot for prediction of disease-free survival in bile duct cancer: integrative analysis of copy number variations. Cancer Genet. 2014;207(3):57–69.
Li L, Lian B, Li C, Li W, Li J, Zhang Y, He X, Li Y, **e L. Integrative analysis of transcriptional regulatory network and copy number variation in intrahepatic cholangiocarcinoma. PLoS ONE. 2014;9(6):e98653.
Cao J, Sun L, Li J, Zhou C, Cheng L, Chen K, Yan B, Qian W, Ma Q, Duan W. A novel threemiRNA signature predicts survival in cholangiocarcinoma based on RNASeq data. Oncol Rep. 2018;40(3):1422–34.
Henneman B, Brouwer TB, Erkelens AM, Kuijntjes GJ, van Emmerik C, van der Valk RA, Timmer M, Kirolos NCS, van Ingen H, van Noort J, et al. Mechanical and structural properties of archaeal hypernucleosomes. Nucleic Acids Res. 2021;49(8):4338–49.
Morrison EA, Baweja L, Poirier MG, Wereszczynski J, Musselman CA. Nucleosome composition regulates the histone H3 tail conformational ensemble and accessibility. Nucleic Acids Res. 2021;49(8):4750–67.
Stevens KM, Swadling JB, Hocher A, Bang C, Gribaldo S, Schmitz RA, Warnecke T. Histone variants in archaea and the evolution of combinatorial chromatin complexity. Proc Natl Acad Sci USA. 2020;117(52):33384–95.
Girardot M, Hirasawa R, Kacem S, Fritsch L, Pontis J, Kota SK, Filipponi D, Fabbrizio E, Sardet C, Lohmann F, et al. PRMT5-mediated histone H4 arginine-3 symmetrical dimethylation marks chromatin at G + C-rich regions of the mouse genome. Nucleic Acids Res. 2014;42(1):235–48.
Guo M, Goudarzi KM, Abedi S, Pieber M, Sjoberg E, Behnan J, Zhang XM, Harris RA, Bartek J, Lindstrom MS, et al. SFRP2 induces a mesenchymal subtype transition by suppression of SOX2 in glioblastoma. Oncogene. 2021;40(32):5066–80.
Lager TW, Conner C, Keating CR, Warshaw JN, Panopoulos AD. Cell surface GRP78 and Dermcidin cooperate to regulate breast cancer cell migration through Wnt signaling. Oncogene. 2021;40(23):4050–9.
Liu Y, Deng H, Liang L, Zhang G, **a J, Ding K, Tang N, Wang K. Depletion of VPS35 attenuates metastasis of hepatocellular carcinoma by restraining the Wnt/PCP signaling pathway. Genes Dis. 2021;8(2):232–40.
O’Geen H, Echipare L, Farnham PJ. Using ChIP-seq technology to generate high-resolution profiles of histone modifications. Methods Mol Biol. 2011;791:265–86.
He HH, Meyer CA, Chen MW, Jordan VC, Brown M, Liu XS. Differential DNase I hypersensitivity reveals factor-dependent chromatin dynamics. Genome Res. 2012;22(6):1015–25.
Ahmed Z, Ucar D. I-ATAC: interactive pipeline for the management and pre-processing of ATAC-seq samples. PeerJ. 2017;5:e4040.
Fu S, Wang Q, Moore JE, Purcaro MJ, Pratt HE, Fan K, Gu C, Jiang C, Zhu R, Kundaje A, et al. Differential analysis of chromatin accessibility and histone modifications for predicting mouse developmental enhancers. Nucleic Acids Res. 2018;46(21):11184–201.
Duttke SH, Chang MW, Heinz S, Benner C. Identification and dynamic quantification of regulatory elements using total RNA. Genome Res. 2019;29(11):1836–46.
Sanborn JZ, Benz SC, Craft B, Szeto C, Kober KM, Meyer L, Vaske CJ, Goldman M, Smith KE, Kuhn RM, et al. The UCSC Cancer Genomics Browser: update 2011. Nucleic Acids Res 2011, 39(Database issue):D951–959.
Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6(269):pl1.
Wang H, Wang MS, Wang Y, Huang YQ, Shi JP, Ding ZL, Wang WJ. Prognostic value of immune related genes in lung adenocarcinoma. Oncol Lett. 2020;20(5):259.
Nematolahi S, Nazari S, Shayan Z, Ayatollahi SMT, Amanati A. Improved Kaplan-Meier estimator in survival analysis based on partially rank-ordered set samples. Comput Math Methods Med. 2020;2020:7827434.
Warde-Farley D, Donaldson SL, Comes O, Zuberi K, Badrawi R, Chao P, Franz M, Grouios C, Kazi F, Lopes CT, et al. The GeneMANIA prediction server: biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res 2010, 38(Web Server issue):W214–220.
McKay SC, Unger K, Pericleous S, Stamp G, Thomas G, Hutchins RR, Spalding DR. Array comparative genomic hybridization identifies novel potential therapeutic targets in cholangiocarcinoma. HPB (Oxford). 2011;13(5):309–19.
Kanehisa M, Sato Y, Kawashima M, Furumichi M, Tanabe M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 2016;44(D1):D457-462.
Torrano J, Al Emran A, Hammerlindl H, Schaider H. Emerging roles of H3K9me3, SETDB1 and SETDB2 in therapy-induced cellular reprogramming. Clin Epigenetics. 2019;11(1):43.
Carvalho S, Freitas M, Antunes L, Monteiro-Reis S, Vieira-Coimbra M, Tavares A, Paulino S, Videira JF, Jeronimo C, Henrique R. Prognostic value of histone marks H3K27me3 and H3K9me3 and modifying enzymes EZH2, SETDB1 and LSD-1 in colorectal cancer. J Cancer Res Clin Oncol. 2018;144(11):2127–37.
Yokoyama Y, Hieda M, Nishioka Y, Matsumoto A, Higashi S, Kimura H, Yamamoto H, Mori M, Matsuura S, Matsuura N. Cancer-associated upregulation of histone H3 lysine 9 trimethylation promotes cell motility in vitro and drives tumor formation in vivo. Cancer Sci. 2013;104(7):889–95.
Leszinski G, Gezer U, Siegele B, Stoetzer O, Holdenrieder S. Relevance of histone marks H3K9me3 and H4K20me3 in cancer. Anticancer Res. 2012;32(5):2199–205.
Dagdemir A, Durif J, Ngollo M, Bignon YJ, Bernard-Gallon D. Histone lysine trimethylation or acetylation can be modulated by phytoestrogen, estrogen or anti-HDAC in breast cancer cell lines. Epigenomics. 2013;5(1):51–63.
Banerjee J, Mishra R, Li X, Jackson RS 2nd, Sharma A, Bhowmick NA. A reciprocal role of prostate cancer on stromal DNA damage. Oncogene. 2014;33(41):4924–31.
Wang P, Yuan D, Guo F, Chen X, Zhu L, Zhang H, Wang C, Shao C. Chromatin remodeling modulates radiosensitivity of the daughter cells derived from cell population exposed to low- and high-LET irradiation. Oncotarget. 2017;8(32):52823–36.
Idrissou M, Boisnier T, Sanchez A, Khoufaf FZH, Penault-Llorca F, Bignon YJ, Bernard-Gallon D. TIP60/P400/H4K12ac plays a role as a heterochromatin back-up skeleton in breast cancer. Cancer Genomics Proteomics. 2020;17(6):687–94.
Chu CH, Wang LY, Hsu KC, Chen CC, Cheng HH, Wang SM, Wu CM, Chen TJ, Li LT, Liu R, et al. KDM4B as a target for prostate cancer: structural analysis and selective inhibition by a novel inhibitor. J Med Chem. 2014;57(14):5975–85.
Monaghan L, Massett ME, Bunschoten RP, Hoose A, Pirvan PA, Liskamp RMJ, Jorgensen HG, Huang X. The emerging role of h3k9me3 as a potential therapeutic target in acute myeloid leukemia. Front Oncol. 2019;9:705.
Wen Z, Chen M, Guo W, Guo K, Du P, Fang Y, Gao M, Wang Q: RORbeta suppresses the stemness of gastric cancer cells by downregulating the activity of the Wnt signaling pathway. Oncol Rep. 2021, 46(2).
Zhang Y, Wang S, Kang W, Liu C, Dong Y, Ren F, Wang Y, Zhang J, Wang G, To KF, et al. CREPT facilitates colorectal cancer growth through inducing Wnt/beta-catenin pathway by enhancing p300-mediated beta-catenin acetylation. Oncogene. 2018;37(26):3485–500.
Lee S, Remark LH, Josephson AM, Leclerc K, Lopez EM, Kirby DJ, Mehta D, Litwa HP, Wong MZ, Shin SY, et al. Notch-Wnt signal crosstalk regulates proliferation and differentiation of osteoprogenitor cells during intramembranous bone healing. NPJ Regen Med. 2021;6(1):29.
Boulter L, Guest RV, Kendall TJ, Wilson DH, Wojtacha D, Robson AJ, Ridgway RA, Samuel K, Van Rooijen N, Barry ST, et al. WNT signaling drives cholangiocarcinoma growth and can be pharmacologically inhibited. J Clin Invest. 2015;125(3):1269–85.
Duspara K, Bojanic K, Pejic JI, Kuna L, Kolaric TO, Nincevic V, Smolic R, Vcev A, Glasnovic M, Curcic IB, et al. Targeting the Wnt signaling pathway in liver fibrosis for drug options: an update. J Clin Transl Hepatol. 2021;9(6):960–71.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science Foundation of China (Grant no. 81760430), Scientific research fund of Yunnan Provincial Department of Education (2022Y209, 202101AU070237).
Author information
Authors and Affiliations
Contributions
XZ and RZ contributed to the study design of the manuscript. SH, XW, TW and LW drafted the manuscript. LL, WR, XL and WZ analyzed the data. WL and ZL collected the data. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for Publication
Not applicable.
Competing interests
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. Result of H3K9me3 Peaks.
Additional file 2
. Full length gels and blots with membrane edges.
Additional file 3
. Differentlly expressed genes in ICC.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hu, S., Wang, X., Wang, T. et al. Differential enrichment of H3K9me3 in intrahepatic cholangiocarcinoma. BMC Med Genomics 15, 185 (2022). https://doi.org/10.1186/s12920-022-01338-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12920-022-01338-1