Abstract
Background
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common malignancy worldwide, characterized by high morbidity, high mortality, and poor prognosis. Collagen triple helix repeat containing 1 (CTHRC1) has been shown to be highly expressed in various cancers. However, its biological functions, potential role as a biomarker, and its relationship with immune infiltrates in HNSCC remain unclear. Our principal objective was to analyze CTHRC1 expression, its prognostic implications, biological functions, and its effects on the immune system in HNSCC patients using bioinformatics analysis.
Methods
The expression matrix was obtained from The Cancer Genome Atlas (TCGA) and Gene Expression Omnibus (GEO). CTHRC1 expression in HNSCC was analyzed between tumor and adjacent normal tissues, different stages were compared, and its impact on clinical prognosis was assessed using Kaplan-Meier analysis. Gene Ontology (GO), Kyoto Encyclopedia of Genes and Genomes (KEGG), and Gene Set Variation Analysis (GSVA) were employed for enrichment analysis. The Search Tool for the Retrieval of Interacting Genes database (STRING) was used to analyze protein-protein interactions. Pearson correlation tests were used to investigate the association between CTHRC1 expression and immune checkpoints. The correlation between CTHRC1 and immune infiltration was investigated using CIBERSORT, TIMER, and ESTIMATE.
Results
Compared to adjacent normal tissues, CTHRC1 was found to be highly overexpressed in tumors. Increased expression of CTHRC1 was more evident in the advanced stage of HNSCC and predicted a poor prognosis. Most genes related to CTHRC1 in HNSCC were enriched in physiological functions of Extracellular matrix(ECM) and tumor. Furthermore, several immune checkpoints, such as TNFSF4 and CD276 have been shown to be associated with CTHRC1 expression. Notably, the level of CTHRC1 expression correlated significantly with immune infiltration levels, particularly activated macrophages in HNSCC.
Conclusions
High expression of CTHRC1 predicts poor prognosis and is associated with immune infiltration in HNSCC, confirming its utility as a tumor marker for HNSCC.
Trial registration
Not applicable. All data are from public databases and do not contain any clinical trials.
Similar content being viewed by others
Introduction
Head and neck squamous cell carcinoma, originates from various subsites of the upper aerodigestive tract, including the oral cavity, sinonasal cavity, pharynx, and larynx [1, 2]. Smoking, alcohol consumption, exposure to environmental pollutants, and infection with HPV and EB viruses are the primary risk factors for HNSCC [GO and KEGG analysis Pearson correlation analysis was performed using R (version 4.3.2) to uncover the correlation between CTHRC1 expression and other genes. Subsequently, the top 500 relevant genes were selected and imported into the database for Annotation, Visualization, and Integrated Discovery (DAVID) (https://david.ncifcrf.gov). The DAVID is a database utilized for GO analysis, including biological process (BP), cellular component (CC), and molecular function (MF). KEGG analysis was also conducted using these genes. The results of the analysis were visualized using the “ggplot2” package. The associated gene sets were obtained from “AmiGO2” (https://amigo.geneontology.org/). The functional enrichment score for each sample was computed using the “GSVA” R package with default parameters. The results were visualized through a heatmap, and pearson correlation analysis was employed to assess the correlation between CTHRC1 and the specified gene sets. To investigate the interaction among related proteins, we constructed a protein-protein interaction network using the STRING database (https://string-db.org/). A minimum required interaction score of 0.4 was set. The protein-protein interaction (PPI) network was visualized using Cytoscape software. Subsequently, GO and KEGG analyses were performed based on the genes encoding these proteins, following the previously mentioned method. A total of 79 immune checkpoints were retrieved from a previous study [39]. Pearson correlation analysis was employed to examine the correlations between CTHRC1 expression and these checkpoints. A P value of less than 0.05 was considered statistically significant. Subsequently, a doughnut diagram was generated using the provided R package in the default parameters of the R environment to visualize the results. Tumor infiltrating immune cell analysis was performed using Cell-type Identification By Estimating Relative Subsets Of RNA Transcripts (CIBERSORT) to characterize the immune features of HNSCC. Immune scores were calculated using the Estimation of Stromal and Immune Cells in Malignant Tumor Tissues using Expression Data (ESTIMATE) algorithm to assess the presence of immune cells. Also, the correlations between CTHRC1 expression levels and the level of immune infiltration were estimated using TIMER, a comprehensive resource for analyzing immune infiltrates in gene expression profiles.GSVA analysis
Construction of protein–protein interaction network
Immune checkpoint testing
Tumor infiltrating immune cell analysis
Results
CTHRC1 expression in normal tissues and tumors
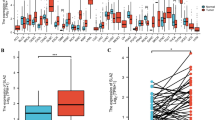
TIMER indicated a significant increase in the expression of CTHRC1 across various tumors, particularly in digestive tract tumors (Fig. 1A). For HNSCC, 1815 genes were up-regulated, while 1947 genes were down-regulated. CTHRC1 ranked 89th in the up-regulated genes. Compared with the adjacent normal tissues, the expression of CTHRC1 was significantly increased in the tumor tissues with a LogFC value of 3.5 (Fig. 1B). Comparable results were observed in the GEPIA database and HPA database (Fig. 1C and E). The results were also confirmed in the GEO database (Fig. 1D).
Expression analysis of CTHRC1 in different databases. A Expression of CTHRC1 in different types of human cancers in TIMER database. B Volcano plot of differentially expressed genes between tumor and normal tissue in HNSCC. C Expression of CTHRC1 in HNSCC from GEPIA database. D Expression of CTHRC1 in HNSCC、dysplasia、and normal tissue in GSE30784. E Expression of CTHRC1 in tumor and normal tissue in HPA database. (Normal: Female, age 59 ID4109. Tumor: Female, age 55, ID4420)
Higher expression of CTHRC1 was found in advanced stage of HNSCC patients and related to poor prognosis
The heatmap illustrates the distribution of clinicopathological features and survival status among patients with increasing CTHRC1 expression in TCGA (Fig. 2). No significant differences were observed among different age groups, genders, M stages and races. CTHRC1 expression was significantly upregulated in the clinical T4 and N3 group (Fig. 3A and B). Further analysis revealed remarkable variation in mRNA expression levels of the CTHRC1 gene among patients with HNSCC at different clinical stages. The expression of CTHRC1 was significantly higher in clinical stage IV, whereas there was no significant difference among stages I, II, and III (Fig. 3C). Regarding pathological staging, the expression levels of the CTHRC1 gene were higher in the T4 and N2&N3 group (Fig. 3D and E). Furthermore, higher expression of CTHRC1 was observed in pathological staging IV (Fig. 3F). Regarding tissue or organ origin, the CTHRC1 expression level was highest in the overlap** lesion of the lip, oral cavity, and pharynx, and lowest in tonsils; however, there was no statistical significance (Fig. 3G).
The Kaplan-Meier Plotter was employed for survival analysis. To assess the OS of patients, 270 cases were classified into the high expression group, and 251 cases were assigned to the low expression group using maximally selected rank statistics in TCGA. Kaplan-Meier curves revealed that high CTHRC1 expression indicated a higher risk of poor overall survival (P = 0.0017). The median overall survival of the high and low CTHRC1 expression groups was 34.6 and 77.3 months, respectively (Fig. 3H). The above results were validated in the GEO database (GSE41613). The high group exhibited worse survival than the low group (P = 0.043). The median survival of the high group was 43.3, and for the low group, it was 78.3(Fig. 3I).
High expression of CTHRC1 is observed in late stage of HNSCC and predicts a poor prognosis. A-F CTHRC1 expression across different stages in HNSCC. G CTHRC1 expression across different organs or tissues. H-I Prognostic impact of CTHRC1: Kaplan-Meier survival curves in TCGA and GSE41613. Statistical significance was determined using either an unpaired t-test or one-way analysis of variance (ANOVA)
Go, KEGG and GSVA analysis
The top 500 co-expression genes were used for GO and KEGG analysis. The GO analysis revealed that these genes were enriched in processes such as extracellular matrix organization (BP: GO: 0030198), collagen fibril organization (BP: GO: 0030199), extracellular matrix (CC: GO:0031012), extracellular region (CC: GO: 0005576), (MF: GO: 0005201) and collagen binding (MF: GO: 0005518) (Fig. 4A and C). Additionally, the KEGG pathway analysis revealed pathways in which these genes were enriched, including ECM-receptor interaction, focal adhesion, protein digestion and absorption, PI3K-Akt signaling pathway, Human papillomavirus infection, and hypertrophic cardiomyopathy (Fig. 4D). Furthermore, GSVA analysis was conducted to reveal the physiological processes in which CTHRC1 might be involved. The results indicated that CTHRC1 might be associated with the formation and organization of collagen and fibril, extracellular matrix, and basement membrane (Fig. 4E).
CTHRC1 is closely associated with ECM-related process. A-C, Biological processes (BP), cellular components (CC), and molecular functions (MF) associated with CTHRC1 in TCGA Database. D Kyoto encyclopedia of genes and genomes (KEGG) pathway analysis of CTHRC1 in TCGA. E Enrichment scores analysis between CTHRC1 expression and ECM-related pathways by GSVA
PPI network construction
The protein-protein interaction network for CTHRC1 was constructed using the STRING database. Thirty genes were found to be closely related to CTHRC1, many of which are involved in cancer biology, including TGFBR1, TGFBR2, TGFB1, MMP2, MMP14, TIMP1, TIM3, WNT5A, ENG, COL1A1, COL1A2, and POSTN (Fig. 5A). To explore the biological role and pathways of the related genes, GO and KEGG were used. Similar to CTHRC1, genes encoding these proteins were closely related to the formation and organization of collagen and fibril, extracellular matrix, and basement membrane. In addition, the result of KEGG indicated that many cancers have been linked to these genes, and many classical tumor-related pathways might be involved (Fig. 5B-E).
Relationship between CTHRC1 and immune checkpoint genes
We explored the relationship between the expression of CTHRC1 and immune checkpoint genes using Pearson correlation analysis. Based on the previous literature, 79 immune checkpoint genes were selected. Finally, 19 genes were confirmed to be positively correlated with CTHRC1 expression in HNSCC (R > 0, P < 0.05), while 24 genes were negatively correlated (R < 0, P < 0.05) (Table 1). The top 6 checkpoints were TNFSF4 (R = 0.474, P = 1.25 × 10− 30), CD276 (R = 0.379, P = 2.94 × 10− 19), CEACAM1 (R=-0.365, P = 7.36 × 10− 18), KIR2DL4 (R=-0.365, P = 7.36 × 10− 18), HLA-DOB (R=-0.241, P = 2.32 × 10− 8), and TNFSF18 (R=-0.208, P = 1.70 × 10− 6) (Fig. 6).
CTHRC1 expression and immune cell infiltration
In this study, we conducted an analysis of immune cell infiltration features using CIBERSORT, TIMER, and ESTIMATE. The CIBERSORT results revealed that 12 out of the 22 types of immune cell types were differentially expressed between CTHRC1 high and low expression groups in TCGA, including plasma cells, T cells CD8, T cells CD4 memory activated, T cells follicular helper, NK cells activated, Monocytes, Macrophages (M0, M1, M2), Dendritic cells resting, Dendritic activated, and Neutrophils (Fig. 7A). In GSE30784, only 4 types of immune cells were discovered (NK cells activited, Macrophages M0, Macrophages M1 and Macrophages M2) (Fig. 7B). Similar findings were also observed, with the exception of CD8 cells, which showed no significant association with CTHRC1 expression in the TIMER database (Fig. 7C). ESTIMATE indicated a correlation between Stromal Score and ESTIMATE score with CTHRC1 expression. However, no correlations were found between CTHRC1 expression and Immune Score (Fig. 7D). Since the ESTIMATE Score is negatively correlated with tumor purity, we infer that tumor purity is negatively correlated with CTHRC1 expression. This finding contradicted the conclusions drawn in the TIMER database, which showed no correlations between CTHRC1 expression and tumor purity.
Immune infiltration analysis of CTHRC1 expression in HNSCC. A Infiltration of different immune cells in CTHRC1 high expression group and low expression group analyzed by CIBERSORT in TCGA. B Infiltration of different immune cells in CTHRC1 high expression group and low expression group in GSE30784. C Correlation of CTHRC1 expression with immune infiltration levels in TIMER. D Pearson correlation between CTHRC1 and immune score using ESTIMATE
Discussion
HNSCC is one of the most prevalent cancers globally, posing a significant threat to human health. The management of HNSCC varies depending on the stage of the disease, anatomical location, and surgical accessibility. In the early stages (stage I and stage II), smaller tumors may undergo extensive resection directly. Intriguingly, diode lasers have gradually been adopted in surgical treatments, enhancing operational efficiency and yielding exceptional aesthetic and functional outcomes [40, 41]. In advanced stages (stage III and stage IV), surgery remains the primary method, with the removal of the primary lesion accompanied by neck lymph node dissection. Postoperative radiotherapy or chemotherapy may be administered subsequently [42, 43]. In recent years, targeted therapies exemplified by EGFR inhibitors, and immunotherapies, such as pembrolizumab hold immense potential in the treatment of advanced HNSCC [26, 44].
The aberrant expression of CTHRC1 has been documented in various cancers, including breast cancer, stomach adenocarcinoma, non-small cell lung cancer, and colon adenocarcinoma [19,20,21,22]. The understanding of the expression pattern of CTHRC1 in tumors will help us to understand the pathogenesis of tumors. Experimental research for HNSCC has indicated that N-Glycosylation collaborates with canonical Wnt pathway to induce CTHRC1 and drive HNSCC cell migration [45]. The high expression of CTHRC1 may be associated with HNSCC progression and poor prognosis, hel** identify high risk individuals. Detecting CTHRC1 aids in the early detection and diagnosis of HNSCC, improving treatment outcomes through noninvasive methods. Further research on CTHRC1 will help develop new diagnostic and therapeutic strategies. In the present study, we found that CTHRC1 expression was significantly higher in tumor tissues consistent with lee’s study in which the HNSCC samples showed a 12.3fold higher expression than normal samples [23]. This suggests that elevated CTHRC1 expression may be associated with a higher risk of HNSCC, and CTHRC1 can serve as a tumor marker to predict the risk of HNSCC occurrence.
Timely diagnosis and early surgery in the initial stages of the tumor are crucial factors affecting the prognosis of HNSCC. It has been asserted that delayed diagnosis and treatment of patients with HNSCC worsen prognosis outcomes and increase undesirable morbidity and mortality in cancer patients [46, 47]. In this study, we observed that CTHRC1 expression tends to be up-regulated in advanced stages of head and neck squamous cell carcinoma. Additionally, the OS of the high CTHRC1 expression group is significantly worse than that of the low expression group. This trend has been confirmed in both the TCGA and GSE41613 datasets. Therefore, high expression of CTHRC1 may predict poor prognosis of patients which is consistent with findings of Lee’s study [23]. The prognostic value of CTHRC1 has also been found in other tumors, such as colon adenocarcinoma, gastric cancer, breast cancer and kidney renal clear cell carcinoma [20, 48, 49]. Our results indicates that CTHRC1 can also serve as a marker to predict the stage and prognosis of HNSCC.
Prior studies have indicated that TME is implicated in various pathological processes of tumor, including tumor growth, metastasis, and invasiveness [29, 30]. Being a dynamic interconnected mesh of macromolecules, ECM stands out as the most abundant component in TME, offering structural support and regulating cellular behavior through mechanical and biochemical cues [50]. It regulates various cellular processes, including proliferation, differentiation, migration, invasion, and survival in cancer [50, 51]. In this study, genes related to CTHRC1 expression in HNSCC were identified using the TCGA database. POSTN, SPARC, PLPP4, GLT8D2, and THY1 were identified as the five genes most closely expressed to CTHRC1, suggesting a robust link between CTHRC1 and the relevant functions of the ECM. Subsequently, we explored the physiological function and related pathways using GO, KEGG, and GSVA analyses. The results indicated that CTHRC1 and its coexpressed genes are enriched in ECM-related processes, such as extracellular matrix organization, collagen fibril organization, cell adhesion, and cell matrix adhesion. In previous studies, Zhao found that CTHRC1 was related to several pathways in gastric cancer, including extracellular matrix organization and vascular development, through enrichment analysis [20]. Also, a similar result was found in meng’s study of colon cancer [48]. Our results were consistent with their studies.
The protein-protein interaction network consists of proteins that interact to participate in various life processes, including biological signal transmission, gene expression regulation, energy and substance metabolism, and cell cycle regulation [52]. In this study, a protein-protein network was constructed, revealing 30 related proteins, including COL3A1, TGFBR1, LTBP1, COL1A2, COL5A1, and POSTN. KEGG analysis showed that, in addition to ECM-related physiological processes, these proteins were also implicated in the initiation and progression of various cancers, as demonstrated in other studies. Zhao indicate that ECM-related genes correlate with immune cells, overall survival, and recurrence of bladder cancer [53]. Also, Keerthi proved that matrisome genes were seen to affect survival across cancers by a pan-cancer analysis [54]. Furthermore, these proteins were involved in several classical signaling pathways that have been proven to play important roles in tumors, such as the Wnt signaling pathway, Hippo signaling pathway, and mTOR signaling pathway [55].
Head and neck cancer inherently exhibits immunosuppression, prompting research into the role of the immune landscape in HNSCC [56]. The advent of immune checkpoint inhibitors represents a noteworthy advancement in oncological therapy. Immune checkpoint blockade (ICB) therapies targeting PD1 and PDL1 have been approved to treat various malignancies, yielding some therapeutic effects. Despite these advancements, only a small percentage of HNSCC patients respond to ICB, with studies indicating a benefit for only 10–20% of HNSCC patients [26, 27]. Therefore, there is an urgent need for the identification of the most suitable treatment regimens and the reduction of immunosuppression in non-responding patients with head and neck cancer. Immune checkpoint agents exhibit antitumor properties by reversing tumor immunosuppressive effects. The study also investigated the correlation between the signature and the levels of various immune checkpoint proteins. Patients with higher CTHRC1 expression exhibited elevated expressions of TNFSF4 and CD276. TNFSF4, a cytokine that promotes the activation and proliferation of T cells, is a well-known immune checkpoint. A previous study demonstrated that TNFSF4 could facilitate chemoresistance in lung adenocarcinoma by inhibiting the apoptosis of tumor cells [57]. The cell surface molecule CD276 serves as an immune checkpoint antigen. Elevated expression of CD276 on tumors contributes to the suppression of anti-tumor T-cell responses and correlates with a poor prognosis [58]. Consequently, the results of this study suggest that TNFSF4 and CD276 may be novel targets for HNSCC immunotherapy.
In addition to stromal cells, immune cells constitute crucial components of the TME and play a pivotal role in the onset and progression of tumors. Studies have indicated that the immune infiltration status of tumors is a crucial factor influencing tumor growth and prognosis [59]. In our study, we assessed the tumor’s immune infiltration status using ESTIMATE, TIMER, and CIBERSORT. ESTIMATE calculates immune and stroma scores by analyzing gene expression signatures specific to immune and stroma cells to predict infiltration by non-tumor cells. The results indicated no correlation between CTHRC1 and the immune score, but it can influence the infiltration status of non-tumor cells by impacting the stromal score. While not influencing the overall immune score, CIBERSORT demonstrated a significant impact of CTHRC1 on the composition of immune cells in tumors, particularly macrophages. Macrophages can polarize into either M1-like or M2-like macrophages. M1-like macrophages are pro-inflammatory and play a role in host defense by producing cytokines like IL-12 and TNF-α, and promoting Th1 responses. In contrast, M2-like macrophages are anti-inflammatory, involved in tissue repair, and secrete cytokines like IL-10 and TGF-β, promoting Th2 responses [60]. Macrophages in the tumor microenvironment are termed tumor-associated macrophages (TAMs), representing a distinctive phenotype of M2-like macrophages [61, 62]. It has been demonstrated that TAMs within the tumor microenvironment promote tumor growth, angiogenesis, and metastasis by secreting pro-tumorigenic factors and suppressing antitumor immune responses across various cancer types [63]. The study revealed a significant increase in M2 macrophages and a corresponding decrease in M1 macrophages in the CTHRC1 high-expression group. This alteration implies that CTHRC1 may be linked to the imbalance in macrophage proportion, a factor deemed crucial in tumor development, immune evasion, and subsequent metastasis and drug resistance, which was also proved in other cancers [22, 49, 61, 64]. Taken together, these findings suggest that CTHRC1 might serve as an indicator of HNSCC prognosis by influencing immune infiltration status, particularly in relation to M2 macrophages. Immunotherapy targeting macrophage polarization may offer potential benefits for improving the treatment outcome of HNSCC patients, with CTHRC1 emerging as a promising target. However, further studies are needed to confirm the causal relationship between them.
In this study, we investigated the relationship between CTHRC1 and HNSCC, aiming to elucidate the underlying mechanisms. Nevertheless, this study has certain limitations. All the data utilized in this study were obtained from online databases, lacking experimental validation and may have biases such as measurement errors and data missing. Also, we did not draw a clear boundary to distinguish high expression and low expression. Additionally, some inconsistent results, such as the disparity between TIMER and ESTIMATE in the assessment of tumor purity, remain unexplained. Therefore, additional analyses and experiments, encompassing in vitro and in vivo validations, are necessary to validate our findings.
Conclusions
High expression of CTHRC1 predicts poor prognosis and is associated with immune infiltration in HNSCC, confirming its utility as a tumor marker for HNSCC.
Data availability
Data that support the findings of this study can be obtained from the public database TCGA-HNSC and GEO database (GSE30784 and GSE41613).
Abbreviations
- BP:
-
Biological process
- CC:
-
Cellular component
- CIBERSORT:
-
Cell-type Identification By Estimating Relative Subsets Of RNA Transcripts
- CTHRC1:
-
Collagen triple helix repeat containing-1
- DAVID:
-
The database for annotation, visualization, and integrated discovery
- DEGs:
-
Differentially expressed genes
- ECM:
-
Extracellular matrix
- ESTIMATE:
-
Estimation of Stromal and Immune Cells in Malignant Tumour Tissues using Expression Data
- GEO:
-
Gene expression omnibus
- GEPIA:
-
Gene Expression Profiling Interactive Analysis
- GO:
-
Gene ontology
- GSVA:
-
Gene set variation analysis
- HNSCC:
-
Head and neck squamous cell carcinoma
- HPA:
-
Human protein atlas database
- ICB:
-
Immune checkpoint blockade
- KEGG:
-
Kyoto encyclopedia of genes and genomes
- MF:
-
Molecular function
- OS:
-
Overall survival
- PPI:
-
Protein-protein interaction
- STRING:
-
The search tool for the retrieval of interacting genes
- TAMs:
-
Tumor-associated macrophages
- TCGA:
-
The cancer genome atlas
- TIMER:
-
Tumor Immune Estimation Resource
- TME:
-
Tumor microenvironment
References
Bhatia A, Burtness B. Treating head and neck cancer in the age of immunotherapy: a 2023 update. Drugs. 2023;83(3):217–48.
Afrah A, Aldelaimi HHE, Tahrir N, Aldelaimi. Khalil Abdulla Mohammed: tumors of Craniofacial Region in Iraq (Clinicopathological study). J Res Med Dent Sci. 2021;9(1):66–71.
Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, **ao W, Kim E, Jiang B, Goodman MT, Sibug-Saber M, Cozen W, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2023;41(17):3081–8.
Kranjčević JK, Čonkaš J, Ozretić P. The role of estrogen and estrogen receptors in head and neck tumors. Cancers (Basel). 2024;16(8).
Afrah A, Khalil SAS. Immunohistochemical expressions of AKT, ATM and Cyclin E in oral squamous cell carcinoma. J Bagh Coll Dentistry. 2016;28(3):44–51.
Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–63.
Caudell JJ, Gillison ML, Maghami E, Spencer S, Pfister DG, Adkins D, Birkeland AC, Brizel DM, Busse PM, Cmelak AJ, et al. NCCN guidelines® insights: head and neck cancers, version 1.2022. J Natl Compr Canc Netw. 2022;20(3):224–34.
Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48.
Jiang P, Liu XS. Big data mining yields novel insights on cancer. Nat Genet. 2015;47(2):103–4.
Al-Ouqaili MTS, MHM SMAA-K. Depending on HPLC and PCR, detection of aflatoxin B1 extracted from aspergillus flavus strains and it’s cytotoxic effect on AFB treated hematopoietic stem cells obtained from human umbilical cord. Asian J Pharm. 2018;12(3):S1048–54.
Vizoso M, Ferreira HJ, Lopez-Serra P, Carmona FJ, Martínez-Cardús A, Girotti MR, Villanueva A, Guil S, Moutinho C, Liz J, et al. Epigenetic activation of a cryptic TBC1D16 transcript enhances melanoma progression by targeting EGFR. Nat Med. 2015;21(7):741–50.
Wang W, Wei C. Advances in the early diagnosis of hepatocellular carcinoma. Genes Dis. 2020;7(3):308–19.
Rafil H, Rasheed, Tahrir NN, Al-Delaimi, Khalil AA. Immunohistochemical expression of CD20, CD43, and CD79 in Burkitt’s lymphoma. New Iraqi J Med. 2010;6(2):66–9.
Afrah A, Khalil SAS. An immunohistochemical expressions of BAD, MDM2, and P21 in oral squamous cell carcinoma. J Bagh Coll Dentistry. 2016;28(2):34–9.
Mei D, Zhu Y, Zhang L, Wei W. The role of CTHRC1 in regulation of multiple signaling and tumor progression and metastasis. Mediators Inflamm. 2020;2020:9578701.
Li Y, Zhou J, Wang J, Chen X, Zhu Y, Chen Y. Mir-30b-3p affects the migration and invasion function of ovarian cancer cells by targeting the CTHRC1 gene. Biol Res. 2020;53(1):10.
Li J, Wang Y, Ma M, Jiang S, Zhang X, Zhang Y, Yang X, Xu C, Tian G, Li Q, et al. Autocrine CTHRC1 activates hepatic stellate cells and promotes liver fibrosis by activating TGF-β signaling. EBioMedicine. 2019;40:43–55.
Singh CK, Fernandez S, Chhabra G, Zaemisch GR, Nihal A, Swanlund J, Ansari N, Said Z, Chang H, Ahmad N. The role of collagen triple helix repeat containing 1 (CTHRC1) in cancer development and progression. Expert Opin Ther Targets. 2024:1–17.
Lai YH, Chen J, Wang XP, Wu YQ, Peng HT, Lin XH, Wang WJ. Collagen triple helix repeat containing-1 negatively regulated by microRNA-30c promotes cell proliferation and metastasis and indicates poor prognosis in breast cancer. J Exp Clin Cancer Res. 2017;36(1):92.
Zhao L, Wang W, Niu P, Luan X, Zhao D, Chen Y. The molecular mechanisms of CTHRC1 in gastric cancer by integrating TCGA, GEO and GSA datasets. Front Genet. 2022;13:900124.
He W, Zhang H, Wang Y, Zhou Y, Luo Y, Cui Y, Jiang N, Jiang W, Wang H, Xu D, et al. CTHRC1 induces non-small cell lung cancer (NSCLC) invasion through upregulating MMP-7/MMP-9. BMC Cancer. 2018;18(1):400.
Liu Y, Chen X, Xu Y, Yang T, Wang H, Wang Z, Hu Z, Chen L, Zhang Z, Wu Y. CTHRC1 promotes colorectal cancer progression by recruiting tumor-associated macrophages via up-regulation of CCL15. J Mol Med (Berl). 2024;102(1):81–94.
Lee CE, Vincent-Chong VK, Ramanathan A, Kallarakkal TG, Karen-Ng LP, Ghani WM, Rahman ZA, Ismail SM, Abraham MT, Tay KK, et al. Collagen triple helix repeat containing-1 (CTHRC1) expression in oral squamous cell carcinoma (OSCC): prognostic value and clinico-pathological implications. Int J Med Sci. 2015;12(12):937–45.
Xu S, Tang L, Li X, Fan F, Liu Z. Immunotherapy for glioma: current management and future application. Cancer Lett. 2020;476:1–12.
Hsieh K, Dickstein DR, Runnels J, Lehrer EJ, Rosenzweig K, Hirsch FR, Samstein RM. Radiotherapy and immunotherapy in lung cancer. Biomedicines. 2023;11(6).
Ferris RL, Blumenschein G Jr., Fayette J, Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE, Even C, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856–67.
Mehra R, Seiwert TY, Gupta S, Weiss J, Gluck I, Eder JP, Burtness B, Tahara M, Keam B, Kang H, et al. Efficacy and safety of pembrolizumab in recurrent/metastatic head and neck squamous cell carcinoma: pooled analyses after long-term follow-up in KEYNOTE-012. Br J Cancer. 2018;119(2):153–9.
Larkins E, Blumenthal GM, Yuan W, He K, Sridhara R, Subramaniam S, Zhao H, Liu C, Yu J, Goldberg KB, et al. FDA approval summary: pembrolizumab for the treatment of recurrent or metastatic head and neck squamous cell carcinoma with disease progression on or after platinum-containing chemotherapy. Oncologist. 2017;22(7):873–8.
Peltanova B, Raudenska M, Masarik M. Effect of tumor microenvironment on pathogenesis of the head and neck squamous cell carcinoma: a systematic review. Mol Cancer. 2019;18(1):63.
Liang B, Tao Y, Wang T. Profiles of immune cell infiltration in head and neck squamous carcinoma. Biosci Rep. 2020;40(2).
Bonaventura P, Shekarian T, Alcazer V, Valladeau-Guilemond J, Valsesia-Wittmann S, Amigorena S, Caux C, Depil S. Cold tumors: a therapeutic challenge for immunotherapy. Front Immunol. 2019;10:168.
Lu T, Zhang Z, Zhang J, Pan X, Zhu X, Wang X, Li Z, Ruan M, Li H, Chen W, et al. CD73 in small extracellular vesicles derived from HNSCC defines tumour-associated immunosuppression mediated by macrophages in the microenvironment. J Extracell Vesicles. 2022;11(5):e12218.
Wculek SK, Cueto FJ, Mujal AM, Melero I, Krummel MF, Sancho D. Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol. 2020;20(1):7–24.
Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–71.
Sacconi A, Muti P, Pulito C, Urbani G, Allegretti M, Pellini R, Mehterov N, Ben-David U, Strano S, Bossi P, et al. Immunosignatures associated with TP53 status and co-mutations classify prognostically head and neck cancer patients. Mol Cancer. 2023;22(1):192.
** Y, Wang Z, Huang S, Liu C, Wu X, Wang H. Identify and validate circadian regulators as potential prognostic markers and immune infiltrates in head and neck squamous cell carcinoma. Sci Rep. 2023;13(1):19939.
Li C, Chen S, Jia W, Li W, Wei D, Cao S, Qian Y, Guan R, Liu H, Lei D. Identify metabolism-related genes IDO1, ALDH2, NCOA2, SLC7A5, SLC3A2, LDHB, and HPRT1 as potential prognostic markers and correlate with immune infiltrates in head and neck squamous cell carcinoma. Front Immunol. 2022;13:955614.
Li C, Guan R, Li W, Wei D, Cao S, Chang F, Wei Q, Wei R, Chen L, Xu C, et al. Analysis of myosin genes in HNSCC and identify MYL1 as a specific poor prognostic biomarker, promotes tumor metastasis and correlates with tumor immune infiltration in HNSCC. BMC Cancer. 2023;23(1):840.
Hu FF, Liu CJ, Liu LL, Zhang Q, Guo AY. Expression profile of immune checkpoint genes and their roles in predicting immunotherapy response. Brief Bioinform. 2021;22(3).
Aldelaimi TN, Khalil AA. Clinical application of diode laser (980 nm) in maxillofacial surgical procedures. J Craniofac Surg. 2015;26(4):1220–3.
Khalil AA, Enezei HH, Aldelaimi TN, Mohammed KA. Advances in diagnosis and treatment of basal cell carcinoma. J Craniofac Surg. 2024;35(2):e204–8.
Pfister DG, Spencer S, Adelstein D, Adkins D, Anzai Y, Brizel DM, Bruce JY, Busse PM, Caudell JJ, Cmelak AJ, et al. Head and neck cancers, version 2.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2020;18(7):873–98.
Lacas B, Carmel A, Landais C, Wong SJ, Licitra L, Tobias JS, Burtness B, Ghi MG, Cohen EEW, Grau C, et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 107 randomized trials and 19,805 patients, on behalf of MACH-NC group. Radiother Oncol. 2021;156:281–93.
Chow LQM. Head and neck cancer. N Engl J Med. 2020;382(1):60–72.
Liu G, Sengupta PK, Jamal B, Yang HY, Bouchie MP, Lindner V, Varelas X, Kukuruzinska MA. N-glycosylation induces the CTHRC1 protein and drives oral cancer cell migration. J Biol Chem. 2013;288(28):20217–27.
Schutte HW, Heutink F, Wellenstein DJ, van den Broek GB, van den Hoogen FJA, Marres HAM, van Herpen CML, Kaanders J, Merkx T, Takes RP. Impact of time to diagnosis and treatment in head and neck cancer: a systematic review. Otolaryngol Head Neck Surg. 2020;162(4):446–57.
Coca-Pelaz A, Takes RP, Hutcheson K, Saba NF, Haigentz M Jr., Bradford CR, de Bree R, Strojan P, Lund VJ, Mendenhall WM, et al. Head and neck cancer: a review of the impact of treatment delay on outcome. Adv Ther. 2018;35(2):153–60.
Meng C, Zhang Y, Jiang D, Wang J. CTHRC1 is a prognosis-related biomarker correlated with immune infiltrates in colon adenocarcinoma. World J Surg Oncol. 2022;20(1):89.
Zhou F, Shen D, **ong Y, Cheng S, Xu H, Wang G, Qian K, Ju L, Zhang X. CTHRC1 is a prognostic biomarker and correlated with Immune infiltrates in kidney renal papillary cell carcinoma and kidney renal clear cell carcinoma. Front Oncol. 2020;10:570819.
Lee DK, Oh J, Park HW, Gee HY. Anchorage dependence and cancer metastasis. J Korean Med Sci. 2024;39(19):e156.
Socovich AM, Naba A. The cancer matrisome: from comprehensive characterization to biomarker discovery. Semin Cell Dev Biol. 2019;89:157–66.
Vella D, Marini S, Vitali F, Di Silvestre D, Mauri G, Bellazzi R. MTGO: PPI network analysis via topological and functional module identification. Sci Rep. 2018;8(1):5499.
Zhao H, Chen Z, Fang Y, Su M, Xu Y, Wang Z, Gyamfi MA, Zhao J. Prediction of prognosis and recurrence of bladder cancer by ECM-related genes. J Immunol Res. 2022;2022:1793005.
Harikrishnan K, Prabhu SS, Balasubramanian N. A pan-cancer analysis of matrisome proteins reveals CTHRC1 and a related network as major ECM regulators across cancers. PLoS ONE. 2022;17(10):e0270063.
Saleh RO, Al-Ouqaili MTS, Ali E, Alhajlah S, Kareem AH, Shakir MN, Alasheqi MQ, Mustafa YF, Alawadi A, Alsaalamy A. lncRNA-microRNA axis in cancer drug resistance: particular focus on signaling pathways. Med Oncol. 2024;41(2):52.
Baysal H, Siozopoulou V, Zaryouh H, Hermans C, Lau HW, Lambrechts H, Fransen E, De Pauw I, Jacobs J, Peeters M, et al. The prognostic impact of the immune signature in head and neck squamous cell carcinoma. Front Immunol. 2022;13:1001161.
Li Y, Chen Y, Miao L, Wang Y, Yu M, Yan X, Zhao Q, Cai H, **ao Y, Huang G. Stress-induced upregulation of TNFSF4 in cancer-associated fibroblast facilitates chemoresistance of lung adenocarcinoma through inhibiting apoptosis of tumor cells. Cancer Lett. 2021;497:212–20.
Harland N, Maurer FB, Abruzzese T, Bock C, Montes-Mojarro IA, Fend F, Aicher WK, Stenzl A, Amend B. Elevated expression of the immune checkpoint ligand CD276 (B7-H3) in urothelial carcinoma cell lines correlates negatively with the cell proliferation. Int J Mol Sci. 2022;23(9).
Xue Y, Tong L, LiuAnwei Liu F, Liu A, Zeng S, **ong Q, Yang Z, He X, Sun Y, Xu C. Tumor–infiltrating M2 macrophages driven by specific genomic alterations are associated with prognosis in bladder cancer. Oncol Rep. 2019;42(2):581–94.
Lin C, Chu Y, Zheng Y, Gu S, Hu Y, He J, Shen Z. Macrophages: plastic participants in the diagnosis and treatment of head and neck squamous cell carcinoma. Front Immunol. 2024;15:1337129.
Li LY, Yin KM, Bai YH, Zhang ZG, Di W, Zhang S. CTHRC1 promotes M2-like macrophage recruitment and myometrial invasion in endometrial carcinoma by integrin-akt signaling pathway. Clin Exp Metastasis. 2019;36(4):351–63.
Griess B, Mir S, Datta K, Teoh-Fitzgerald M. Scavenging reactive oxygen species selectively inhibits M2 macrophage polarization and their pro-tumorigenic function in part, via Stat3 suppression. Free Radic Biol Med. 2020;147:48–60.
Li C, Xu X, Wei S, Jiang P, Xue L, Wang J. Tumor-associated macrophages: potential therapeutic strategies and future prospects in cancer. J Immunother Cancer. 2021;9(1).
Wang Z, Zhang S, Zheng C, **a K, Sun L, Tang X, Zhou F, Ouyang Y, Tang F. CTHRC1 is a potential prognostic biomarker and correlated with macrophage infiltration in breast cancer. Int J Gen Med. 2022;15:5701–13.
Acknowledgements
All the authors are thankful to the kindly sharing from Nansihuanxilu.
Funding
The study is funded by the Dean’s research fund of **an stomatology hospital.
Author information
Authors and Affiliations
Contributions
Chao Xu provided design of the work. Zhichao Zhang was responsible for the acquisition, analysis and writing the manuscript. Xusheng Ren, Yiling wang, ** Liu, Peng Lin and Shumei ** made contributions in the interpretation of data. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, Z., Ren, X., Wang, Y. et al. CTHRC1 is a prognostic biomarker correlated with immune infiltration in head and neck squamous cell carcinoma. BMC Oral Health 24, 742 (2024). https://doi.org/10.1186/s12903-024-04525-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12903-024-04525-x