Abstract
Background
Childhoods in urban or rural environments may differentially affect the risk of neuropsychiatric disorders, possibly through memory processing and neural response to emotional stimuli. Genetic factors may not only influence individuals’ choices of residence but also modulate how the living environment affects responses to episodic memory.
Methods
We investigated the effects of childhood urbanicity on episodic memory in 410 adults (discovery sample) and 72 adults (replication sample) with comparable socioeconomic statuses in Bei**g, China, distinguishing between those with rural backgrounds (resided in rural areas before age 12 and relocated to urban areas at or after age 12) and urban backgrounds (resided in cities before age 12). We examined the effect of childhood urbanicity on brain function across encoding and retrieval sessions using an fMRI episodic memory paradigm involving the processing of neutral or aversive pictures. Moreover, genetic association analyses were conducted to understand the potential genetic underpinnings that might contribute to memory processing and neural mechanisms influenced by early-life urban or rural environments.
Results
Episodic memory retrieval accuracy for more difficult neutral stimuli was similar between those with urban and rural childhoods, whereas aversive stimuli elicited higher retrieval accuracy in the urban group (P = 0.023). For aversive stimuli, subjects with urban childhood had relatively decreased engagement of the striatum at encoding and decreased engagement of the hippocampus at retrieval. This more efficient striatal encoding of aversive stimuli in those with urban childhoods was associated with common variation in neurotrophic tyrosine kinase receptor type 2 (NTRK2) (right striatum: P = 1.58×10−6). These findings were confirmed in the replication sample.
Conclusions
We suggest that this differential striatal processing of aversive stimuli observed in individuals with urban or rural childhoods may represent mechanisms by which childhood urbanicity may affect brain circuits, heightening behavioral responses to negative stressors associated with urban environments. NTRK2-associated neural processes in the striatum may play a role in these processes.
Similar content being viewed by others
Background
Episodic memory is the memory of autobiographical events that occur at a particular time and place, engaging the medial temporal lobe (MTL) and prefrontal cortex (PFC) [1,2,3]. Episodic memory can be categorized into emotional and non-emotional stimuli [4]. Aversive content and emotional arousal have consistently been shown to increase memory of previously encoded items compared to neutral ones. The hippocampus plays an important role in processing negative or stressful information. Therefore, inhibiting negative memory engrams in the hippocampus could be a novel therapeutic approach for treating the cognitive symptoms of depression [5]. The striatum is also important for episodic memory formation, and successful memory is associated with greater activity in the striatum during encoding [6].
Deficits in both emotional and non-emotional episodic memories have been observed in various neuropsychiatric disorders [6]. In a neutral encoding task, patients with schizophrenia and their healthy siblings showed reduced parahippocampal activation and hippocampal-parietal coupling during the encoding of neutral stimuli compared with normal control participants [7]. Patients with depression demonstrated impairments in selecting relevant positive information [8]. Individuals with anxiety disorders showed enhanced memory for threatening disorder-related material [9], but impaired memory for neutral information [10]. In addition, police officers with post-traumatic stress disorder (PTSD) exhibited smaller hippocampal volumes, higher cortisol levels, and memory impairments [11]. Other studies have suggested that the amygdala has heightened responsivity in symptomatic states of PTSD during the processing of trauma-unrelated affective information, whereas the responsivity of the medial prefrontal cortex (MPFC) is inversely associated with PTSD symptom severity [12].
Urbanicity is a major socio-ecological change especially in this century. By 2050, the urban population is expected to increase to 66%, whereas the rural population is expected to decline [13]. Previous studies have suggested that the environment during childhood affects brain development [14]. Urban childhood was negatively correlated with the gray matter volume (GMV) of the MPFC in developed and develo** countries [15, 16], while positively correlated with the GMV of the dorsal lateral prefrontal cortex (DLPFC) only in develo** countries [16]. Meanwhile, activation of the pregenual anterior cingulate cortex (pACC) in a social stress task was affected by childhood urbanicity [17] and interacts with polygenic risk score to affect brain activation under social-stress working memory task [18]. Different urban and rural childhood environments can affect early memory development. Among children aged 10 to 13 years, those with early rural childhoods were more likely to remember information about social interactions, while those with early urban childhoods were more likely to report individual memories, and these memories appeared to contain more words [19]. However, the impact of childhood urbanicity on the neural correlates of episodic memory remains poorly understood.
Although urban environments can facilitate a higher average quality of life [20], they may also be accompanied by an increased risk of neuropsychiatric disorders, including depression, autistic spectrum disorders, and psychosis [21,22,23,24]. Additionally, a higher genetic risk for psychiatric disorders has been reported to affect individuals’ choice of residence [25]. From twin studies, living in an urban environment is itself partially heritable [26]. Although the idea that childhood environment is heritable may seem counterintuitive, work on behavioral genetics has long documented the heritability of many exposures perceived as environmental [27]. This heritability is referred to as gene–environment correlation (rGE), and potential rGE mechanisms may be posited to explain the heritability of childhood environments [28]. One such mechanism is “active” rGE, where individuals with genetic variants associated with certain behavioral phenotypes may be more prone to selecting adverse situations. For instance, genetic factors related to individuals’ nature experiences may lead children who experience more nature to benefit more from it [29]. Therefore, we hypothesized that differences in episodic memories affected by urbanicity may have genetic influences, as reflected in brain activity. The significant loci found in genome-wide association studies may provide clues about the mechanism of partial heritability on the impact of urbanicity on episodic memory.
China has undergone large-scale urbanization since the 1980s, accompanied by its economic development [30]. This gave us a unique opportunity to leverage China’s recent urbanization to examine different childhood rural-urban environmental effects on brain development, which are currently poorly understood. The aim of this study was to explore how the childhood environment could affect episodic memory brain function. To achieve this, we first compared aversive or neutral episodic memory performance across participants from rural and urban childhood environments. Second, we explored the effect of urbanicity on brain activity and investigated the correlation between brain activity and performance. Third, considering that the mechanism of urbanization effects on the brain activity associated with episodic memories remains unknown and may be influenced by genetic variation, we also conducted genome-wide genoty** of the discovery sample. Subsequently, we performed a genome-wide association study with urban-rural differences in brain activity as the dependent variable to explore the potential genetic effects. Participants in this study were balanced between their current urban environments and genetic backgrounds [18], thus maximizing the effect of childhood urban versus rural upbringing.
Methods
Participants
A total of 522 healthy subjects were recruited from the local community using social media and posters and 410 subjects were included in this study, all of whom had been living in Bei**g for at least 1 year and had different childhood urbanicities. In this study, we divided subjects into two main groups: the urban group (N=220) comprised adult subjects who lived in cities before the age of 12 years, while the rural group (N=190) comprised those who only moved to cities after the age of 12 years. We also conducted analysis with an increased grou** resolution of four groups (born in and continue to live in cities, born in rural areas, and lived in cities since before 12 years, born in rural areas and lived in cities since 12–18, born and lived in rural areas for >18 years since birth) and utilizing urbanicity scores [17]. The detailed recruitment methods are described in the Additional file 1: Supplementary Methods [31,32,1: Supplementary Methods. Principal component analysis (PCA) was performed to verify the genetic backgrounds of the rural and urban groups (Additional file 1: Figure S1). Finally, nine participants were excluded after quality control. A total of 4,388,740 variants across 401 individuals were included in the genetic association analysis. Logistic regression under an additive genetic model was used to evaluate the associations between the allele dosages and the urban-rural differences in activity that survived at P < 0.05, FWE cluster-wise with P < 0.001 voxel-wise corrected for the whole brain in the encoding and retrieval task in PLINK v1.90. Age, sex, educational level, and 10 principal components were entered as covariates. The threshold was set at P < 5×10−8 to reveal significant loci, and exploratory P < 5×10−6 to reveal suggestive results.
Results
Demographics
In the discovery sample, participants with childhood urbanicity were slightly younger than that with rural childhood (Table 1). Both groups were currently living in Bei**g and were not different in sex distribution, educational, and occupational levels. They were genetically homogeneous, with no significant differences between the first 10 principal components from the genome-wide genoty** (Additional file 1: Figure S1). In the replication sample, the participants with childhood urbanicity were younger. Similar to the discovery sample, no significant differences in gender distribution, educational level, or population stratification were observed between the urban and rural groups (Table 2).
Behavioral results
Discovery sample
In the encoding task, two-way repeated-measures ANOVA with age, gender, and educational level as covariates showed a significant interaction effect between urbanicity and stimulus valence (aversive or neutral) [partial eta squared (η2p) = 0.021, P = 0.003, Figure 1b]. The accuracy of the aversive task was generally lower than that of the neutral task in both groups (P < 0.001); however, this effect was more pronounced in the rural group (rural group: η2p = 0.316; urban group: η2p = 0.210; considering effect size η2p >0.14 as large; around 0.06 are medium; and <0.01 small [40]). Simple effect analysis showed that the urban group exhibited significantly higher accuracy than the rural group for aversive stimuli (η2p = 0.018, P = 0.006), but not for neutral stimuli (η2p = 0.0002, P = 0.802, Table 1).
In the retrieval task, there was a significant interaction effect between urbanicity and valence (η2p = 0.012, P = 0.015, Figure 1b). The accuracy of the aversive task was higher than that of the neutral task in both groups (P < 0.001); however, this effect was more pronounced in the urban group (rural group: η2p = 0.127; urban group: η2p = 0.261). The urban group exhibited significantly higher accuracy than the rural group for aversive stimuli (η2p = 0.012, P = 0.027), but not for neutral stimuli (η2p = 0.002, P = 0.367, Table 1).
Replication sample
In the encoding task, two-way repeated-measures ANOVA with age, gender, and educational level as covariates showed there were no significant differences between the rural and urban groups in terms of accuracy (Table 2), and there was no significant interaction effect between urbanicity and valence (η2p = 0.036, P = 0.116, Figure 1c). The accuracy of the aversive task was generally lower than that of the neutral task in both groups; however, this effect was more pronounced in the rural group (rural group: η2p = 0.292, P < 0.001; urban group: η2p = 0.103, P = 0.007).
In the retrieval task, there was a significant interaction effect between urbanicity and valence (η2p = 0.111, P = 0.005, Figure 1c). The accuracy of the aversive task was higher than that of the neutral task in urban groups (η2p = 0.313, P < 0.001), but not in the rural group (η2p = 0.026, P = 0.184). The urban group exhibited significantly lower accuracy than the rural group for neutral stimuli (η2p = 0.129, P = 0.002), but not for aversive stimuli (η2p = 0.001, P = 0.806, Table 2).
fMRI task activation
During the encoding and retrieval sessions under both neutral and aversive stimuli, regions in the DLPFC, occipital visual cortex, parts of the temporal and parietal lobes, hippocampus, striatum, and amygdala exhibited robust engagement. Conversely, decreased engagement was observed in parts of the MPFC, posterior cingulate cortex (PCC), insula, and precuneus (Additional file 1: Figure S2, Table S1 and Table S2, whole-brain FWE-corrected P <0.05, k > 100).
Effects of valence
To investigate the effects of stimulus valence on the neural circuitry of declarative memory, the aversive condition was compared with the neutral condition across encoding and retrieval sessions. During the encoding session, significantly greater activation in response to aversive scenes was observed in many brain regions, including the bilateral MPFC, DLPFC, precuneus, hippocampus, amygdala, striatum, fusiform, thalamus, and parts of the temporal lobe (including the temporal pole). Conversely, significantly decreased activity was observed in the bilateral insula, inferior parietal lobule, and angular gyrus (Additional file 1: Figure S2 and Table S1, whole-brain FWE-corrected P <0.05, k > 100). During the retrieval session, significantly greater activity was observed for aversive scenes in similar brain regions, with only the right superior temporal gyrus showing decreased activation compared to that of neutral stimuli (Additional file 1: Figure S2 and Table S2, whole-brain FWE-corrected P <0.05, k > 100).
Effects of urbanicity
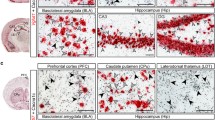
We explored the rural-urban difference in two contrast images (i.e., neutral-baseline and aversive-baseline) during both the encoding and retrieval sessions. During the encoding session, the rural group showed increased activation relative to the urban group in the bilateral caudate, putamen, and bilateral middle frontal gyrus under aversive stimuli (Figure 1d, Table 3, whole-brain cluster-wise FWE-corrected P < 0.05). Under neutral stimuli, there were no regions with differential activity that survived with FWE-corrected P < 0.05. Only the right caudate and putamen showed greater activation in the rural group than the urban group, while the urban group exhibited greater angular activation than the rural group (Table 3, P < 0.001, uncorrected, k >100).
During the retrieval session, the rural group showed greater activation than the urban group in the bilateral hippocampus, right amygdala, left thalamus, and fusiform under aversive stimuli (Figure 1d, Table 3, whole-brain cluster-wise FWE-corrected P < 0.05). Under neutral stimuli, there were no regions surviving FWE-corrected P < 0.05. The rural group only showed higher activation of the left thalamus and left fusiform than that of the urban group (Table 3, P <0.001, uncorrected, k > 100).
Notably, similar results were observed using ANOVA with individual t-contrast maps when the grou** resolution was increased to four groups: those who were born in and continued to live in cities, those who have lived in cities since before age 12, those who lived in rural areas between birth and age 18, and those who lived in rural areas for ≥18 years since birth (Additional file 1: Figure S3 and S4). Additionally, similar results were obtained using urbanicity scores [17, 41] (Additional file 1: Figure S5 and S6). Notably, participants exhibited progressive weaker brain activations with gradual exposure to levels of urbanicity (Additional file 1: Figure S4 and S6).
Brain-behavior correlations
To assess which brain regions were associated with recognition accuracy during encoding, simple regressions were performed between encoding activation maps and recognition accuracy, with age and sex as covariates. We found that the activation of the right DLPFC (peak at [44 22 42], t = 4.55, cluster size = 372) and the right striatum (peat at [24 12 -6], t = 4.11, cluster size = 579) was associated with neutral retrieval accuracy (whole-brain cluster-wise FWE-corrected P < 0.05, Figure 2). Specifically, under neutral stimuli, the retrieval accuracy was positively correlated with encoding brain activations of the DLPFC (P < 0.001; r = 0.245) and the striatum (P < 0.001, r = 0.213). When we used d-prime as the dependent variable (Additional file 1: Table S5), or included age, sex, and socioeconomic status (SES) as covariates, similar results were obtained (Additional file 1: Table S6 and S7). We did not find any significant correlations under aversive stimuli.
Brain-behavior relationship between encoding activation and retrieval accuracy for neutral stimuli. Encoding brain activation was associated with retrieval accuracy under neutral stimuli (N = 410, P < 0.05, whole-brain cluster-wise FWE corrected, controlled for age and gender). a The DLPFC activation during encoding was associated with retrieval accuracy (Peat at [44 22 42], t = 4.55, cluster size = 372). b Scatter plot showing a positive correlation between encoding DLPFC activation and retrieval accuracy (P < 0.001, r = 0.245). c The striatum activation during encoding was associated with retrieval accuracy (Peat at [24 12 -6], t = 4.11, cluster size = 579). b Scatter plot showing a positive correlation between encoding DLPFC activation and retrieval accuracy (P < 0.001, r = 0.213)
Genetic association
In the genome-wide association study, we extracted the average BOLD response of the most differentially activated urban-rural regions during the encoding and retrieval tasks as dependent variables. By selecting an ROI in each representative brain region, a total of 8 ROIs were drawn centered on the peak activation difference, with a 6-mm radius. These ROIs were as follows: for the encoding task, the middle frontal gyrus (peak at [24 32 40]), the right striatum (peak at [16 12 12]), the left striatum (peak at [-14 12 12]); the middle frontal gyrus (peak at [-28 10 64]); for the retrieval task, the amygdala (peak at [34 -8 -14]), the hippocampus (peak at [18 -24 -10]), the thalamus (peak at [-24 -30 2]), and the middle temporal gyrus (peak at [-38 -58 6]) (Table 3). The time series of each voxel within the ROIs were extracted and the average BOLD response obtained. We identified an exploratory single-nucleotide polymorphism (SNP) located within genes with minor allele frequency > 0.10, and |Beta| > 0.10 in the discovery sample.
The results revealed that rs7042458, an intron of the neurotrophic tyrosine kinase receptor type 2 (NTRK2) gene, was correlated with the BOLD response in the bilateral striatum under aversive stimuli during the encoding task (right striatum: P = 1.58×10−6, Beta = −0.1679; left striatum: P = 1.33×10−6, Beta = −0.1639, Figure 3). AA homozygotes (n=306) showed significantly higher activity in the bilateral striatum than AT/TT heterozygotes (n=95). Furthermore, the chi-square test showed that there were more individuals with the T-carrier genotype in the urban group (χ2 = 4.039, P = 0.044). Public expression data from the Brainace Database indicated that the common NTRK2 rs7042458 variant affected gene expression in the putamen and frontal cortex, with the AT/TT group exhibiting higher expression (Additional file 1: Figure S7). The explained variance (adjusted R2) of the average BOLD response of the right striatum was increased from 5.23% (including genetic variant) to 9.59% (including both genetic variant and urbanicity). The 4.36% variance was still explained by urbanicity if genetic variants were used as predictors. The explained variance (adjusted R2) of the average BOLD response of the left striatum was increased from 4.23 to 8.70%. The 4.47% variance was still explained by urbanicity if genetic variants were used as predictors. Furthermore, rs9320231 (P = 3.46×10−6, Beta = −0.1846,) located within the Scm Polycomb Group Protein Like 4 (SCML4) gene, and rs1562086 (P = 3.79×10−6, Beta = −0.2686), located within the Alpha-1,6-Mannosylglycoprotein 6-Beta-N-Acetylglucosaminyltransferase B (MGAT5B) gene, were correlated with the BOLD response of the right middle frontal gyrus under aversive stimuli during the encoding task.
Striatal emotional encoding function was associated with the common variant in NTRK2. a, b Rs7042458, an intron of neurotrophic tyrosine kinase receptor type 2 (NTRK2) gene, was correlated with BOLD response of urban-rural different right striatum under aversive stimuli during encoding task (P = 1.58×10−6, Beta = −0.1679). c, d Rs7042458 was also associated with BOLD response of urban-rural different left striatum under aversive stimuli during encoding task (P = 1.33×10−6, Beta = −0.1639)
Under aversive stimuli in the retrieval task, rs6442936, an intron of the ADP ribosylation factor like GTPase 8 B (ARL8B) gene involved in lysosomal function, was significantly correlated with the BOLD response in the right amygdala (P = 3.52×10−9, Beta = 0.3796) in a small number of participants with a CG genotype (17/397). Rs423158 (P = 3.84×10−6, Beta = 0.1026), located within the Solute Carrier Family 25 member 18 (SCL25A18) gene, was also correlated with the BOLD response of the right amygdala. Rs71471176 (P = 3.44×10-6, Beta = 0.2044), located within the Glutamate Ionotropic Receptor Delta Type Subunit 1 (GRID1) gene, was correlated with the BOLD response in the right hippocampus. Rs152591 (P = 1.64×10−6, Beta = −0.1263), located within the Ephrin A5 (EFN5A) gene, was correlated with the BOLD response of the left middle temporal gyrus.
Independent replication
We focused on replicating the contrast of interest (aversive vs. baseline) that showed significant brain activation associated with urbanicity. During the encoding session, the rural group showed higher activation compared to the urban group in the right caudate, left inferior frontal gyrus, and middle temporal gyrus in response to aversive stimuli. During the retrieval session, the rural group showed higher activation in the right caudate, medial frontal gyrus, and anterior cingulate cortex under aversive stimuli than that in the urban group (P < 0.001, uncorrected, k > 30, Figure 1e, Table 3). Although the regions did not survive with FWE-corrected P < 0.05 mainly due to the limited sample size, we observed a consistent role of urban-rural striatal activity difference in response to aversive stimuli during the encoding session. After extracting the replicated urban-rural striatal activity differences in the encoding task under aversive stimuli, we found that rs7042458 AA homozygotes (n=45) showed significantly higher activity in the right striatum than the AT/TT group (n=14, P = 0.042, with age and sex as covariates), which was consistent with the discovery sample.
Discussion
In this study, we examined the effects of urban and rural childhood on episodic memory and related brain function in a large and genetically homogeneous sample of young healthy Han Chinese adults. Despite having similar current educational and occupational status, these individuals differed in their urban or rural childhood during China’s rapid and large-scale urbanization. Rural childhood appeared to result in stronger brain activation during encoding, especially in the striatum; and stronger brain activation during retrieval, especially in the hippocampus. These findings were replicated in an independent sample. The different urban–rural striatal emotional encoding functions were associated with the NTRK2 common variant, which exhibited a higher distribution of the T allele in the urban group. These findings suggest that effects on striatal function may be related to childhood urbanicity, emotional memory processes, and genetic variability within NTRK2.
Both groups exhibited reduced accuracy to aversive stimuli during the encoding session, indicating a tendency to avoid aversive stimuli. However, the urban group demonstrated a smaller decrease, which suggests that the urban participants were less likely to neglect aversive stimuli compared to their rural counterparts. The absence of a significant interaction effect between urbanicity and valence in the replication sample may be attributed to the limited sample size, necessitating a more cautious interpretation of behavioral measures. During the retrieval session, episodic memory for aversive stimuli was generally better than for neutral stimuli, consistent with the memorability and ease of recall of aversive stimuli [38]. However, participants with rural childhoods showed less of this effect under aversive stimuli in both cohorts, which may reflect a tendency toward enhanced memory for negative stimuli among urban participants.
During the encoding phase, the rural group showed greater activation in the dorsal striatum than the urban group in both the discovery and replication samples, particularly in response to aversive stimuli. The striatum, especially the caudate nucleus [42], is involved in the formation of declarative memory, the process by which episodic elements are bound to a complete memory trace. One possible explanation for this activation is that networks involved in incremental learning, including the striatum, contribute to the binding process in the formation of integrated episodes [43]. Considering the role of the striatum in processing reward stimuli, the removal of an image may serve as a reward. The lower engagement of the striatum in the urban group may reflect weaker encoding of this positive stimuli. Given that striatal activation has also been associated with aversive learning, a potential reason may be that participants are contemplating how to avoid the potentially negative outcome [44]. In our sample, rural volunteers, when confronted with aversive stimuli, exhibited higher levels of brain activation and lower recall accuracy, aligning with this hypothesis. Furthermore, the higher accuracy demonstrated by the urban group when facing aversive stimuli may suggest a more automatic processing of negative information among adults with an urban upbringing.
During the retrieval phase, the rural group showed greater activation in the hippocampus and parahippocampal gyrus compared to the urban group, particularly in response to aversive stimuli. The hippocampus is involved in emotional memory recall and regulation [45, 46]. While rural participants exhibited increased hippocampal engagement, suggesting a greater investment in memory recall, they still displayed lower accuracy than their urban counterparts when facing aversive stimuli. However, their accuracy levels were similar when encountering neutral stimuli. These results may reflect a protective mechanism of emotional regulation that successfully inhibits negative memory recall.
Previous studies have hypothesized that memory encoding competes for striatal processing in the hippocampus [47, 48]. If this hypothesis holds true, the stronger striatal activation observed in rural participants during encoding could indicate selective neglect of aversive episodic memory through inhibition of hippocampal function. During the retrieval session, despite rural participants displaying increased hippocampal activity, their accuracy remained lower than that of the urban participants. This could be attributed to their selective suppression of the encoding of negative stimuli. Therefore, we suggest that rural participants possess an adaptive striatal function that is less engaged by aversive stimuli, and therefore less affected by or more resilient to stress.
On the other hand, another possibility is that urban participants may more automatically focus on and remember aversive stimuli than their rural counterparts. The pathological process of enhancing negative memories or neglecting positive memories has been observed in conditions including depression, anxiety, and PTSD [8, 10, 49]. A meta-analysis indicated that, when exposed to negative stimuli, patients with depression exhibited lower striatal response levels compared to healthy volunteers, possibly due to decreased striatal dopamine levels when confronted with negative information [50]. Compared to rural volunteers, the brain activation patterns of urban volunteers were analogous to that observed in the depression models. In addition, the reduced prefrontal engagement of urban participants under aversive stimuli may indicate hypersensitivity to negative events, suggesting a potential “sensitizing effect” in urban participants [51].
Our exploratory Genome-Wide Association Studies (GWAS) analysis suggesting genetic variability within NTRK2 gene affected the striatal processing of aversive stimuli in relation to childhood urbanicity was based on a modest sample of over 400 participants (power = 98.62% calculated by Quanto [52]). NTRK2 (also known as Tropomyosin receptor kinase B, TrkB) is activated by several neurotrophins and serves as a high-affinity receptor for brain-derived neurotrophic factor (BDNF). Although these findings are consistent with previous suggestions that correlations between SNP data and task-related brain imaging data can offer clues about genetic mechanisms [53], some caution is warranted. Firstly, we note that GWAS of complex diseases including in psychiatry, and in psychological constructs (e.g., intelligence quotient, psychological traits) [54, 55] has generally implicated genetic variants with small effect sizes. Our limited sample size thus limits the power to detect additional small genetic and/or environmental effects that could be present, despite the large environmental differences in this unique, genetically homogenous sample, and the relatively large neuroimaging samples herein. For similar reasons, it is also possible that effect sizes here are overestimated. Replication in independent samples is thus needed, which we provide. Here, we again observe the differential striatal activity between two genotype groups, suggesting that the involvement of BDNF signaling in these effects could be robust. Nevertheless, we suggest that future work should be needed in larger populations, and indeed in populations with improved characterization of features in the urban environment (e.g., local-level pollution, density, noise). BDNF expression and downstream signaling through the TrkB receptor are essential for memory formation in aversive domains [56]. BDNF-TrkB signaling within the ventral tegmental area (VTA) - nucleus accumbens (NAc) circuit has also been reported as a pathological mechanism during periods of chronic stress, resulting in depression [57]. Accumulating evidence suggest a relationship between NTRK2 and a broad range of psychiatric disorders, especially those associated with stress, including depression, schizophrenia, and anxiety disorders. Genotype-dependent differences in NTRK2 have been observed in white matter properties among patients with depression [58] and have been linked to emotional arousal in healthy individuals [59]. Furthermore, NTRK2 plays a role in modulating fear learning and synaptic plasticity in the amygdala [60]. Our discovery of NTRK2 common variations associated with differential urban-rural striatal encoding activities in aversive conditions through genome-wide association analysis supports the potential role of NTRK2 in emotion dysregulation. The high expression levels of NTRK2 in the putamen and frontal cortex throughout the lifespan, along with genotype-related expression patterns, suggest its involvement in general neurodevelopmental processes underlying stress and emotion, which may have implications for the mental health of individuals undergoing urban migration.
This study had several limitations. Firstly, the rural group of our subjects was slightly older, and this could potentially have influenced the results, although we did control for age as a factor. Secondly, because our analysis was performed on a population of relatively highly educated individuals in large cities, it may not fully represent people who still reside in rural areas. Further research should aim to include participants with varying socioeconomic statuses, encompassing both patients and healthy individuals, while incorporating variables that could directly reflect early life rural–urban features and considering additional potential confounding factors. Thirdly, our GWAS findings that suggest the involvement of BDNF signaling in the striatal processing of aversive stimuli were exploratory. Although the differential striatal activity had been observed between two genotype groups in the replication sample, more replications were needed in a larger sample. Fourthly, the use age 12 in defining childhood urbanicity is not meant to suggest any empirical evidence that the age of 12 constitutes some critical juncture for the impact of urbanicity on neurodevelopmental processes. Rather, this category variable was selected on the basis that at least in our population context (and many others), age 12 is a convenient demarcation between elementary and middle or secondary school. Moreover, we note that if instead of using such a categorical urbanicity variable, we use a continuous urbanicity variable such as the urbanicity score [17] (Additional file 1: Figure S5), similar results are obtained.
Conclusions
In conclusion, childhood in rural or urban environments appears to be associated with behavioral and brain physiological differences, particularly in the neural processing of aversive episodic memory within the striatal brain regions. Rural individuals may possess an adaptive striatal function that enhances less aversive memory and inhibits aversive memory. In contrast, urban individuals might have sensitized brain function to negative stress and memory processes. NTRK2 may play a significant role in the impact of childhood urbanicity on striatal encoding of aversive memory.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- ARL8B:
-
ADP ribosylation factor like GTPase 8 B
- BDNF:
-
Brain-derived neurotrophic factor
- DLPFC:
-
Dorsal-lateral prefrontal cortex
- EFN5A:
-
Ephrin A5
- fMRI:
-
Functional magnetic resonance imaging
- FWE:
-
Family-wise error rate
- GLM:
-
General linear model
- GMV:
-
Gray matter volume
- GRID1:
-
Glutamate ionotropic receptor delta type subunit 1
- MGAT5B:
-
Alpha-1,6-Mannosylglycoprotein 6-Beta-N-Acetylglucosaminyltransferase B
- MPFC:
-
Medial prefrontal cortex
- MTL:
-
Medial temporal lobe
- NAc:
-
Nucleus accumbens
- NTRK2:
-
Neurotrophic tyrosine kinase receptor type 2
- pACC:
-
Pregenual anterior cingulate cortex
- PCA:
-
Principal component analysis
- PCC:
-
Posterior cingulate cortex
- PFC:
-
Prefrontal cortex
- PTSD:
-
Post-traumatic stress disorder
- rGE:
-
Gene–environment correlation
- ROIs:
-
Regions of interest
- SCL25A18:
-
Solute carrier family 25 member 18
- SCML4:
-
scm polycomb group protein like 4
- SES:
-
Socioeconomic status
- SNP:
-
Single-nucleotide polymorphism
- TrkB:
-
Tropomyosin receptor kinase B
- VTA:
-
Ventral tegmental area
References
Tulving E, Markowitsch HJ. Episodic and declarative memory: role of the hippocampus. Hippocampus. 1998;8(3):198–204.
Nyberg L, Marklund P, Persson J, Cabeza R, Forkstam C, Petersson KM, et al. Common prefrontal activations during working memory, episodic memory, and semantic memory. Neuropsychologia. 2003;41(3):371–7.
Dickerson BC, Eichenbaum H. The episodic memory system: neurocircuitry and disorders. Neuropsychopharmacology. 2010;35(1):86–104.
Hamann S. Cognitive and neural mechanisms of emotional memory. Trends Cogn Sci. 2001;5(9):394–400.
Zhang TR, Larosa A, Di Raddo ME, Wong V, Wong AS, Wong TP. Negative memory engrams in the hippocampus enhance the susceptibility to chronic social defeat stress. J Neurosci. 2019;39(38):7576–90.
Dere E, Pause BM, Pietrowsky R. Emotion and episodic memory in neuropsychiatric disorders. Behav Brain Res. 2010;215(2):162–71.
Rasetti R, Mattay VS, White MG, Sambataro F, Podell JE, Zoltick B, et al. Altered hippocampal-parahippocampal function during stimulus encoding: a potential indicator of genetic liability for schizophrenia. JAMA Psychiatry. 2014;71(3):236–47.
Levens SM, Gotlib IH. Impaired selection of relevant positive information in depression. Depression and Anxiety. 2009;26:403–10.
Zeijlmans van Emmichoven IA, IMH V, de Ruiter C, Brosschot JF. Selective processing of threatening information: effects of attachment representation and anxiety disorder on attention and memory. Dev Psychopathol. 2003;15(1):219–37.
Airaksinen E, Larsson M, Forsell Y. Neuropsychological functions in anxiety disorders in population-based samples: evidence of episodic memory dysfunction. J Psychiatr Res. 2005;39(2):207–14.
Lindauer RJ, Olff M, van Meijel EP, Carlier IV, Gersons BP. Cortisol, learning, memory, and attention in relation to smaller hippocampal volume in police officers with posttraumatic stress disorder. Biol Psychiatry. 2006;59(2):171–7.
Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann N Y Acad Sci. 2006;1071:67–79.
World Urbanization Prospects: The 2014 Revision https://esa.un.org/unpd/wup/Publications/Files/WUP2014-Highlights.pdf
Jiang H, Zhang X, Zhang Y, Yan H, Yu H, Tan HY, et al. Effects of parenting styles on adult personality traits, depressive trait, and brain structure. Asian Journal of Psychiatry. 2024;93:103939.
Haddad L, Schafer A, Streit F, Lederbogen F, Grimm O, Wust S, et al. Brain structure correlates of urban upbringing, an environmental risk factor for schizophrenia. Schizophr Bull. 2015;41(1):115–22.
Zhang X, Yan H, Yu H, Zhang Y, Tan HY, Zhang D, et al. The effects of environmental factors associated with childhood urbanicity on brain structure and cognition. BMC Psychiatry. 2023;23(1):598.
Lederbogen F, Kirsch P, Haddad L, Streit F, Tost H, Schuch P, et al. City living and urban upbringing affect neural social stress processing in humans. Nature. 2011;474(7352):498–501.
Zhang X, Yan H, Yu H, Zhao X, Shah S, Dong Z, et al. Childhood urbanicity interacts with polygenic risk for depression to affect stress-related medial prefrontal function. Transl Psychiatry. 2021;11(1):522.
Goz I, Ceven ZI, Tekcan AI. Urban-rural differences in children's earliest memories. Memory (Hove, England). 2017;25(2):214–9.
Global report on urban health http://www.who.int/kobe_centre/measuring/urban-global-report/en/
Pedersen CB, Mortensen PB. Evidence of a dose-response relationship between urbanicity during upbringing and schizophrenia risk. Arch Gen Psychiatry. 2001;58(11):1039–46.
Peen J, Schoevers RA, Beekman AT, Dekker J. The current status of urban-rural differences in psychiatric disorders. Acta Psychiatrica Scandinavica. 2010;121(2):84–93.
van Os J, Kenis G, Rutten BP. The environment and schizophrenia. Nature. 2010;468(7321):203–12.
Lauritsen MB, Astrup A, Pedersen CB, Obel C, Schendel DE, Schieve L, et al. Urbanicity and autism spectrum disorders. J Autism Dev Disord. 2014;44(2):394–404.
Maxwell JM, Coleman JRI, Breen G, Vassos E. Association between genetic risk for psychiatric disorders and the probability of living in urban settings. JAMA Psychiatry. 2021;78(12):1355–64.
Whitfield JB, Zhu G, Heath AC, Martin NG. Choice of residential location: chance, family influences, or genes? Twin Res Hum Genet. 2005;8(1):22–6.
Dalvie S, Maihofer AX, Coleman JRI, Bradley B, Breen G, Brick LA, et al. Genomic influences on self-reported childhood maltreatment. Transl Psychiatry. 2020;10(1):38.
Jaffee SR, Price TS. Gene-environment correlations: a review of the evidence and implications for prevention of mental illness. Mol Psychiatry. 2007;12(5):432–42.
Chang CC, Cox DTC, Fan Q, Nghiem TPL, Tan CLY, Oh RRY, et al. People's desire to be in nature and how they experience it are partially heritable. PLoS Biol. 2022;20(2):e3001500.
Gong P, Liang S, Carlton EJ, Jiang Q, Wu J, Wang L, et al. Urbanisation and health in China. Lancet. 2012;379(9818):843–52.
Ministry of Housing and Urban-Rural Development PRC: China Urban Construction Statistical Yearbook. 2014:66-99.
Koch K, Myers NE, Göttler J, Pasquini L, Grimmer T, Förster S, et al. Disrupted intrinsic networks link amyloid-β pathology and impaired cognition in prodromal Alzheimer's Disease. Cerebral Cortex. 2015;25(12):4678–88.
**e K, ** Z, ** D-G, Zhang J, Li L. Shared and distinct structure-function substrates of heterogenous distractor suppression ability between high and low working memory capacity individuals. NeuroImage. 2022;260:119483.
Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): technical manual and affective ratings. NIMH Center for the Study of Emotion and Attention. 1997;1:39–58.
Hariri AR, Goldberg TE, Mattay VS, Kolachana BS, Callicott JH, Egan MF, et al. Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J Neurosci. 2003;23(17):6690–4.
Bertolino A, Rubino V, Sambataro F, Blasi G, Latorre V, Fazio L, et al. Prefrontal-hippocampal coupling during memory processing is modulated by COMT val158met genotype. Biol Psychiatry. 2006;60(11):1250–8.
Meyer-Lindenberg A, Buckholtz JW, Kolachana B, Hariri AR, Pezawas L, Blasi G, et al. Neural mechanisms of genetic risk for impulsivity and violence in humans. Proc Natl Acad Sci U S A. 2006;103(16):6269–74.
Murty VP, Sambataro F, Das S, Tan HY, Callicott JH, Goldberg TE, et al. Age-related alterations in simple declarative memory and the effect of negative stimulus valence. J Cogn Neurosci. 2009;21(10):1920–33.
Gescheider GA: Psychophysics: The Fundamentals. 1997.
Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol. 2013;4:863.
Mortensen PB, Pedersen CB, Westergaard T, Wohlfahrt J, Ewald H, Mors O, et al. Effects of family history and place and season of birth on the risk of schizophrenia. N Engl J Med. 1999;340(8):603–8.
Blumenfeld RS, Parks CM, Yonelinas AP, Ranganath C. Putting the pieces together: the role of dorsolateral prefrontal cortex in relational memory encoding. J Cogn Neurosci. 2011;23(1):257–65.
Ben-Yakov A, Dudai Y. Constructing realistic engrams: poststimulus activity of hippocampus and dorsal striatum predicts subsequent episodic memory. J Neurosci. 2011;31(24):9032–42.
Delgado MR, Li J, Schiller D, Phelps EA. The role of the striatum in aversive learning and aversive prediction errors. Philos Trans R Soc B: Biol. 2008;363:3787–800.
Buzsaki G, Moser EI. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat Neurosci. 2013;16(2):130–8.
Schumacher A, Villaruel FR, Ussling A, Riaz S, Lee ACH, Ito R. Ventral hippocampal CA1 and CA3 differentially mediate learned approach-avoidance conflict processing. Curr Biol. 2018;28(8):1318–1324 e1314.
Wimmer GE, Braun EK, Daw ND, Shohamy D. Episodic memory encoding interferes with reward learning and decreases striatal prediction errors. J Neurosci. 2014;34(45):14901–12.
Packard MG, Knowlton BJ. Learning and memory functions of the Basal Ganglia. Annu Rev Neurosci. 2002;25:563–93.
Wessa M, Jatzko A, Flor H. Retrieval and emotional processing of traumatic memories in posttraumatic stress disorder: peripheral and central correlates. Neuropsychologia. 2006;44(10):1683–96.
Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, Gotlib IH. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of base line activation and neural response data. Am J Psychiatry. 2012;169(7):693–703.
Rutter M. Resilience as a dynamic concept. Dev Psychopathol. 2012;24(2):335–44.
Gauderman WJ. Sample size requirements for matched case-control studies of gene-environment interaction. Stat Med. 2002;21(1):35–50.
Elliott LT, Sharp K, Alfaro-Almagro F, Shi S, Miller KL, Douaud G, et al. Genome-wide association studies of brain imaging phenotypes in UK Biobank. Nature. 2018;562(7726):210–6.
Als TD, Kurki MI, Grove J, Voloudakis G, Therrien K, Tasanko E, et al. Depression pathophysiology, risk prediction of recurrence and comorbid psychiatric disorders using genome-wide analyses. Nat Med. 2023;29(7):1832–44.
Hill WD, Marioni RE, Maghzian O, Ritchie SJ, Hagenaars SP, McIntosh AM, et al. A combined analysis of genetically correlated traits identifies 187 loci and a role for neurogenesis and myelination in intelligence. Mol Psychiatry. 2019;24(2):169–81.
Choi DC, Gourley SL, Ressler KJ. Prelimbic BDNF and TrkB signaling regulates consolidation of both appetitive and aversive emotional learning. Transl Psychiat. 2012;2:e205.
Koo JW, Labonte B, Engmann O, Calipari ES, Juarez B, Lorsch Z, et al. Essential role of mesolimbic brain-derived neurotrophic factor in chronic social stress-induced depressive behaviors. Biol Psychiat. 2016;80(6):469–78.
Murphy ML, Carballedo A, Fagan AJ, Morris D, Fahey C, Meaney J, et al. Neurotrophic tyrosine kinase polymorphism impacts white matter connections in patients with major depressive disorder. Biol Psychiat. 2012;72(8):663–70.
Spalek K, Coynel D, Freytag V, Hartmann F, Heck A, Milnik A, et al. A common NTRK2 variant is associated with emotional arousal and brain white-matter integrity in healthy young subjects. Transl Psychiat. 2016;6:e758.
Musumeci G, Sciarretta C, Rodriguez-Moreno A, Al Banchaabouchi M, Negrete-Diaz V, Costanzi M, et al. TrkB modulates fear learning and amygdalar synaptic plasticity by specific docking sites. Journal of Neuroscience. 2009;29(32):10131–43.
Acknowledgements
We are grateful to all the funding supporters, as well as to all our research assistants and volunteers.
Funding
The current study was supported by the National Natural Science Foundation of China (82001416, X. ZHANG; 82301687, Y. ZHANG; 82330042 and 81825009, W. YUE; 81361120395, D. ZHANG; 81771443 and 82071505, H. Yan; 81901358, H. YU); CAMS Innovation Fund for Medical Sciences (2021-I2M-C&T-B-099 and 2019-I2M-5-006, W. YUE); National Key R&D Program of China (2023YFE0119400, W. YUE); the U.S. National Institutes of Health (R01MH101053, Tan); Natural Science Foundation of Shandong Province (ZR2019BH001 and ZR2021YQ55, H.YU); Young Taishan Scholars of Shandong Province (tsqn201909146, H.YU).
Author information
Authors and Affiliations
Contributions
D.Z., W.Y., H.YAN, and H.Y.T. conceived and designed the project. X.Z., Y.Z., H.YAN, H.Y.T., W.Y., and D.Z. performed or supervised the data acquisition. X.Z., Y.Z., and H.YU analyzed the data. V.S.M. advised on data analysis and findings interpretation. X.Z., Y.Z., H.Y.T., and W.Y. wrote or edited the manuscript for critical intellectual content. X.Z and Y.Z contributed equally to this study and are co-first authors. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Peking University Institute of Mental Health (2013-13) and Johns Hopkins University School of Medicine (NA_00088322). Written consent was obtained from each subject after description of the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1:
Supplementary Methods. Figure S1. Principal component analysis of the discovery sample. Figure S2. Activations at different episodic memory conditions. Figure S3. Brain activation of episodic memory task across four groups with different urbanicity. Figure S4. Participants exhibited progressive weaker brain activations with gradual exposure to levels of urbanicity across the four groups. Figure S5. Early-life urbanicity effect on episodic memory task using the urbanicity score. Figure S6. Participants exhibited progressive weaker brain activations with gradual exposure to urbanicity score. Figure S7. Gene expression profiles of NTRK2 gene and rs3177121. Figure S8. The overlapped brain regions showing the effects of urbanicity in the discovery and replication sample. Table S1. Brain activation during encoding. Table S2. Brain activation during retrieval. Table S3. Rural subjects have more brain activation during encoding session. Table S4. Rural subjects have more brain activation during retrieval session. Table S5. Brain-Behavior correlation using d-prime under the neutral stimulation as the dependent variable. Table S6. Brain-Behavior correlation using recognition accuracy under the neutral stimulation as the dependent variable included socioeconomic status as covariate. Table S7. Brain-Behavior correlations using d-prime under the neutral stimulation as the dependent variable included socioeconomic status as covariate.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, X., Zhang, Y., Yan, H. et al. Childhood urbanicity is associated with emotional episodic memory-related striatal function and common variation in NTRK2. BMC Med 22, 146 (2024). https://doi.org/10.1186/s12916-024-03365-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-024-03365-4