Abstract
Background
Glutathione S-transferases (GSTs) play important roles in protecting cells against oxidative stress and toxic chemicals. This study aimed to investigate the distribution of GSTM1, GSTT1, and GSTP1 variants and their roles in periodontitis susceptibility in a Caucasian population.
Methods
We analyzed 406 participants, including 204 healthy controls and 203 periodontitis patients. A multiplex polymerase chain reaction (PCR) approach was used to analyze GSTM1 and GSTT1 loci. GSTP1 variants were detected by PCR-haploty** method in a subgroup of participants (N = 350). Chi-square or Fisher´s exact tests were used to compare genotypic and allelic differences. The Bonferroni method was applied to correct for multiple comparisons (pcorr).
Results
The GSTM1 genotype distribution did not differ significantly between controls and periodontitis patients (p = 0.44). Additionally, the wild/null genotypes of GSTT1, Ile105Val and Ala114Val frequencies of GSTP1 were not significantly different between the two groups after correction for multiple comparisons (p = 0.05, p = 0.55, p = 0.02, pcorr>0.05, respectively). The GSTM1 and GSTP1 Ile105Val gene variants were similarly distributed between non-smokers and smokers in both groups (p = 0.38, p = 0.20, and p = 0.14, p = 0.35, respectively). However, the wild genotype of the GSTT1 and Ala114Ala variant of the GSTP1 genes were present more frequently in non-smoking periodontitis patients than in non-smoking controls (p = 0.03, pcorr>0.05, and p = 0.009, pcorr>0.05, respectively) although their frequencies did not differ between smoking periodontitis patients and smoking controls (p = 0.23, p = 0.68, respectively).
Conclusions
This study in a Czech Caucasian population did not confirm a highly significant association between GST gene variants and susceptibility to periodontitis, as previously reported by Arshad and colleagues in Pakistanis. However, a weak relationship between GSTT1 and GSTP1 rs1138272 polymorphisms and periodontitis in non-smokers was observed.
Similar content being viewed by others
Background
Periodontal disease affects up to 90% of the population, making it one of the most common oral problems [1]. Periodontitis is a complex infectious disease attributed to the presence of bacteria in dental plaque and is characterized by chronic inflammation and destruction of the supporting structures of teeth. Although dental plaque bacteria are necessary for disease development, the immune response of the body is essential. The host immune responses that contribute to periodontitis are also associated with oxidative stress [2]. Antioxidant mechanisms act against reactive oxygen species (ROS) before they cause oxidative damage to tissues and cells [3]. Additionally, smoking is a strong risk factor for periodontitis, and tobacco smoke can release several substances capable of inhibiting cellular immune responses. For detoxification, conjugation with glutathione phase II enzymes, such as glutathione-S-transferases (GSTs) is necessary [4].
The primary role of glutathione-S-transferases (GSTs) is to detoxify reactive electrophilic compounds, including environmental toxins and oxidative stress products. The GSTs include seven classes: alpha (α, GSTA), mu (µ, GSTM), pi (π, GSTP), sigma (σ, GSTS), theta (θ, GSTT), omega (ω, GSTO), and zeta (ξ, GSTZ) [5]. The GSTM1 gene is located on chromosome 1 (1p13.3) and the GSTT1 gene is located on chromosome 22 (22p11.23). The most common mutation in these two genes is the whole null genotype, which results in a lack of enzymatic activity and may cause upregulation of oxidative stress [6]. The human GSTP1 gene is located on chromosome 11 (11q13.2) and contains two common polymorphisms in exon 5, namely, rs1695 (A313G, Ile105Val), and in exon 6, namely, rs1138272 (C341T, Ala114Val), with possible functional effects. Consequently, the changes of Ile or Ala to Val, which are in the electrophile-binding active site of the GSTP peptide, may result in subtle alterations in the a-helical and/or superhelical structure, which can affect H-site architecture and ultimately result in differences in substrate binding affinities and catalytic activities between the GSTP enzymes [7].
A recent case-control study in a Pakistani population reported that the absence of GSTM1, the presence of GSTT1, and the mutant allele (G) at rs1695 in the GSTP1 gene may be associated with susceptibility to periodontitis [8]. This study aimed to determine the frequencies of GSTM1, T1, and P1 variants and analyze their role in periodontitis susceptibility in a Caucasian (Czech) population of non-smokers and smokers.
Methods
Study design, clinical examination, and sample collection
This case-control study follows the principles of the Declaration of Helsinki. This study was approved by the Ethics Committee of Masaryk University, Faculty of Medicine (No. 15/2009, No. 13/2013) and St. Anne’s Faculty Hospital Brno (without No. /2005). All participants provided written informed consent prior to inclusion in the study. All DNA samples were obtained from the biobank of the Clinic of Stomatology, St. Anne´s Faculty Hospital and Faculty of Medicine, and Department of Pathophysiology, Faculty of Medicine, from participants recruited from 2005 to 2015.
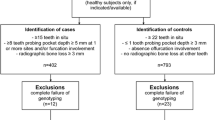
The participants with periodontitis (N = 203) were patients at the Clinic of Stomatology. All participants had at least 20 remaining teeth and were in good general health. Their Community Periodontal Index of Treatment Needs (CPITN) [9] was 3 or 4. Periodontitis was diagnosed based on clinical examination and radiographic evaluation. Probing depth (PD) and clinical attachment loss (CAL) were determined using a UNC-15 periodontal probe (HuFriedy, Chicago, IL, USA) from six sites on each tooth. The gingival health status was determined using the modified Löe-Silness gingival index (GI) [10] as described previously [11], and alveolar bone loss was determined radiographically and assessed using the Mühlemann index [12]. All periodontitis patients fulfilled the diagnostic criteria for chronic periodontitis (CP) defined according to CAL levels by the International Workshop for the Classification of Periodontal Diseases and Conditions for Chronic Periodontitis [13]. The inclusion criteria for patients with generalized CP were as follows: ≥ 30% of the teeth were affected, PD of ≥ 4 mm, and the amount of CAL was consistent with the presence of mineralized plaque. All our patients would have belonged to Stages 2–4 and most of them to Grade B according to the new (currently valid) classification [14].
Controls (N = 204) were recruited from patients who were referred to the Clinic of Stomatology for various reasons (e.g., preventive dental examinations, dental caries, and orthodontic consultations) during the same period as the periodontitis patients. They were of similar age, sex, smoking status, and had no previous diagnosis of periodontitis. Moreover, they had at least 20 remaining teeth, were in good general health, and with a CPITN of < 3.
The exclusion criteria were the presence of systemic diseases such as diabetes mellitus, cardiovascular disorders, pregnancy or lactation, malignant diseases; use of anti-inflammatory drugs or antibiotics for at least six weeks before the recruitment period; and an inability to provide consent, as described previously [15].
The participants were classified into the groups of non-smokers (participants who never smoked) and smokers (former smokers for ≥ 5 pack-years or current smokers) [16]. Pack-years were calculated by multiplying the number of cigarettes smoked per day by the number of years that a person smoked.
Isolation of genomic DNA
Genomic DNA was isolated from peripheral blood leukocytes by standard proteinase K digestion and phenol-chloroform extraction method [17].
Genetic analysis
Analysis of GSTM1 and GSTT1 gene variants
The analysis of GSTM1 and GSTT1 genes was simultaneously performed in a single assay using a slightly modified multiplex polymerase chain reaction (PCR) method [18] with primers listed in Table 1 and the details described previously [19]. PCR was performed using a SensoQuest Labcycler (Schoeller, Germany). The presence or absence of GSTM1 and GSTT1 genes was detected as a band at 215 bp (corresponding to GSTM1), 480 bp (corresponding to GSTT1), and 312 bp (corresponding to CYP1A1 as an internal control).
Analysis of GSTP1 genetic polymorphisms
To detect two GSTP1 polymorphisms (Ile105Val and Ala114Val) simultaneously, an assay with forward and reverse allele-specific primers enabling the identification of cis/trans orientation (PCR haploty**) [20], with slight modifications, was applied. The primers and product sizes are listed in Table 2. All reaction mixtures contained control primers for human growth hormone as described previously [19].
Statistical analysis
The power analysis for Fisher’s exact test was computed with the following settings: assumed sample size for the control/treatment group N = 200/200, power 80%, and level of statistical significance a = 0.05 [21, 22]. The chi-square test was used to assess Hardy-Weinberg equilibrium (HWE) and to compare genotype differences. Allele frequencies were calculated from the observed genotypes using Fisher’s exact test. The Bonferroni method was employed to eliminate the issue of multiple hypothesis testing (pcorr). One-way analysis of variance (ANOVA) and Kruskal-Wallis ANOVA were used to compare continuous variables between independent groups. All statistical analyses were performed using Statistica version 14 (StatSoft Inc., Tulsa, Okla., USA).
Results
Power calculations were performed for a wide range of endpoint relative frequencies in the control and treatment cohorts (0.25–0.75). Given the settings, the planned sample size allowed the detection of differences in the occurrence of examined markers ± 11% as statistically significant, which was confirmed by the estimation of 95% confidence limits of odds ratio (OR).
The characteristics of 407 participants comprising 204 healthy controls (108/96 males/females; 48.6 ± 6.0 years, mean age ± standard deviation, SD) and 203 periodontitis patients (99/104 males/females; 54.3 ± 7.4 years) are listed in Table 3. Smoking status was known in 187 (91.7%) healthy controls and 187 (92.1%) periodontitis patients. The groups did not differ in the male/female ratio (p > 0.05) or smoking status (p > 0.05); however, the periodontitis patients were older than the healthy individuals (p < 0.05). Periodontal health parameters, such as PD, CAL, GI, and Mühlemann index, were significantly different between healthy participants and periodontitis patients (p < 0.001; Table 3).
Table 4 shows the distribution of GST variants in healthy controls and periodontitis patients. The genotype frequencies in healthy controls were in accordance with HWE (p > 0.05, data not shown). We found no significant differences in the GSTM1, GSTT1, or GSTP1 genotype frequencies between the two groups (pcorr > 0.05).
Table 5 shows the GSTs frequencies in healthy controls and periodontitis patients according to their smoking status. The GSTM1 gene variants were similarly distributed between non-smokers and smokers. However, the wild genotype of the GSTT1 and Ala114Ala variant of the GSTP1 genes were present more frequently in the non-smoking periodontitis patients than in non-smoking controls (p = 0.03, pcorr > 0.05; p < 0.009, pcorr > 0.05, respectively). Conversely, the GST variants did not differ significantly between smoking periodontitis patients and smoking controls (p > 0.05).
Discussion
Periodontitis is a chronic and extremely widespread disease, the multifactorial etiology of which is affected by complex environmental, behavioral, and genetic factors [23]. Recently, the genetic variability of GSTs has been reported as a susceptibility factor for periodontitis in a Pakistani population. In an investigated set of 201 controls and 203 periodontitis patients, the researchers detected that the absence of GSTM1, presence of GSTT1, and mutant allele (G) at rs1695 in the GSTP1 gene might be associated to susceptibility to periodontitis [8]. However, this is not consistent with the results of this study, which involved a similar number of participants (204 healthy controls and 203 periodontitis patients) but found no significant differences in the distribution of GSTM1, GSTT1, and GSTP1 gene variants after correction for multiple comparisons between the two groups. Furthermore, as harmful products derived from cigarette smoke reportedly affect the association between several genes and periodontal status [24,25,26], we also explored the interaction between GST polymorphisms and smoking in the development of periodontitis. In our study, the GSTM1 gene variants were similarly distributed between non-smokers and smokers. However, the wild genotype of GSTT1 and Ala114Ala variant of the GSTP1 genes were present more frequently in non-smoking periodontitis patients than in non-smoking controls.
GSTs are involved in the neutralization of hydroperoxides derived from lipoperoxidation processes during oxidative stress caused by periodontal inflammatory processes and in the detoxification of xenobiotics, especially tobacco-derived substances, such as polycyclic aromatic hydrocarbons and hydroxylated metabolites of benzo-α-pyrene [27,28,29]. Kim et al. [30] first described a significantly increased risk of periodontal disease among participants (115 with chronic periodontitis and 126 controls) with GSTM1(+) genotype (OR = 2.1, 95%CI = 1.3–3.6) in a Korean population. Those with the GSTM1(+) genotype had a significant increase in periodontitis risk among smokers (OR = 3.1, 95%CI = 1.5–6.6) and a moderate risk increase among non-smokers (OR = 1.8, 95%CI = 1.0-3.1). Conversely, Concolino et al. [4] showed a significant association between both aggressive (N = 14) and chronic (N = 69) forms of periodontitis and the GSTM1-null variant in Italians (OR = 3.59, 95%CI = 1.66–7.84) regardless of smoking, patients’ age, sex, and hygienic habits. However, no significant differences were found in GSTT1 genotypes. In addition, in their pilot study, Ortega et al. [31] analyzed GSTM1, GSTT1, and GSTP1 polymorphisms in 60 Mexicans with chronic periodontitis (30 non-smokers and 30 smokers). Polymorphisms in the GSTT1 and GSTP1 genes were not significantly different between smokers and non-smokers; however, the GSTM1(+) genotype was significantly more frequent in smokers with periodontitis (p ≤ 0.05). However, compared to historical data from a healthy Mexican population, periodontitis patients showed a higher frequency of null and mutant polymorphisms in GSTM1, T1, and P1 [31,32,33]. Finally, a recent study by Saravanan et al. in 100 participants from a South Indian population did not reveal any significant association between GSTP1 (rs1695) polymorphism and periodontitis [34]. However, the GSTM1 and GSTT1 null genotypes have been found to be associated with an increased risk of apical periodontitis [35] and a significantly enhanced risk of develo** oral cancer [36].
The inconsistent conclusions reported in various studies could be attributed to the different ethnic backgrounds, as the frequency of GSTs polymorphic alleles between populations can vary significantly; different inclusion and exclusion criteria; or different methodological approaches to the analyses [37].
Certain limitations of this study should be considered. The main drawback is that the number of smokers is relatively small. This may lead to potential biases that could influence the study findings. Moreover, case-control study designs tend to produce false-positive results when cases and controls are from different population strata. However, in this study, the participants were selected from a small region of South Moravia in the Czech Republic, and thus the population studied was homogeneous. Furthermore, the participants in this study were all Caucasians; therefore, the effect of ethnicity could not be assessed. Finally, the association between gene polymorphisms and serum GST levels was not examined in this study, although it has been reported previously [38].
Conclusion and clinical relevance
This study did not find a highly significant association between GST gene variants and susceptibility to periodontitis in a Caucasian population, as previously reported for Pakistanis. Weak relationships between GSTT1 and GSTP1 rs1138272 polymorphisms and periodontitis in non-smokers were observed in this study. From a clinical perspective, the analyzed GST polymorphisms cannot be used as markers of an increased risk of develo** periodontitis in the Caucasian population, in contrast to the Asian population, where the presence of GSTT1 and a mutant allele (G) at rs1695 in the GSTP1 gene may be considered risk factors for genetic susceptibility to these disorders. The validity of these results must be confirmed in studies with robust sample sizes and independent cohorts of different ethnic origins.
Data availability
The datasets used and/or analyzed in the current study are available from the corresponding author upon reasonable request.
Abbreviations
- Ala:
-
Alanine
- ANOVA:
-
Analysis of variance
- bp:
-
base pair
- CAL:
-
Clinical attachment loss
- CI:
-
Confidence interval
- CP:
-
Chronic periodontitis
- CPITN:
-
Community Periodontal Index of Treatment Needs
- CYP1A1:
-
Cytochrome P450 A1
- DNA:
-
Deoxyribonucleotide acid
- GI:
-
Gingival index
- GSTs:
-
Glutathione S-transferases
- GSTM1:
-
Glutathione-S-transferase µ-1
- GSTP1:
-
Glutathione-S-transferase π -1
- GSTT1:
-
Glutathione-S-transferase θ -1
- HWE:
-
Hardy-Weinberg equilibrium
- Ile:
-
Isoleucine
- LD:
-
Linkage disequilibrium
- N:
-
Number of individuals
- NA:
-
Non-applicable
- OR:
-
Odds ratio
- PCR:
-
Polymerase chain reaction
- PCR-SSCP:
-
Polymerase chain reaction – sequence-specific primers
- PD:
-
Probing depth
- ROS:
-
Reactive oxygen species
- SD:
-
Standard deviation
- Val:
-
Valine
References
Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366(9499):1809–20. https://doi.org/10.1016/S0140-6736(05)67728-8.
Chen M, Cai W, Zhao S, Shi L, Chen Y, Li X, et al. Oxidative stress-related biomarkers in saliva and gingival crevicular fluid associated with chronic periodontitis: a systematic review and meta-analysis. J Clin Periodontol. 2019;46(6):608–22. https://doi.org/10.1111/jcpe.13112.
Chapple ILC, Matthews JB. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontol 2000. 2007;43:160–232. https://doi.org/10.1111/j.1600-0757.2006.00178.x.
Concolino P, Cecchetti F, D´Autilia C, Santonocito C, Di Stasio E, Zuppi C, et al. Association of periodontitis with GSTM1/GSTT1-null variants – A pilot study. Clin Biochem. 2007;40(13–14):939–45. https://doi.org/10.1016/j.clinbiochem.2007.04.012.
Sheehan D, Meade G, Foley VM, Dowd CA. Structure, function and evolution of glutathione transferases: implications for classification of non-mammalian members of an ancient enzyme superfamily. Biochem Soc. 2001;360(1):1–16. https://doi.org/10.1042/bj3600001.
Onaran I, Güven G, Ozaydin A, Ulutin T. The influence of GSTM1 null genotype on susceptibility to in vitro oxidative stress. Toxicology. 2001;157:195–205. https://doi.org/10.1016/s0300-483x(00)00358-9.
Ali-Osman F, Akande O, Antoun G, Mao JX, Buolamwini J. Molecular cloning, characterization, and expression in Escherichia coli of full-length cDNAs of three human glutathione S-transferase pi gene variants. Evidence for differential catalytic activity of the encoded proteins. J Biol Chem. 1997;272:10004–12. https://doi.org/10.1074/jbc272.15.10004.
Arshad K, Ishfaq U, Asif M, Akbar A, Pitafi KF, Mulghani MR, et al. Association of GSTT1, M1 and polymorphism in GSTP1 with chronic periodontal disease in a Pakistani population. Genes. 2023;14:455. https://doi.org/10.3390/genes14020455.
World Health Organisation., Oral health surveys: basic methods, 1997. 4th ed., WHO, Geneva.
Silness J, Löe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand. 1964;22:121–35. https://doi.org/10.3109/00016356408993968.
Bartosova M, Borilova Linhartova P, Musilova K, Broukal Z, Kukletova M, Kukla L, Izakovicova Holla L. Association of the CD14 -260 C/T polymorphism with plaque-induced gingivitis depends on the presence of Porphyromonas gingivalis. Int J Pediatr Dent. 2022;32:223–31. https://doi.org/10.1111/ipd.12847.
Mühlemann HR, Mazor ZS, Röntgendiagnostik. Schweiz Monattschr Zahnkeilkd. 1955;65:1005–13.
Armitage GC. Development of a classification system for periodontal diseases and conditions. Annals Periodontology. 1999;4:1–6. https://doi.org/10.1902/annals.1999.4.1.1.
Papapanou PN, Sanz M, Buduneli N, Dietrich T, Feres M, Fine DH, et al. Periodontitis: consensus report of workgroup 2 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J Periodontol. 2017;89:173–82. https://doi.org/10.1002/JPER.17-0721.
Izakovicova Holla L, Hrdlickova B, Vokurka J, Fassmann A. Matrix metalloproteinase 8 (MMP8) gene polymorphisms in chronic periodontitis. Arch Oral Biol. 2012;57:188–96. https://doi.org/10.1016/j.archoralbio.2011.08.018.
Izakovicova Holla L, Fassmann A, Stejskalova A, Znojil V, Vanek J, Vacha J. Analysis of the interleukin-6 gene promoter polymorphisms in Czech patients with chronic periodontitis. J Periodontol. 2004;75:30–6. https://doi.org/10.1902/jop.2004.75.1.30.
Sambrook J, Fritsch EF, Maniatis T, editors. Molecular cloning: a laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. pp. 9–17.
Abdel-Rahman SZ, El-Zein RA, Anwar WA, Au WW. A multiplex PCR procedure for polymorphic analysis of GSTM1 and GSTT1 genes in population studies. Cancer Lett. 1996;107:229–33. https://doi.org/10.1016/0304-3835(96)04832-x.
Kala Z, Dolina J, Marek F, Izakovicova Holla L. Polymorphisms of glutathione S-transferase M1, T1 and P1 in patients with reflux esophagitis and Barrett´s esophagus. J Hum Genet. 2007;52:527–34. https://doi.org/10.1007/s10038-007-0148-z.
Marshall SE, Bordea C, Haldar NA, Mullighan CG, Wojnarowska F, Morris PJ, et al. Glutathione S-transferase polymorphisms and skin cancer after renal transplantation. Kidney Int. 2000;58:2186–93. https://doi.org/10.1111/j.1523-1755.2000.00392.x.
Chow SC, Shao J, Wang H. 2008. Sample Size Calculations in Clinical Research, Second Edition. Chapman & Hall/CRC. Boca Raton, Florida.
Ryan TP. Sample size determination and Power. Hoboken, New Jersey: Wiley; 2013.
Di Stefano M, Polizzi A, Santonocito S, Romano A, Lombardi T, Isola G. Impact of oral microbiome in periodontal health and periodontitis: a critical review on prevention and treatment. Int J Mol Sci. 2022;23(9):5142. https://doi.org/10.3390/ijms23095142.
Freitag-Wolf S, Munz M, Wiehe R, Junge O, Graetz C, Jockel-Schneider Y, et al. Smoking modifies the genetic risk for early-onset periodontitis. J Dent Res. 2019;98(12):1332–9. https://doi.org/10.1177/0022034519875443.
Kocher T, Sawaf H, Fanghänel J, Timm R, Meisel P. Association between bone loss in periodontal disease and polymorphism of N-acetyltransferase (NAT2). J Clin Periodontol. 2002;29(1):21–7. https://doi.org/10.1034/j.1600-051x.2002.290104.x.
Izakovicova Holla L, Buckova D, Fassmann A, Benes P, Znojil V. Plasminogen-activator-inhibitor-1 promoter polymorphism as a risk factor for adult periodontitis in non-smokers. Genes Immun. 2002;3(5):292–4. https://doi.org/10.1038/sj.gene.6363874.
Borges I Jr, Moreira EA, Filho DW, de Oliveira TB, da Silva MB, Fröde TS. Proinflammatory and oxidative stress markers in patients with periodontal disease. Mediators Inflamm. 2007;45794. https://doi.org/10.1155/2007/45794.
Eaton DL, Bammler TK. Concise review of the glutathione S-transferases and their significance to toxicology. Toxicol Sci. 1999;49(2):156–64. https://doi.org/10.1093/toxsci/49.2.156.
Ketterer B, Harris JM, Talaska G, Myeyer DJ, Pemble SE, Taylor JB, et al. The human glutathione S-transferase supergene family, its polymorphism, and its effects on susceptibility to lung cancer. Environ Health Perspect. 1992;98:87–94. https://doi.org/10.1289/ehp.929887.
Kim JS, Park JY, Chung WY, Choi MA, Cho KS, Park KK. Polymorphisms in genes coding for enzymes metabolizing smoking-derived substances and the risk of periodontitis. J Clin Periodontol. 2004;31:959–64. https://doi.org/10.1111/j.1600-051X.2004.00587.x.
Camargo Ortega VR, Bravo López LD, Saldago AV, Sanchez FM, Caden JC. Polymorphisms in glutathione S-transferase M1, T1, and P1 in patients with chronic periodontitis: a pilot study. Int Sch Res Notices. 2014;2014:25. https://doi.org/10.1155/2014/135368.
Montero R, Araujo A, Carranza P, Mejía-Loza V, Serrano L, Albores A, et al. Genotype frequencies of polymorphic GSTM1, GSTT1, and cytochrome P450 CYP1A1 in mexicans. Hum Biol. 2007;79(3):299–312. https://doi.org/10.1353/hub.2007.0037.
Pérez-Morales R, Castro-Hernández C, Gonsebatt ME, Rubio J. Polymorphism of CYP1A1*2 C, GSTM1*0, and GSTT1*0 in a Mexican mestizo population: a similitude analysis. Hum Biol. 2008;80(4):457–65. https://doi.org/10.3378/1534-6617-80.4.457.
Saravanan RV, Pandi A, Murthykumar K, Selvi SGA, Arumugam P, Jayaseelan VP. Genetic association between rs1695 in glutathione S-transferase P1 and risk of periodontitis: a pilot study. Mol Biol Res Commun. 2023;12(4):133–7. https://doi.org/10.22099/mbrc.2023.46999.1815.
Jakovljevic A, Nikolic N, Carkic J, Beljic-Ivanovic K, Soldatovic I, Miletic M, et al. Association of polymorphisms in TNF-α, IL-1β, GSTM, and GSTT genes with apical periodontitis: is there a link with herpesviral infection? Int Endod J. 2020;53:895–904. https://doi.org/10.1111/iej.13298.
Vinod Kumar K, Goturi A, Nagaraj M, Soma Sekhar Goud EV. Null genotypes of glutathione S-transferase M1 and T1 and risk of oral cancer: a meta-analysis. J Oral Maxillofac Pathol. 2022;26:592. https://doi.org/10.4103/jomfp.jomfp_435_21.
Garte S, Gaspari L, Alexandrie AK, Ambrosone C, Autrup H, Autrup JL, et al. Metabolic gene polymorphism frequencies in control populations. Cancer Epidemiol Biomarkers Prev. 2001;10:1239–48.
Burattia FM, Darneyb K, Vichia S, Turcoa L, Di Consiglioa E, Lautzb LS, et al. Human variability in glutathione-S-transferase activities, tissue distribution and major polymorphic variants: Meta-analysis and implication for chemical risk assessment. Toxicol Lett. 2021;337:78–90. https://doi.org/10.1016/jtoxlet.2020.11.007.
Acknowledgements
We thank Mrs. Andrea Stejskalova for laboratory assistance.
Funding
The study was supported by project MUNI/A/1607/2023.
Author information
Authors and Affiliations
Contributions
Conceptualization: LIH; Methodology: LIH; Investigation: LIH, AF; Statistical analysis: LIH, LD; Resources: LIH; Writing – original draft preparation: PI; Writing – review and editing: LIH, AF, LD; Project administration: LIH; Funding acquisition: LIH.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Ethics Committee of Masaryk University, Faculty of Medicine (No.15/2009, No. 13/2013), and St. Anne’s Faculty Hospital Brno (without No./2005) approved this study. Written informed consent was obtained from all participants prior to their inclusion in the study according to the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest. The funders had no role in the design of the study; collection, analyses, or interpretation of data; writing the manuscript; or decision to publish the results.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Izakovicova, P., Fassmann, A., Dusek, L. et al. Glutathione S-transferase M1, T1, and P1 polymorphisms and periodontitis in a Caucasian population: a case-control study. BMC Oral Health 24, 288 (2024). https://doi.org/10.1186/s12903-024-04034-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12903-024-04034-x