Abstract
Background
To evaluate the cost-effectiveness of six diagnostic strategies involving magnetic resonance imaging (MRI) targeted biopsy for diagnosing prostate cancer in initial and repeat biopsy settings from the Singapore healthcare system perspective.
Methods
A combined decision tree and Markov model was developed. The starting model population was men with mean age of 65 years referred for a first prostate biopsy due to clinical suspicion of prostate cancer. The six diagnostic strategies were selected for their relevance to local clinical practice. They comprised MRI targeted biopsy following a positive pre-biopsy multiparametric MRI (mpMRI) [Prostate Imaging – Reporting and Data System (PI-RADS) score ≥ 3], systematic biopsy, or saturation biopsy employed in different testing combinations and sequences. Deterministic base case analyses with sensitivity analyses were performed using costs from the healthcare system perspective and quality-adjusted life years (QALY) gained as the outcome measure to yield incremental cost-effectiveness ratios (ICERs).
Results
Deterministic base case analyses showed that Strategy 1 (MRI targeted biopsy alone), Strategy 2 (MRI targeted biopsy ➔ systematic biopsy), and Strategy 4 (MRI targeted biopsy ➔ systematic biopsy ➔ saturation biopsy) were cost-effective options at a willingness-to-pay (WTP) threshold of US$20,000, with ICERs ranging from US$18,975 to US$19,458. Strategies involving MRI targeted biopsy in the repeat biopsy setting were dominated. Sensitivity analyses found the ICERs were affected mostly by changes to the annual discounting rate and prevalence of prostate cancer in men referred for first biopsy, ranging between US$15,755 to US$23,022. Probabilistic sensitivity analyses confirmed Strategy 1 to be the least costly, and Strategies 2 and 4 being the preferred strategies when WTP thresholds were US$20,000 and US$30,000, respectively.
Limitations and conclusions
This study found MRI targeted biopsy to be cost-effective in diagnosing prostate cancer in the biopsy-naïve setting in Singapore.
Similar content being viewed by others
Introduction
Prostate cancer is the second most common cancer diagnosed and the fifth most fatal cancer amongst men globally [1]. In Singapore, prostate cancer is the third most common cancer in men, accounting for 14.1% of cancers diagnosed and 5.8% of total cancer deaths in men from 2013 to 2017 [2]. The discordant incidence and mortality reflect prostate cancer’s indolent growth and low fatality especially when diagnosed without metastasis [3, 4], and potential overdiagnosis of clinically insignificant cancer partly contributed by the limitations of prostate-specific antigen (PSA) testing [5, 6]. Currently, there is no population-wide screening recommended for the early detection of prostate cancer in Singapore. Individual men aged 50 to 70 years with life expectancy exceeding 10 years may be offered PSA testing after discussing its potential benefits and harms [7].
Distinguishing clinically significant prostate cancer from clinically insignificant ones is central to the management of prostate cancer. While the definition of clinical significance continues to evolve [8], the underpinning approach is accurate detection and characterization of clinically significant cancer to improve morbidity and mortality while minimizing adverse effects of unnecessary treatments. Limiting treatment of clinically insignificant cancers that do not threaten life expectancy can reduce overdiagnosis and overtreatment [9].
Contemporary non-targeted transrectal or transperineal prostate biopsies rely on real-time ultrasound guidance. Despite good imaging of the prostate gland boundaries and its adjacent organ structures, ultrasound cannot distinguish malignant lesions from benign ones [10]. Ultrasound alone is also insufficient to target specific lesions as about 40% are isoechoic [11]. The inability to target specific lesions can lead to sampling errors and inaccurate risk stratification which can affect subsequent clinical management decisions. Targeted biopsy techniques can potentially circumvent these limitations. Accumulating evidence supports the use of prebiopsy multiparametric magnetic resonance imaging (mpMRI) followed by magnetic resonance imaging (MRI) targeted biopsy as they detect more high-grade cancers with fewer biopsy cores, while reducing detection of low-grade clinically insignificant cancers [12,13,14,15]. To assess the value of such tests, cost-effectiveness studies can be conducted to simulate costs and effects of a new testing strategy and subsequent treatment options compared to existing strategies. Most published cost-effectiveness studies focus on a single biopsy protocol which do not reflect the use of MRI targeted biopsy in real life when multiple diagnostic strategies are used. A comprehensive comparison of all clinically relevant diagnostic strategies involving MRI targeted biopsy positioned in various diagnostic sequences can shed light on resource allocation in prostate cancer diagnosis in the initial and repeat biopsy settings. This is particularly pertinent as mpMRI and MRI targeted biopsy are becoming the standard of care in diagnosing prostate cancer [16, 17].
This study aims to evaluate the cost-effectiveness of six diagnostic strategies involving MRI targeted biopsy for diagnosing prostate cancer in initial and repeat biopsy settings from the Singapore healthcare system perspective.
Methods
Patient population
The starting model population was men aged 65 years clinically suspected of having localized prostate cancer based on elevated serum PSA above 4 ng/ml, abnormal digital rectal examination (DRE), or both, and referred for a first prostate biopsy. The starting age of 65 years was used as it corresponded with the estimated age recording a marked increase in age-specific incidence rate of prostate cancer in Singapore [18]. Men presenting de novo with metastases due to prostate malignancy were excluded as they almost never require MRI targeted biopsy for initial prostate cancer diagnosis due to the presence of locally advanced cancer in addition to their metastases.
Diagnostic strategies
Table 1 shows six diagnostic strategies relevant to the local practice that were evaluated in the model. They involved MRI targeted biopsy, systematic biopsy, and saturation biopsy, employed in different testing combinations and sequences. In Singapore’s context, MRI targeted biopsy refers to MRI-ultrasound (US) fusion targeted biopsy combined with systematic biopsy as a combined technique has been shown to improve detection of clinically significant cancer [19,20,21,22]. Locally, a 12-core systematic biopsy is the more common systematic biopsy performed. This definition of MRI targeted biopsy is consistent with a recent Cochrane review [23].
A positive prebiopsy mpMRI with a Prostate Imaging – Reporting and Data System (PI-RADS) score of 3 to 5 was subjected to further testing by MRI targeted biopsy. In the initial biopsy setting, patients with negative mpMRI did not proceed to biopsy; in repeat biopsy settings, patients with negative mpMRI received systematic biopsy alone if there was persistent clinical suspicion. Although saturation biopsy is also a form of systematic biopsy, it typically involves extracting 20 or more cores [24]. Saturation biopsy was assumed to be template prostate map** biopsy using a 5 mm sampling frame [25]. All prostate biopsies can be performed transrectally or transperineally with local or general anesthesia, and are associated with bleeding, infection, and urinary retention risks [26, 27]. The model assumed that patients received a maximum of three biopsies for initial diagnosis of prostate cancer if clinical suspicion remained.
Model structure and key specifications
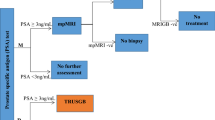
A model combining a decision tree and Markov sub-models was developed in consultation with local clinicians to ensure face validity (see Fig. 1). The decision tree described the detection of prostate cancer and evaluated the likelihood of men classified as having no cancer, or localized prostate cancer of various risk levels conditional on their true disease status. The Markov sub-models described disease progression conditional upon the risk level of the detected localized prostate cancer. These sub-models included the natural history of localized prostate cancer, downstream management strategies and death.
Simplified schema of model structure. Abbreviations: CRPC, castration-resistant prostate cancer; mpMRI, multiparametric magnetic resonance imaging; PI-RADS, Prostate Imaging – Reporting and Data System scores. Notes. 1. MRI targeted biopsy refers to the administration of MRI targeted biopsy combined with systematic biopsy following a positive mpMRI. 2. Diagnostic tests used only in repeat biopsy settings (i.e. as second or third biopsy) are represented in dotted lines.
The model was simulated over 20 years using annual cycle length, in line with the estimated life expectancy for men at age 65 years in Singapore [28]. During the simulated time horizon, patients moved through the model based on different transition probabilities to accrue costs and effects for each diagnostic strategy.
The outcome measure of the model was quality-adjusted life years (QALYs). Incremental cost-effectiveness ratios (ICERs) were calculated from pairwise comparisons of incremental costs and QALYs of the six diagnostic strategies. An annual discounting rate of 3% was applied to both costs and effectiveness. All analyses were conducted from the Singapore healthcare system perspective, and all costs were in 2020 United States (US) dollars ($, USD1 = SGD1.36) [29].
The model was built using TreeAge Pro 2018 R2.0 (TreeAge Software, Inc., MA). Analyses were performed using Monte Carlo simulation (seed number set at 16).
Model input parameters
Model input parameters were obtained from published data where possible and available. To overcome the dearth of real-world local data on downstream management pathways and resources, distribution of care strategies, and distribution of localized prostate cancer across cancer risks, a survey was co-developed and administered to clinician experts from October 2018 to January 2019. Each of the five local public healthcare institutions offering MRI targeted biopsy, and a specialty cancer center, was represented by a clinician expert to provide and coordinate inputs from their practice setting. A total of six responses were received. Clinician experts that provided inputs included urologists, radiologists, and medical oncologist.
Natural history and management strategies
Based on the number of patients suspected of prostate cancer referred to public healthcare institutions for the first biopsy in the past 5 years, the estimated prevalence of prostate cancer in men referred for the first biopsy was 37.7%. The risk of disease progression from localized prostate cancer to metastasis and the risk of death depends on age, tumor risk, and prostate cancer management strategy [30]. The model used the European Association of Urology (EAU) risk groups for localized prostate cancer – low-risk, intermediate-risk, and high-risk prostate cancer defined by PSA levels, International Society of Urology Pathology (ISUP) grades, or clinical tumor categories [16]. Low-risk prostate cancers were clinically insignificant, while intermediate or high-risk prostate cancers were clinically significant [31].
Conventional prostate cancer management strategies considered in the model included active surveillance which involved active disease monitoring and potentially curative therapy if the cancer progressed [9]; watchful waiting which is palliative in nature [16]; and active treatment involving radical prostatectomy or radiotherapy with or without androgen deprivation therapy [16]. In the model, after a biopsy-confirmed diagnosis of localized prostate cancer, management strategies were assigned based on the patient’s risk stratification, disease status, and whether the treatment intent was curative or palliative (see Additional file 1 for distribution of care strategies for localized prostate cancer of various risks). Upon detection of intermediate or high-risk localized prostate cancer, patients experienced watchful waiting, active treatment, metastasis, or death; low-risk localized prostate cancer patients including true low-risk or misclassified intermediate-risk experienced watchful waiting, active treatment, active surveillance, metastasis, or death. Patients on active surveillance remained on the same management strategy unless prompted by disease progression to undergo active treatment, or transition to watchful waiting from age of 75 years, whichever earlier [16] .
Undiagnosed patients and patients on active surveillance were assumed to follow progression rates associated with watchful waiting [30]. Patients with true intermediate-risk cancer but misclassified as low-risk cancer received treatments for low-risk localized prostate cancer but followed progression rates for intermediate-risk localized prostate cancer. Only patients with misclassified low-risk prostate cancer on active surveillance were likely to experience metastasis before they switched to active treatment. In line with clinician experts’ inputs, an estimated 50% of patients with misclassified low-risk prostate cancer in the active surveillance cohort switched to active treatment every year. Patients receiving radical prostatectomy and radiotherapy experienced the same progression risk [16]. Patients whose cancer diagnoses were missed by biopsy remained under observation or watchful waiting at age of 75 years and did not receive any active treatment until onset of metastatic disease.
A proportion of patients receiving active treatment could experience biochemical recurrence to trigger further treatments such as salvage treatment [32]. When symptomatic metastases developed, they either received active or palliative care. Some patients with metastatic disease may further progress to castration-resistant prostate cancer (CRPC) despite hormonal therapy with or without the early use of docetaxel or novel oral hormonal agents in the castrate-sensitive state, necessitating the use of other CRPC therapies such as abiraterone, enzalutamide, radium-223, docetaxel and cabazitaxel (see Additional file 2 for distribution of treatments for metastatic cancer and castration-resistant prostate cancer and Additional file 3 for percentage of treatment-related complications).
All prostate cancer-related deaths were assumed to occur following metastatic disease. In any health state, men could die from causes other than prostate cancer by applying the general population’s all-cause mortality rates for resident males [33].
Model validation
To ensure face validity, inputs from surveyed clinician experts provided insights to diagnostic and management pathways for prostate cancer, model inputs and assumptions used in the model. For external validity, 15-year overall survival rates from the PREDICT Prostate multivariate model were compared with the current model’s predicted overall survival output (see Additional file 4 for more details on this comparison) [34].
Diagnostic performance and uptake
Table 2 shows the diagnostic performance of various tests and the estimated biopsy uptake rates in local clinical practice. Diagnostic performance of the various tests varied in initial and repeat biopsy settings due to differing prostate cancer risks [23, 35]. Patients with positive biopsy findings had their disease staged and received appropriate management. The model assumed that only patients who were truly intermediate-risk could be susceptible to be misclassified and managed as low-risk. In negative biopsies, all false negative cases were assumed to have persistently elevated PSA level, while an estimated 75% of those with true negative results remained clinically suspicious for prostate cancer. These were indications for a subsequent biopsy a year later. The distribution of patients with localized prostate cancer of varying risk was 31% low risk, 44% intermediate risk, and 25% high risk (see Additional file 5 for more details on this distribution).
Health utilities
A QALY is an outcome measure derived by adjusting the length of time by quality of life measured in health utilities on a scale of 0 (death) to 1 (perfect health) [36]. Table 3 shows health utilities inputs in the model. Utilities for men with the modelled starting age of 65 years with no diagnosed cancer was taken from a multi-country population norms study using the EQ-5D-3L [37]. The model considered utility decrements associated with age [38], utilities associated with localized prostate cancer of various risk levels and metastases [39], and impact of biopsies on utilities [31, 35]. Varying complication rates arising from saturation or systematic biopsies led to different utility decrements [31, 35]. Patients with undetected cancer or on active surveillance were assumed to have the same utilities as patients on watchful waiting. Utilities of patients with CRPC were lower than those without CRPC assuming no substantial decline in utilities until CRPC developed [40]. Metastatic patients receiving palliative care were assumed to have the same utilities as those not receiving CRPC care. Health utility benefits associated with active treatment was assumed to be equal for low, intermediate, and high-risk localized cancers, and remained constant until metastasis occurred. Treatment complications and biochemical recurrence were associated with utility decrements [41, 42].
Costs
In line with a healthcare system perspective, only direct medical resources were considered. Table 4 shows that cost inputs included charges associated with the diagnostic strategies, management and treatment of prostate cancer, and management of complications.
Sensitivity analyses
One-way sensitivity analyses were conducted over the range of predefined values for specific model parameters using the reported 95% confidence intervals from published literature or ± 10% of point estimate. Except for costs of mpMRI and biopsies in the evaluated diagnostic strategies, costs were considered known parameters and were not evaluated in the one-way sensitivity analyses. If more than two strategies were considered cost-effective, one-way sensitivity analyses were performed for the pair of strategies with the highest ICER in the deterministic base case analyses. The least costly strategy was used as the reference strategy for the pairwise comparison.
Multivariate probabilistic sensitivity analyses with 1000 second-order Monte Carlo simulations (50,000 first-order simulation trials) were performed. The uncertainty of model inputs was explored by simultaneously and randomly sampling the parameters from assigned distributions. Cost-effectiveness acceptability curves presented the cost-effectiveness probability of each diagnostic strategy over a range of willingness-to-pay (WTP) thresholds.
Results
Base case analyses
Figure 2 showed strategies 1, 2 and 4 on the cost-effectiveness plane, indicating that these strategies achieved the most QALYs per USD spent. Strategy 1 had the lowest cost, while Strategies 2 and 4 gave ICERs ranging from US$18,975 to US$19,458 when compared to Strategies 1 and 2 respectively (see Table 5). When Strategy 4 was compared to Strategy 1, the ICER was US$19,175. Strategies 1, 2, 4 involved MRI targeted biopsy used in the biopsy-naïve patients in the initial biopsy setting and were considered cost-effective, assuming a WTP threshold of US$20,000. On the other hand, strategies that involved MRI targeted biopsy in the repeat biopsy setting (Strategies 5 and 6) were dominated, incurring more costs with less QALY gain. Similarly, Strategy 3 was dominated, suggesting saturation biopsy, when used, should be reserved as the last option within a testing sequence.
Cost-effectiveness plane of base case analyses. Abbreviations: mpMRI, multiparametric magnetic resonance imaging; MRI, magnetic resonance imaging; QALY, quality-adjusted life years; USD, US dollars. Notes. 1. Strategy 1: MRI targeted biopsy. Strategy 2: MRI targeted biopsy ➔ Systematic biopsy. Strategy 3: MRI targeted biopsy ➔ Saturation biopsy. Strategy 4: MRI targeted biopsy ➔ Systematic biopsy ➔ Saturation biopsy. Strategy 5: Systematic biopsy ➔ MRI targeted biopsy. Strategy 6: Systematic biopsy ➔ MRI targeted biopsy ➔ Saturation biopsy. 2. MRI targeted biopsy refers to the administration of MRI targeted biopsy combined with systematic biopsy following a positive mpMRI. 3. Strategies 1, 2, 4 are on the cost-effectiveness frontier, indicating that they achieve the most QALY per USD spent; Strategies 3, 5, 6 are dominated as they are more costly and gave fewer QALYs.
Sensitivity analyses
One-way sensitivity analyses were performed for Strategy 4 compared with Strategy 1 as the reference strategy as Strategy 1 had the lowest cost. Figure 3 showed that ICER was most sensitive to annual discounting rate, prevalence of prostate cancer in men referred for first biopsy, probability of detecting low-risk cancer given true low-risk cancer using systematic biopsy as first biopsy, and the probability of low-risk cancer classified as suspicious clinically significant cancer by mpMRI (see Additional file 6 for summary of one-way sensitivity analyses parameters and results of Strategy 4 vs Strategy 1). All ICERs remained between US$15,755 to US$23,022 per QALY gained when model parameters were varied (see Additional file 7 for full chart of ICER tornado diagram for Strategy 4 vs Strategy 1).
ICER tornado diagram for Strategy 4 vs Strategy 1 (top 10 drivers). Abbreviations: ICER, incremental cost-effectiveness ratio; MRI, magnetic resonance imaging; mpMRI, multi-parametric magnetic resonance imaging; PSA, prostate-specific antigen. Notes. 1. Strategy 1: MRI targeted biopsy. Strategy 4: MRI targeted biopsy ➔ Systematic biopsy ➔ Saturation biopsy. 2. MRI targeted biopsy refers to the administration of MRI targeted biopsy combined with systematic biopsy following a positive mpMRI. 3. Blue bars denote the ICERs when the parameter’s lower bound limit is tested; red bars denote the ICERs when the parameter’s upper bound limit is tested.
Probabilistic sensitivity analyses in Fig. 4 found that Strategies 1, 2, 4, and 6 were on the cost-effectiveness frontier, while Strategies 3 and 5 remained dominated. The ICERs for Strategies 1, 2, 4, and 6 ranged from US$15,990 to US$58,097 (see Additional file 8 for probabilistic sensitivity analyses of all strategies for base case). The cost-effectiveness acceptability curves in Fig. 5 confirmed that Strategy 1 was the least costly, and Strategy 2 was potentially cost-effective at WTP of US$20,000 and Strategy 4 at WTP of US$30,000. However, the probability of Strategy 2 being cost-effective was less than 50%. Strategy 6 could only be considered cost-effective when WTP increased to US$60,000.
Cost-effectiveness plane of probabilistic sensitivity analyses. Abbreviations: mpMRI, multiparametric magnetic resonance imaging; MRI, magnetic resonance imaging; QALY, quality-adjusted life years; USD, US dollars. Notes. 1. Strategy 1: MRI targeted biopsy. Strategy 2: MRI targeted biopsy ➔ Systematic biopsy. Strategy 3: MRI targeted biopsy ➔ Saturation biopsy. Strategy 4: MRI targeted biopsy ➔ Systematic biopsy ➔ Saturation biopsy. Strategy 5: Systematic biopsy ➔ MRI targeted biopsy. Strategy 6: Systematic biopsy ➔ MRI targeted biopsy ➔ Saturation biopsy. 2. MRI targeted biopsy refers to the administration of MRI targeted biopsy combined with systematic biopsy following a positive mpMRI. 3. Strategies 1, 2, 4, 6 are on the cost-effectiveness frontier, indicating that they achieve the most QALY per USD spent; Strategies 3, 5 are dominated as they are more costly and gave fewer QALYs.
Cost-effectiveness acceptability curves. Abbreviations: mpMRI, multiparametric magnetic resonance imaging; MRI, magnetic resonance imaging; QALY, quality-adjusted life years; USD, US dollars. Notes. 1. Strategy 1 (shown in blue squares): MRI targeted biopsy. Strategy 2 (shown in red triangles): MRI targeted biopsy ➔ Systematic biopsy. Strategy 3 (shown in yellow circles): MRI targeted biopsy ➔ Saturation biopsy. Strategy 4 (shown in green triangles): MRI targeted biopsy ➔ Systematic biopsy ➔ Saturation biopsy. Strategy 5 (shown in blue diamonds): Systematic biopsy ➔ MRI targeted biopsy. Strategy 6 (shown in purple ovals): Systematic biopsy ➔ MRI targeted biopsy ➔ Saturation biopsy. 2. MRI targeted biopsy refers to the administration of MRI targeted biopsy combined with systematic biopsy following a positive mpMRI
Discussion
This study found that all strategies including MRI targeted biopsy in the initial biopsy setting were cost-effective except when saturation biopsy was used immediately as a second biopsy. Many published cost-effectiveness analyses only evaluated MRI targeted biopsy as a first biopsy compared with systematic transrectal ultrasound-guided (TRUS) biopsy based on a single biopsy protocol [43,44,45,46]. With MRI targeted biopsy becoming more commonly used [16, 17], a more pertinent resource allocation question should be – what are the cost-effective ways to deploy MRI targeted biopsy, given the many possible diagnostic sequences within a clinical care pathway. By varying the position of MRI targeted biopsy in six diagnostic strategies, single and multiple biopsy strategies containing MRI targeted biopsy could address how its introduction in a testing strategy could impact its cost-effectiveness. To the best of our knowledge, no other study has evaluated the cost-effectiveness of MRI targeted biopsy within a testing sequence.
This study also incorporated relevant clinical considerations to estimate long-term costs and effectiveness of various diagnostic strategies in single and multiple biopsy settings. Diagnostic accuracy inputs on MRI targeted biopsy for different risk groups were drawn mainly from a recent Cochrane review which comprehensively reviewed the evidence on MRI targeted biopsies based on 17 studies in initial biopsy setting and 8 studies in the repeat biopsy setting [23]. This avoids mixing cost and health impact of detecting clinically significant and clinically insignificant prostate cancer [45, 47]. Downstream management strategies were comprehensively modelled to ensure that diagnostic outcomes and follow-up care included expensive CRPC drug therapies such as abiraterone, enzalutamide, and cabazitaxel. While early detection of treatable clinically significant cancer is the aim of any prostate biopsy, new developments in downstream management of patients with detected cancer or those with persistent clinical suspicion of prostate cancer despite previous negative biopsies can potentially create an undesirable situation where their consequent management accrue higher costs and lower utilities.
The consistently favorable results of Strategies 1, 2 and 4 showed that early use of MRI targeted biopsy as a first biopsy was potentially cost-effective but could be influenced by when saturation biopsy was used. Strategy 3 where saturation biopsy was the second biopsy was shown to be dominated, whereas Strategy 4 had an ICER of US$19,458 when saturation biopsy was used as the third biopsy, implying later use of saturation biopsy could improve the cost-effectiveness of MRI targeted biopsy in the initial biopsy setting. Despite high sensitivity of 95% in detecting localized prostate cancer, saturation biopsy gave greater utility decrements and costs arising from higher complication rates than systematic biopsy.
The discordant results for Strategy 6 in the deterministic base case analyses and probabilistic sensitivity analyses could be explained by the modest difference in the incremental effectiveness between Strategy 4 and Strategy 6, and the multiplicative effect of input parameters in a nonlinear model. In both initial and repeat biopsies, MRI targeted biopsy improved detection of clinically significant cancer and clinically insignificant cancer [23]. Compared to Strategy 4, Strategy 6 had marginally higher detection rate of clinically significant cancer by 0.02%, and clinically insignificant cancer by 0.2% [23]. For strategies with similar diagnostic performance like Strategies 4 and 6, any change in costs and QALYs resulting from a complex nonlinear model with multiplicative effects of input parameters can introduce further uncertainty in the cost-effectiveness result. As probabilistic sensitivity analyses are preferred for estimating mean costs and outcomes in nonlinear models [48, 49], the results lend support for using MRI targeted biopsy in the initial and repeat biopsy settings. Although Strategy 1, a single biopsy protocol, is least costly, it has limited applicability in cases with persistently high clinical suspicion.
Despite a lack of published literature comparing diagnostic strategies that varied position of the MRI targeted biopsy, published literature found greater inconsistency in the cost-effectiveness of MRI targeted biopsy in the repeat biopsy setting. MRI targeted biopsy compared with TRUS systematic biopsy in the initial biopsy setting were consistently cost-effective [43,44,45,46,47, 50]; published literature on MRI targeted biopsy as a second biopsy reported wide ranging results from £5778 per QALY to being dominated when compared with TRUS systematic biopsy [31, 35, 50], indicating less consistent findings in this setting. This could be due to the mounting diagnostic challenge as the yield of clinically significant cancer progressively declines with each subsequent biopsy [51] and the variable downstream care strategies in different practice settings. Recent guidelines also indicated varied strength when recommending MRI targeted biopsy use in repeat biopsy settings [16, 17].
Successful implementation of any diagnostic strategies in a practice setting requires several considerations. The effectiveness of MRI targeted biopsy depends on the quality assurance of mpMRI. Although PI-RADS scores help standardize the acquisition, interpretation and reporting of prostate mpMRI, the learning curve of interpreting mpMRI remains steep and inter-observer differences persist. Radiology practices performing prostate mpMRI should engage in in-house training and continual quality improvement programs to ensure a minimum competency standard is maintained [46, 52]. With evidence supporting prostate mpMRI as a triage in the initial biopsy setting [35], the utilization of mpMRI and its corresponding waiting time are likely to increase but potentially with corresponding reduction in unnecessary biopsies.
This study has several limitations. First, the diagnostic input of MRI targeted biopsy was based upon a review that included all MRI targeted biopsy techniques including cognitive MRI targeted biopsy and in-bore MRI targeting [23]. Variations in these targeted techniques, scanning protocols, varying thresholds for mpMRI positivity to trigger biopsy could contribute to heterogeneity. Despite these sources of heterogeneity, current evidence has not demonstrated clear superiority of one MRI-based biopsy technique over another [16]. Sensitivity analyses performed in this study using published 95% confidence intervals also helped ensure the robustness of the results.
Second, probabilities of metastatic progression and treatment effects were sourced from the SPCG-4 trial which provided up to 18 years’ follow-up data [30, 53]. Although the trial provided good long-term follow-up data, it recruited patients between 1989 and 1999 when PSA testing was not yet widely adopted. Recent improvements in contemporary treatment modalities such as robotic surgery and more precise radiotherapy are expected to give better QALY benefits. The more recent PIVOT trial enrolled men from 1994 to 2002 during the early era of PSA testing but did not report time to progression for each risk group as required by this model [54, 55]. As such, the progression rates of patients receiving radical prostatectomy in the SPCG-4 trial represented a conservative estimate of the treatment modality, while those receiving watchful waiting best approximated the natural history of prostate cancer without treatment [31].
Third, this study did not include a potential diagnostic strategy with two consecutive MRI targeted biopsies due to its low frequency of use in local clinical practice and lack of clarity in the guidelines. This diagnostic strategy could take place if there were concerns that the first MRI targeted biopsy was not optimal due to technical reasons or if the second mpMRI showed changes in the lesion(s) after a negative initial biopsy. Future studies could consider including this strategy when there is more published data or greater clarity in guidelines.
Fourth, model inputs such as the prevalence of prostate cancer in men referred for biopsy, the distribution of localized prostate cancer risks, and allocation of care and treatment strategies were informed through surveys of clinician experts working in the local public healthcare institutions. This was due to a lack of published real-world local data. To ensure representativeness of the findings, each public healthcare institution offering prostate biopsy had at least one urologist providing input. Additional inputs from radiologists were also sought. As model inputs and assumptions were developed for a starting age of 65 years, its findings cannot readily apply to other starting ages without a separate undertaking to ensure rigor and relevance of model input and assumptions.
Fifth, health utility scores were from published literature derived from Australia, Canada, Netherlands, United Kingdom, and USA due to a lack of published local information [39,40,41, 56, 57]. Given a lack of local published health utilities, these sources present the best available published evidence that met the data needs of the model. While the emotional toll of a cancer diagnosis could adversely impact quality of life, similar utilities between no cancer and localized low-risk prostate cancer were applied based on available published utilities. The small difference is expected given the typically slow-progressing nature of prostate cancer. When varied in one-way sensitivity analyses, they did not materially impact the ICERs (see Additional file 8 for probabilistic sensitivity analyses of all strategies for base case).
Conclusions
To conclude, this study found Strategy 1, Strategy 2, and Strategy 4 – where MRI targeted biopsy was used in biopsy-naïve patients in the initial biopsy setting – to be cost-effective diagnostic options for prostate cancer. The findings are useful to inform decision making in funding different diagnostic options within the Singapore public healthcare system.
Availability of data and materials
The dataset(s) supporting the conclusions of this article are included within the article and (and its additional file(s)).
Abbreviations
- CRPC:
-
Castration-resistant prostate cancer
- DRE:
-
Digital rectal examination
- EAU:
-
European Association of Urology
- ICER:
-
Incremental cost-effectiveness ratio
- ISUP:
-
International Society of Urology Pathology
- mpMRI:
-
Multiparametric magnetic resonance imaging
- MRI:
-
Magnetic resonance imaging
- MRI-US:
-
Magnetic resonance imaging - ultrasound
- PI-RADS:
-
Prostate Imaging – Reporting and Data System
- PSA:
-
Prostate-specific antigen
- QALY:
-
Quality-adjusted life year
- TRUS:
-
Transrectal ultrasound-guided
- US:
-
United States
- USD:
-
United States dollar
- WTP:
-
Willingness-to-pay
References
Cancer IAfRo. Global Cancer Registry (GLOBOCAN). Lyon: World Health Organization: International Agency for Research on Cancer; 2018.
Registry; SC. 50 years of cancer registration - Singapore Cancer Registry: Singapore; 2019.
Rawla P. Epidemiology of prostate Cancer. World J Oncol. 2019;10(2):63–89. https://doi.org/10.14740/wjon1191.
American Cancer Society. Cancer Facts & Figures 2020. Atlanta: American Cancer Society; 2020.
Draisma G, Etzioni R, Tsodikov A, Mariotto A, Wever E, Gulati R, et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst. 2009;101(6):374–83. https://doi.org/10.1093/jnci/djp001.
Etzioni R, Penson DF, Legler JM, di Tommaso D, Boer R, Gann PH, et al. Overdiagnosis due to prostate-specific antigen screening: lessons from U.S. prostate Cancer incidence trends. J Natl Cancer Inst. 2002;94(13):981–90. https://doi.org/10.1093/jnci/94.13.981.
Singapore; AoM. Report of the Screening Test Review Committee. Singapore: Academy of Medicine, Singapore; 2019.
Matoso A, Epstein JI. Defining clinically significant prostate cancer on the basis of pathological findings. Histopathology. 2019;74(1):135–45. https://doi.org/10.1111/his.13712.
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN guidelines) - prostate Cancer (version 2.2020): National Comprehensive Cancer Network; 2002.
Hegde JV, Mulkern RV, Panych LP, Fennessy FM, Fedorov A, Maier SE, et al. Multiparametric MRI of prostate cancer: an update on state-of-the-art techniques and their performance in detecting and localizing prostate cancer. J Magn Reson Imaging. 2013;37(5):1035–54. https://doi.org/10.1002/jmri.23860.
Panebianco V, Barchetti F, Sciarra A, Ciardi A, Indino EL, Papalia R, et al. Multiparametric magnetic resonance imaging vs. standard care in men being evaluated for prostate cancer: a randomized study. Urol Oncol. 2015;33(1):17 e1–7.
Turkbey B, Mani H, Shah V, Rastinehad AR, Bernardo M, Pohida T, et al. Multiparametric 3T prostate magnetic resonance imaging to detect cancer: histopathological correlation using prostatectomy specimens processed in customized magnetic resonance imaging based molds. J Urol. 2011;186(5):1818–24. https://doi.org/10.1016/j.juro.2011.07.013.
Siddiqui MM, Rais-Bahrami S, Truong H, Stamatakis L, Vourganti S, Nix J, et al. Magnetic resonance imaging/ultrasound-fusion biopsy significantly upgrades prostate cancer versus systematic 12-core transrectal ultrasound biopsy. Eur Urol. 2013;64(5):713–9. https://doi.org/10.1016/j.eururo.2013.05.059.
Rastinehad AR, Turkbey B, Salami SS, Yaskiv O, George AK, Fakhoury M, et al. Improving detection of clinically significant prostate cancer: magnetic resonance imaging/transrectal ultrasound fusion guided prostate biopsy. J Urol. 2014;191(6):1749–54. https://doi.org/10.1016/j.juro.2013.12.007.
Wysock JS, Rosenkrantz AB, Huang WC, Stifelman MD, Lepor H, Deng FM, et al. A prospective, blinded comparison of magnetic resonance (MR) imaging-ultrasound fusion and visual estimation in the performance of MR-targeted prostate biopsy: the PROFUS trial. Eur Urol. 2014;66(2):343–51. https://doi.org/10.1016/j.eururo.2013.10.048.
Mottet N, van den Bergh RCN, Briers E, Cornford P, De Santis M, Fanti S, et al. EAU-EANM-ESUR-ESTRO-SIOG guidelines on prostate Cancer. Amhem: EAU; 2019.
NICE. NICE guideline on Prostate cancer: diagnosis and management: National Institute for Health and Care Excellence; 2019.
Singapore Cancer Registry. 50 years of cancer registry. Singapore: Health Promotion Board; 2019.
Elkhoury FF, Felker ER, Kwan L, Sisk AE, Delfin M, Natarajan S, et al. Comparison of targeted vs systematic prostate biopsy in men who are biopsy naive: the prospective assessment of image registration in the diagnosis of prostate Cancer (PAIREDCAP) study. JAMA Surg. 2019;154(9):811–8. https://doi.org/10.1001/jamasurg.2019.1734.
Lee AY, Yang XY, Lee HJ, Law YM, Hong HH, Lau WK, et al. Multiparametric MRI-Ultrasound Software Fusion Prostate Biopsy - Initial Results Using A Stereotactic Robotic Assisted Transperineal Prostate Biopsy Platform Comparing Systematic Versus Targeted Biopsy. BJU Int. 2020;126(5):568–76. https://doi.org/10.1111/bju.15118.
Radtke JP, Kuru TH, Boxler S, Alt CD, Popeneciu IV, Huettenbrink C, et al. Comparative analysis of transperineal template saturation prostate biopsy versus magnetic resonance imaging targeted biopsy with magnetic resonance imaging-ultrasound fusion guidance. J Urol. 2015;193(1):87–94. https://doi.org/10.1016/j.juro.2014.07.098.
Rouvière O, Puech P, Renard-Penna R, Claudon M, Roy C, Mège-Lechevallier F, et al. Use of prostate systematic and targeted biopsy on the basis of multiparametric MRI in biopsy-naive patients (MRI-FIRST): a prospective, multicentre, paired diagnostic study. Lancet Oncol. 2019;20(1):100–9. https://doi.org/10.1016/S1470-2045(18)30569-2.
Drost FJH, Osses DF, Nieboer D, Steyerberg EW, Bangma CH, Roobol MJ, et al. Prostate MRI, with or without MRI-targeted biopsy, and systematic biopsy for detecting prostate cancer (review). Cochrane Database Syst Rev. 2019;4(4):CD012663. https://doi.org/10.1002/14651858.CD012663.pub2.
Scattoni V, Maccagnano C, Zanni G, Angiolilli D, Raber M, Roscigno M, et al. Is extended and saturation biopsy necessary? Int J Urol. 2010 May;17(5):432–47. https://doi.org/10.1111/j.1442-2042.2010.02479.x.
South A, Ahmed H, Brown L, Dudderidge T, Hindley R, Kaplan R, et al. MRC CTU at UCL briefing paper: multi-parametric MRI scans prior to biopsy for improving diagnosis of prostate cancer. 2017.
Borghesi M, Ahmed H, Nam R, Schaeffer E, Schiavina R, Taneja S, et al. Complications after systematic, random, and image-guided prostate biopsy. Eur Urol. 2017;71(3):353–65. https://doi.org/10.1016/j.eururo.2016.08.004.
Loeb S, Vellekoop A, Ahmed HU, Catto J, Emberton M, Nam R, et al. Systematic review of complications of prostate biopsy. Eur Urol. 2013;64(6):876–92. https://doi.org/10.1016/j.eururo.2013.05.049.
Department of Statistics Singapore. Death and Life Expectancy - Latest Data. 2020 [cited 2020 14 Jun]; Available from: https://www.singstat.gov.sg/find-data/search-by-theme/population/death-and-life-expectancy/latest-data
Monetary Authority of Singapore. Exchange Rate. 2020 [cited 2020 15 May]; Available from: https://secure.mas.gov.sg/msb/ExchangeRates.aspx
Bill-Axelson A, Holmberg L, Garmo H, Rider JR, Taari K, Busch C, et al. Radical prostatectomy or watchful waiting in early prostate Cancer. N Engl J Med. 2014;370(10):932–42. https://doi.org/10.1056/NEJMoa1311593.
NICE. Guidelines NG131: Prostate cancer update health economic model report 2019.
Menon M, Bhandari M, Gupta N, Lane Z, Peabody JO, Rogers CG, et al. Biochemical recurrence following robot-assisted radical prostatectomy: analysis of 1384 patients with a median 5-year follow-up. Eur Urol. 2010;58(6):838–46. https://doi.org/10.1016/j.eururo.2010.09.010.
Department_of_Statistics. Department of Statistics, Complete life tables for Singapore Resident Population, 2016–2017. 2018. Available at https://www.singstat.gov.sg/publications/population/-/media/Files/publications/population/lifetable16-17. Accessed 30 Nov 2018.
Thurtle DR, Greenberg DC, Lee LS, Huang HH, Pharoah PD, Gnanapragasam VJ. Individual prognosis at diagnosis in nonmetastatic prostate cancer: Development and external validation of the PREDICT Prostate multivariable model. PLoS Med. 2019;16(3):e1002758. https://doi.org/10.1371/journal.pmed.1002758.
Brown LC, Ahmed HU, Faria R, Bosaily AE-S, Gabe R, Kaplan RS, et al. Multiparametric MRI to improve detection of prostate cancer compared with transrectal ultrasound-guided prostate biopsy alone: the PROMIS study. Health Technol Assessment (Winchester, England). 2018;22(39):1.
Drummond MF, Sculpher MJ, Torrance GW, O'Brien BJ, Stoddart GL. Methods for the economic evaluation of health care Programmes. 3rd ed. New York: Oxford University Press; 2005.
Clemens S, Begum N, Harper C, Whitty JA, Scuffham PA. A comparison of EQ-5D-3L population norms in Queensland, Australia, estimated using utility value sets from Australia, the UK and USA. Qual Life Res. 2014;23(8):2375–81. https://doi.org/10.1007/s11136-014-0676-x.
Ara R, Brazier JE. Populating an economic model with health state utility values: moving toward better practice. Value Health. 2010;13(5):509–18. https://doi.org/10.1111/j.1524-4733.2010.00700.x.
Stewart ST, Lenert L, Bhatnagar V, Kaplan RM. Utilities for prostate cancer health states in men aged 60 and older. Med Care. 2005;43(4):347–55. https://doi.org/10.1097/01.mlr.0000156862.33341.45.
Bayoumi AM, Brown AD, Garber AM. Cost-effectiveness of androgen suppression therapies in advanced prostate cancer. J Natl Cancer Inst. 2000;92(21):1731–9. https://doi.org/10.1093/jnci/92.21.1731.
Krahn M, Ritvo P, Irvine J, Tomlinson G, Bremner KE, Bezjak A, et al. Patient and community preferences for outcomes in prostate cancer: implications for clinical policy. Med Care. 2003 Jan;41(1):153–64. https://doi.org/10.1097/00005650-200301000-00017.
Ramsay C, Pickard R, Robertson C, Close A, Vale L, Armstrong N, et al. Systematic review and economic modelling of the relative clinical benefit and cost-effectiveness of laparoscopic surgery and robotic surgery for removal of the prostate in men with localised prostate cancer. Health Technol Assess. 2012;16(41):1–313. https://doi.org/10.3310/hta16410.
Pahwa S, Schiltz NK, Ponsky LE, Lu Z, Griswold MA, Gulani V. Cost-effectiveness of MR imaging–guided strategies for detection of prostate cancer in biopsy-naive men. Radiology. 2017;285(1):157–66. https://doi.org/10.1148/radiol.2017162181.
Venderink W, Govers TM, de Rooij M, Fütterer JJ, Sedelaar JM. Cost-effectiveness comparison of imaging-guided prostate biopsy techniques: systematic transrectal ultrasound, direct in-bore MRI, and image fusion. Am J Roentgenol. 2017;208(5):1058–63. https://doi.org/10.2214/AJR.16.17322.
Cerantola Y, Dragomir A, Tanguay S, Bladou F, Aprikian A, Kassouf W. Cost-effectiveness of multiparametric magnetic resonance imaging and targeted biopsy in diagnosing prostate cancer. Urol Oncol. 2016;34(3):119.e1–9. https://doi.org/10.1016/j.urolonc.2015.09.010.
Barnett CL, Davenport MS, Montgomery JS, Wei JT, Montie JE, Denton BT. Cost-effectiveness of magnetic resonance imaging and targeted fusion biopsy for early detection of prostate cancer. BJU Int. 2018;122(1):50–8. https://doi.org/10.1111/bju.14151.
National Collaborating Centre for Cancer. Prostate Cancer: diagnosis and treatment - Clinical Guideline. UK: National Collaborating Centre for Cancer; 2014.
National Institute for Health and Care Excellence. Guide to the methods of technology appraisal 2013. UK: NICE; 2013.
Claxton K. Exploring uncertainty in cost-effectiveness analysis. Pharmacoeconomics. 2008;26(9):781–98. https://doi.org/10.2165/00019053-200826090-00008.
Gordon LG, Tuffaha H, James R, Scuffham P. Economic modelling of healthcare services for prostate cancer. Australia: Griffith University, Prostate Cancer Foundation of Australia; 2016.
Roehl KA, Antenor JAV, Catalona WJ. Serial biopsy results in prostate cancer screening study. J Urol. 2002;167(6):2435–9. https://doi.org/10.1016/S0022-5347(05)64999-3.
American Urological Association. Prostate MRI and MRI-Targeted Biopsy in Patients with Prior Negative Biopsy; 2016.
Bill-Axelson A, Holmberg L, Garmo H, Taari K, Busch C, Nordling S, et al. Radical prostatectomy or watchful waiting in prostate Cancer - 29-year follow-up. N Engl J Med. 2018;379(24):2319–29. https://doi.org/10.1056/NEJMoa1807801.
Wilt TJ, Brawer MK, Jones KM, Barry MJ, Aronson WJ, Fox S, et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med. 2012;367(3):203–13. https://doi.org/10.1056/NEJMoa1113162.
Wilt TJ, Jones KM, Barry MJ, Andriole GL, Culkin D, Wheeler T, et al. Follow-up of prostatectomy versus observation for early prostate Cancer. N Engl J Med. 2017;377(2):132–42. https://doi.org/10.1056/NEJMoa1615869.
Stewart SB, Scales CD Jr, Moul JW, Reed SD. Does variation in either age at start of therapy or duration of therapy make chemoprevention with finasteride cost-effective? Prostate Cancer Prostatic Dis. 2012;15(4):380–5. https://doi.org/10.1038/pcan.2012.26.
Korfage IJ, Essink-Bot ML, Borsboom GJ, Madalinska JB, Kirkels WJ, Habbema JDF, et al. Five-year follow-up of health-related quality of life after primary treatment of localized prostate cancer. Int J Cancer. 2005;116(2):291–6. https://doi.org/10.1002/ijc.21043.
Acknowledgements
This study was part of a health technology assessment (HTA) evaluation on MRI-US fusion targeted biopsy conducted by the Agency for Care Effectiveness (ACE), Ministry of Health, Singapore in 2019. The authors would like to thank Mr. Chris Foteff for his inputs to the initial draft of this manuscript, and Mr. Mohamed Ismail Abdul Aziz and Ms. Cher Boon Piang for their inputs to the revision of this manuscript.
Funding
This study did not receive any sponsorship or funding.
Author information
Authors and Affiliations
Contributions
Conception and design: L.C., S.S., C.T., K.N. Analysis and interpretation of the data: L.C., S.S., T.T., C.T., T.L., K.T., W.L., B.A., E.C., K.N. Drafting of the paper or revising it critically for intellectual content: L.C., S.S., T.T., C.T., T.L., K.T., W.L., B.A., E.C., K.N. Final approval of the version to be published: L.C., S.S., T.T., C.T., T.L., K.T., W.L., B.A., E.C., K.N. All authors agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
No permission was required to access aggregated cost and utilisation data for MOH, and published literature.
Consent for publication
Not applicable.
Competing interests
E.C. received speaker honorarium from Athena medical company.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Distribution of care strategies for localized prostate cancer of various risks.
Additional file 2: Table S2.
Distribution of treatments for metastatic cancer and castration-resistant prostate cancer.
Additional file 3: Table S3.
Percentage of the treatment-related complications.
Additional file 4: Table S4.
Comparison of overall survival of men from published prostate cancer data and current study’s modelled population.
Additional file 5: Table S5.
Distribution of patients with localised prostate cancer across risk status.
Additional file 6: Table S6.
Summary of one-way sensitivity analyses parameters and results - Strategy 4 vs Strategy 1.
Additional file 7: Fig. S1.
ICER tornado diagram for Strategy 4 vs Strategy 1 (full chart).
Additional file 8: Table S7.
Probabilistic sensitivity analyses of all strategies for base case.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Cheng, LJ., Soon, S.S., Tan, T.W. et al. Cost-effectiveness of MRI targeted biopsy strategies for diagnosing prostate cancer in Singapore. BMC Health Serv Res 21, 909 (2021). https://doi.org/10.1186/s12913-021-06916-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12913-021-06916-0