Abstract
Objectives
To evaluate the diagnostic value of plasma exosomal miR-223 and its combination with CA125 for the diagnosis of early-stage epithelial ovarian cancer (EOC).
Patients and methods
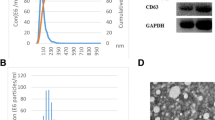
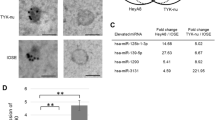
Exosomes derived from the plasma of 78 EOC patients, 40 patients with epithelial benign ovarian tumors, and 52 healthy participants were isolated using the ultracentrifugation method and identified by transmission electron microscopy (TEM) and western blot.
Results
The expression of exosomal miR-223 was significantly upregulated in the plasma of EOC patients compared to that in healthy subjects and patients with benign diseases. The combination of exosomal miR-223 and CA125 from plasma had an equivalent area under the ROC curve (AUC) to CA125 alone for discriminating between EOC and non-EOC cases, including healthy subjects and benign ovarian tumors. However, the AUC value of the combination was 0.944 (95% CI: 0.899–0.990) for differentially diagnosing early-stage EOC from healthy subjects, slightly higher than that of CA125 alone (0.928, 95% CI: 0.875–0.981), with a sensitivity and specificity of 0.9784 and 0.885, respectively.
Conclusion
Our data suggest that plasma exosomal miR-223 can be used as a complement to CA125 to increase the diagnostic power for differentiating early-stage EOC from healthy subjects.
Similar content being viewed by others
Introduction
Ovarian cancer (OC) is a leading death cause for gynecological cancers, characterized by poor prognosis and high mortality rates. The most prevalent type of ovarian cancer is epithelial ovarian cancer (EOC), accounting for 90% of cases. EOC originates from the flat surface epithelial cells that cover the ovary, subserosal inclusion cysts, or the fimbriated end of the fallopian tubes [1]. Unfavorable outcomes in OC are mainly attributed to late-stage diagnosis, with an overall 5-year survival rate below 30% [2]. Conversely, early-stage boasts a 5-year survival rate exceeding 90%, highlighting the potential for improved prognoses through accurate early-stage diagnosis. Presently, serum cancer antigen 125 (CA125) test and transvaginal ultrasonography (TVUS) are commonly used screening tools for OC [3]. However, they often fail to detect OC at earlier stage, leading to limited reduction in OC-related mortality. Notably, only 50–60% of stage I-II OC patients exhibit increased CA125 levels [4]. Consequently, there is an urgent need for novel diagnostic markers to identify early-stage OC.
Exosomes are small membranous particles (40–160 nm), which are released into the extracellular space through the fusion of multivesicular bodies with the cell membrane [5]. They transmit functionally informative molecules, including proteins, nucleic acids, lipids, and even organelles into recipient cells to mediate intercellular communications [6]. Exosomes can be found in various body fluids, such as blood, urine, and cerebrospinal fluid. Accumulating evidence suggests that exosomes play fundamental roles in the progression, metastasis, and drug resistance of OC [7, 8]. MicroRNAs (miRNAs) are one of most abundant types in the RNA cargo of exosomes [9]. Exosomal miRNAs are usually tumor specific [10], thus attracting increasing attention in cancer diagnosis and prognosis due to their non-invasiveness, easy accessibility and stability [11]. For instance, plasma levels of exosomal miR-139-3p may serve as a novel biomarker for the early diagnosis and metastasis monitoring in colorectal cancer [12]. Plasma exosomes of breast cancer patients show selective enrichment of miR-1246, which is significantly increased compared to healthy controls [13].MiR-223, a multifunctional miRNA, plays a central role in innate immunity, including regulating immune cell differentiation and macrophage polarization, it also has immunomodulatory effects in certain tissues [14]. Recently, numerous studies have reported that miR-223 is involved in the development of varius types of cancer including hepatocellular [15], gastro-esophageal [16], breast [17] and lung cancers [ Plasma exosomal miR-223 was significantly up-regulated in EOC patients and correlated with disease progression. Our study clearly demonstrates that exosomal miR-223, as a non-invasive biomarker, may complement CA125 to enhance the diagnostic accuracy for differentiating early-stage EOC from healthy subjects.Conclusion
Availability of data and materials
The data used and/or analyzed during the current study are available from the corresponding author.
References
Nebgen DR, Lu KH, Bast RC Jr. Novel approaches to Ovarian Cancer Screening. Curr Oncol Rep. 2019;21(8):75.
Coughlan AY, Testa G. Exploiting epigenetic dependencies in ovarian cancer therapy. Int J Cancer. 2021;149(10):1732–43.
Matulonis UA, Sood AK, Fallowfield L, Howitt BE, Sehouli J, Karlan BY. Ovarian cancer. Nat Rev Dis Primers. 2016;2:16061.
Kobayashi H, Yamada Y, Sado T, Sakata M, Yoshida S, Kawaguchi R, Kanayama S, Shigetomi H, Haruta S, Tsuji Y, et al. A randomized study of screening for ovarian cancer: a multicenter study in Japan. Int J Gynecol Cancer. 2008;18(3):414–20.
van Niel G, Porto-Carreiro I, Simoes S, Raposo G. Exosomes: a common pathway for a specialized function. J Biochem. 2006;140(1):13–21.
Isaac R, Reis FCG, Ying W, Olefsky JM. Exosomes as mediators of intercellular crosstalk in metabolism. Cell Metab. 2021;33(9):1744–62.
Feng W, Dean DC, Hornicek FJ, Shi H, Duan Z. Exosomes promote pre-metastatic niche formation in ovarian cancer. Mol Cancer. 2019;18(1):124.
Zhu X, Shen H, Yin X, Yang M, Wei H, Chen Q, Feng F, Liu Y, Xu W, Li Y. Macrophages derived exosomes deliver miR-223 to epithelial ovarian cancer cells to elicit a chemoresistant phenotype. J Exp Clin Cancer Res. 2019;38(1):81.
Redis RS, Calin S, Yang Y, You MJ, Calin GA. Cell-to-cell miRNA transfer: from body homeostasis to therapy. Pharmacol Ther. 2012;136(2):169–74.
Preethi KA, Selvakumar SC, Ross K, Jayaraman S, Tusubira D, Sekar D. Liquid biopsy: exosomal microRNAs as novel diagnostic and prognostic biomarkers in cancer. Mol Cancer. 2022;21(1):54.
De Rubis G, Rajeev Krishnan S, Bebawy M. Liquid biopsies in Cancer diagnosis, monitoring, and prognosis. Trends Pharmacol Sci. 2019;40(3):172–86.
Liu W, Yang D, Chen L, Liu Q, Wang W, Yang Z, Shang A, Quan W, Li D. Plasma Exosomal miRNA-139-3p is a Novel Biomarker of Colorectal Cancer. J Cancer. 2020;11(16):4899–906.
Hannafon BN, Trigoso YD, Calloway CL, Zhao YD, Lum DH, Welm AL, Zhao ZJ, Blick KE, Dooley WC, Ding WQ. Plasma exosome microRNAs are indicative of breast cancer. Breast Cancer Res. 2016;18(1):90.
Jiao P, Wang XP, Luoreng ZM, Yang J, Jia L, Ma Y, Wei DW. miR-223: an Effective Regulator of Immune Cell differentiation and inflammation. Int J Biol Sci. 2021;17(9):2308–22.
Wang X, He Y, Mackowiak B, Gao B. MicroRNAs as regulators, biomarkers and therapeutic targets in liver diseases. Gut. 2021;70(4):784–95.
Zhou X, ** W, Jia H, Yan J, Zhang G. MiR-223 promotes the cisplatin resistance of human gastric cancer cells via regulating cell cycle by targeting FBXW7. J Exp Clin Cancer Res. 2015;34(1):28.
Citron F, Segatto I, Vinciguerra GLR, Musco L, Russo F, Mungo G, D’Andrea S, Mattevi MC, Perin T, Schiappacassi M, et al. Downregulation of miR-223 expression is an early event during Mammary Transformation and confers resistance to CDK4/6 inhibitors in luminal breast Cancer. Cancer Res. 2020;80(5):1064–77.
Luo P, Wang Q, Ye Y, Zhang J, Lu D, Cheng L, Zhou H, **e M, Wang B. MiR-223-3p functions as a tumor suppressor in lung squamous cell carcinoma by miR-223-3p-mutant p53 regulatory feedback loop. J Exp Clin Cancer Res. 2019;38(1):74.
D’Antona P, Cattoni M, Dominioni L, Poli A, Moretti F, Cinquetti R, Gini E, Daffre E, Noonan DM, Imperatori A, et al. Serum miR-223: a validated biomarker for detection of early-stage Non-small Cell Lung Cancer. Cancer Epidemiol Biomarkers Prev. 2019;28(11):1926–33.
Yu G, Yin Z, He H, Zheng Z, Chai Y, Xuan L, Lin R, Wang Q, Li J, Xu D. Low serum miR-223 expression predicts poor outcome in patients with acute myeloid leukemia. J Clin Lab Anal. 2020;34(3):e23096.
Laios A, O’Toole S, Flavin R, Martin C, Kelly L, Ring M, Finn SP, Barrett C, Loda M, Gleeson N, et al. Potential role of miR-9 and miR-223 in recurrent ovarian cancer. Mol Cancer. 2008;7:35.
O’Shea AS. Clinical staging of Ovarian Cancer. Methods Mol Biol. 2022;2424:3–10.
Wang S, Song X, Wang K, Zheng B, Lin Q, Yu M, **e L, Chen L, Song X. Plasma exosomal miR-320d, miR-4479, and mir-6763-5p as diagnostic biomarkers in epithelial ovarian cancer. Front Oncol. 2022;12:986343.
Zhang Y, Xu H. Serum exosomal miR-378 upregulation is associated with poor prognosis in non-small-cell lung cancer patients. J Clin Lab Anal. 2020;34(6):e23237.
Wang B, Mao JH, Wang BY, Wang LX, Wen HY, Xu LJ, Fu JX, Yang H. Exosomal mir-1910-3p promotes proliferation, metastasis, and autophagy of breast cancer cells by targeting MTMR3 and activating the NF-kappaB signaling pathway. Cancer Lett. 2020;489:87–99.
Liu H, Liu Y, Sun P, Leng K, Xu Y, Mei L, Han P, Zhang B, Yao K, Li C, et al. Colorectal cancer-derived exosomal miR-106b-3p promotes metastasis by down-regulating DLC-1 expression. Clin Sci (Lond). 2020;134(4):419–34.
Fang G, Liu J, Wang Q, Huang X, Yang R, Pang Y, Yang M. MicroRNA-223-3p regulates ovarian cancer cell proliferation and invasion by targeting SOX11 expression. Int J Mol Sci. 2017;18(6):1208.
Zhang J, Luo X, Li H, Yue X, Deng L, Cui Y, Lu Y. MicroRNA-223 functions as an oncogene in human colorectal cancer cells. Oncol Rep. 2014;32(1):115–20.
Hu Y, Yi B, Chen X, Xu L, Zhou X, Zhu X. MiR-223 promotes Tumor Progression via Targeting RhoB in gastric Cancer. J Oncol. 2022;2022:6708871.
Qi P, Cheng SQ, Wang H, Li N, Chen YF, Gao CF. Serum microRNAs as biomarkers for hepatocellular carcinoma in Chinese patients with chronic hepatitis B virus infection. PLoS ONE. 2011;6(12):e28486.
Funding
This study was supported by Tangshan Talent Funding Project (A202010008) and Tangshan Talent Funding Project A202010008 (C202303032).
Author information
Authors and Affiliations
Contributions
ZY: Conceptualization, and Writing—original draft. LY: Formal analysis; ZL: Investigation; NQ and LT: Methodology; All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The present study was approved by the institutional research ethics committee of Tangshan ’Workers’ Hospital. Informed consent was obtained from each participant.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yang, L., Yang, Z., Liu, Z. et al. Diagnostic value of plasma-derived exosomal miR-223 for epithelial ovarian cancer. BMC Women's Health 24, 150 (2024). https://doi.org/10.1186/s12905-024-02976-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12905-024-02976-6