Abstract
Background
This in vitro study examined the effect of the inflammatory cytokines (tumour necrosis factor-α (TNF-α), interleukin (IL)-1β, and IL-6) on osteogenic, chondrogenic, and adipogenic differentiation of dental pulp stem cells (DPSCs) which have significant relevance in future regenerative therapies.
Methods
DPSCs were isolated from the impacted third molar dental pulp and determined with flow cytometry analysis. DPSCs were divided into into 5 main groups with 3 subdivisions for each group making a total of 15 groups. Experimental groups were stimulated with TNF-α, IL-1β, IL-6, and a combination of all three to undergo osteogenic, chondrogenic, and adipogenic differentiation protocols. Next, the differentiation of each group was examined with different staining procedures under a light microscope. Histological analysis of osteogenic, chondrogenic, and adipogenic differentiated pellets was assessed using a modified Bern score. Statistical significance determined using one-way analysis of variance, and correlations were assessed using Pearson’s test (two-tailed).
Results
Stimulation with inflammatory cytokines significantly inhibited the osteogenic, chondrogenic and adipogenic differentiation of DPSCs in terms of matrix and cell formation resulting in weak staining than the unstimulated groups with inflammatory cytokines. On contrary, the unstimulated groups of MSCs have shown to be highly proliferative ability in terms of osteogenic, chondrogenic, and adipogenic differentiation.
Conclusions
DPSCs have high osteogenic, chondrogenic, and adipogenic differentiation capabilities. Pretreatment with inflammatory cytokines decreases the differentiation ability in vitro, thus inhibiting tissue formation.
Similar content being viewed by others
Background
Stem cell-based technologies are an ideal source for regenerative medicine, immunological studies, and cell therapy because they induce tissue repair and regeneration [1, 2]. Mesenchymal stem cells (MSCs) play a key role in tissue regeneration treatment. They are rapidly adherent, clonogenic, and capable of extended proliferation in vitro [3]. In addition, they maintain stem cell properties such as self-renewal, long-term viability, and differentiation potential into mesodermal origin osteocytes, chondrocytes, and adipocytes [4, 5]. As a result of their capacity to differentiate into various cell types, MSCs play a key role in tissue and organ regeneration and have recently attracted great interest in tissue engineering [6, 7].
Even though MSCs can be isolated from many sources, such as cord blood, bone marrow, or adipose tissue [8], a very promising source is the relatively easily obtainable dental tissue. There are five types of human dental stem cells: dental pulp stem cells (DPSCs) [9], stem cells from exfoliated deciduous teeth (SHED) [10], periodontal ligament stem cells (PDLSCs) [11], dental follicle stem cells [12], and stem cells from apical papilla [13]. These MSCs express specific MSC markers, such as CD29, CD73, CD90, CD105, and CD166, and can differentiate into odontoblasts, chondrocytes, and adipocytes under appropriate circumstances [9, 10]. DPSCs can easily be isolated from the dental pulp tissue of newly extracted teeth, making the procedure relatively more straightforward and avoiding ethical dilemmas [14]. DPSCs are commonly used in the regeneration and reconstruction of dental structures in addition to bone tissue engineering after undergoing osteogenic differentiation [15, 16].
Tissue engineering techniques, including the use of MSCs, often require scaffolds and cytokines serving as inductive factors [17]. Some inflammatory cytokines alter stem cell functions as well as immune or inflammatory cells [18]. In vitro studies have revealed that cytokines can affect the differentiation process of mesenchymal progenitor cells during tissue formation. Most of these in vitro studies have used MSCs by isolating them because of their ability to adhere to plastic [19, 20].
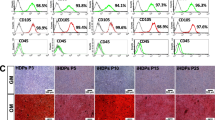
Cytokines are commonly used superior markers of inflammation, modulating immune and inflammatory responses [21]. Tumour necrosis factor-α (TNF-α) is defined as a proinflammatory cytokine expressed in injured tissues as well as in ischaemic situations [22]. TNF-α also plays a major role in the repair process of injured tissues and promotes MSC recruitment [23,24,50, 51]. The two most commonly used techniques for isolating DPSCs from dental pulp tissue are the explant method and enzymatic digestion. Hilkens et al. [52] reported no difference in the tissue differentiation potential of DPSCs regarding the isolation method. In our study, the enzymatic digestion method used to isolate DPSCs was based on previous studies [9, 10]. After isolation, the cells displayed fibroblast-shaped morphology and were adherent to plastic Petri plates. Flow cytometry analysis declared that the cells showed positive expression for CD29, CD105, CD146, and CD73 markers and negative for CD3, CD4, and CD20 markers, which agree with the criteria of the International Society of Cellular Therapy [48]. Osteogenic, chondrogenic, and adipogenic differentiation procedures were performed on characterised DPSCs, and Alizarin red, Alcian blue, and oil red stains were used, respectively, to determine their differentiation into cell lines in accordance with previous studies [2, 53, 54]. Tarte et al. [55] also used staining procedures to compare the proliferation and differentiation of SHEDs and PDLSCs, but used von Kossa staining instead of Alcian blue to determine chondrogenic differentiation. MSCs can differentiate in vitro spontaneously or by the induction of biologically active molecules [56]. DPSCs can also proliferate and differentiate, as can other stem cells of dental origin [2, 9, 10, 51].
Effect of cytokine on differentiation
Cytokines modulate immune and inflammatory responses and are markers of inflammation [21, 57]. In many situations, certain tissues need to be regenerated due to injury. Whether the tissue injury is caused by microorganisms (e.g. pulpitis) or trauma (e.g. bone fractures), proinflammatory cytokines are the superior markers of inflammatory responses [32, 58]. Both positive and negative impacts of cytokines on MSC differentiation and tissue healing have been reported [32, 47]. The present study determined how DPSCs might behave in an inflammatory environment set up with some key proinflammatory cytokines TNF-α, IL-1β, and IL-6. Many previous in vitro and in vivo studies have evaluated their roles in osteogenic and chondrogenic differentiation of MSCs. Kondo et al. [59]. indicated that in the early stages, TNF-α, IL-1β, and IL-6 contribute to fracture healing and bone remodelling. **e et al. also [54] demonstrated that IL-6 promotes osteogenic differentiation in BM-MSCs in vitro. In vitro studies demonstrating the effects of TNF-α, IL-1β and IL-6 have mostly involved osteogenesis with MSCs other than dental origin and have not directly compared their effects on differentiation [32, 47]. Contrary to our results, Liu et al. [51] demonstrated that TNF-α promoted the osteogenic differentiation of DPSCs in vitro. Similarly, Feng et al. [25] demonstrated that TNF-α activates the NF-κB pathway and promotes osteogenic differentiation of DPSCs in vitro. Another in vitro study showed increased calcium deposits following IL-1β pretreatment when culturing BM-MSCs in osteogenic medium.
On contrary, Kondo et al. [59] also reported bone resorption can be induced under IL-6 stimulation. Lacey et al. [32] compared the effects of TNF-α and IL-1β on the osteogenic capacity of murine MSCs and found that these cytokines inhibited MSC differentiation to osteoblasts, which agrees with our findings. Liu et al. [51] investigated osteogenic differentiation of DPSCs promoted by TNF-α; this was similar to our study with the difference of evaluating transcriptome changes. Additionally, relatively long-term exposure to inflammatory mediators were reported to suppresses DPSC differentiation ability [60].
Considering the chondrogenic differentiation of BM-MSCs, Mumme et al. demonstrated the most intense staining for cartilage with low-dose IL-1β (10 and 50 pg/mL) [41]. The discrepancies in the outcomes among these studies and our study can be explained by the differences in the concentrations of proinflammatory cytokines and in the origin of the stem cells.
A limitation of the present study was that it was an in vitro analysis and not in vivo. The cytokine concentrations used in the study were used as the highest possible concentrations to assess the differentiation potential of DPSCs into the inflammatory niche. These concentrations may not be similar in vivo. In addition, the differentiation potential of DPSCs may vary in the presence of anti-inflammatory drugs such as anti-TNF-α, anti-IL6, which are used in some autoimmune or inflammatory diseases. Nonetheless, our findings might be useful for further studies for understanding the mechanisms and outcomes of DPSC differentiation with specific cytokine modulation both in vitro and in vivo because the functions and expressions of proinflammatory cytokines during certain tissue differentiations remain unclear in vivo [54]. In addition, stem cells of dental origin are expected to be preferred more frequently in future research because they are easy to obtain. Future studies should be designed to include different concentrations of inflammatory cytokines, evaluation of gene expression, and use of dental stem cells with different origins.
Conclusion
Our results indicated that DPSCs are highly proliferative MSCs in terms of osteogenic, chondrogenic, and adipogenic differentiation. In the present in vitro study, TNF-α, IL-1β, and IL-6 were demonstrated to inhibit DPSC differentiation and tissue formation. Further studies, including in vivo applications with different dental MSCs origins and diverse amount, type and appliance durations are required to more comprehensively understand the underlying molecular mechanisms for application in stem cell therapies.
Availability of data and materials
The datasets generated during and analyzed during the current study are not publicly available due to the protocol submitted to the Ethics Committee Of X University but are available from the corresponding author on reasonable request.
References
Akkoç T, Genç D. Asthma immunotherapy and treatment approaches with mesenchymal stem cells. Immunotheraphy. 2020;12:9.
Yıldırım S, Zibandeh N, Genç D, et al. The comparision of the immunologic properties of stem cells isolated from human exfoliated deciduous teeth dental pulp and dental folicles. Stem Cells Int. 2016;2016:1–16.
Keating A. Mesenchymal stromal cells: new directions. Cell Stem Cell. 2012;10:709–16.
Suchánek J, Visek B, Soukup T, et al. Stem cells from human exfoliated deciduous teeth-isolation, long term cultivation and phenotypical analysis. Acta Medica. 2010;53:93–9.
Schaffler A, Buchler C. Consice rewiew: adipose tissue-derived stromal cells – basic and clinical implications for novel cell-based theraphies. Stem Cells. 2007;25:818–27.
Ankrum J, Karp JM. Mesenchymal stem cell theraphy: two steps forward, one step back. Trends Mol Med. 2010;16:203–9.
Kolf CM, Cho E, Tuan RS. Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis Res Ther. 2007;9:204.
da Silva MI, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post natal organs and tissues. J Cell Sci. 2006;119:2204–13.
Gronthos S, Mankani M, Brahim J, et al. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000;97:13625–30.
Miura M, Gronthos S, Zhao M, et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA. 2003;100:5807–12.
Seo BM, Miura M, Gronthos S, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–55.
Morsczeck C, Gotz W, Schierholz J, et al. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol. 2005;24:155–65.
Sonoyama W, Liu Y, Fang D, et al. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS ONE. 2006;1:e79.
Huang GT, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res. 2009;88:792–806.
Tatullo M, Marrelli M, Shakesheff KM, et al. Dental pulp stem cells: function, isolation and applications in regenerative medicine. J Tissue Eng Regen Med. 2015;9:1205e1216.
Laino G, D’Aquino R, Graziano A, et al. A new population of human adult dental pulp stem cells: a useful source of living autologous fibrous bone tissue (LAB). J Bone Mineral Res. 2005;20:1394e1402.
Ringe J, Sittinger M. Selecting the right biological scaffold for tissue engineering. Nat Rev Rheumatol. 2014;10:388–9.
Szabó E, Fajka-Boja R, Kriston-Pál É, et al. Licensing by inflammatory cytokines abolishes heterogeneity of immunosuppressive function of mesenchymal stem cell population. Stem Cells and Dev. 2015;18:2171–80.
Murakami S, Lefebvre V, de Crombrugghe B. Potent inhibition of the master chondrogenic factor Sox9 gene by interleukin-1 and tumor necrosis factor-alpha. J Biol Chem. 2000;275:3687e92.
Suzawa M, Takada I, Yanagisawa J, et al. Cytokines suppress adipogenesis and PPAR gamma function through the TAK1/TAB1/NIK cascade. Nat Cell Biol. 2003;5:224e30.
Ashida H, Mimuro H, Ogawa M, et al. Cell death and infection: a double-edged sword for host and pathogen survival. J Cell Biol. 2011;195:931–42.
Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501.
Jiang B, Liao R. The paradoxical role of inflammation in cardiac repair and regeneration. J Cardiovasc Transl Res. 2010;3:410–6.
Kon T, Cho TJ, Aizawa T, et al. Expression of osteoprotegerin, receptor activator of NF-κB ligand (osteoprotegerin ligand) and related proinflammatory cytokines during fracture healing. J Bone Miner Res. 2001;16:1004–14.
Feng X, Feng G, **ng J, et al. TNF-a triggers osteogenic differentiation of human dental pulp stem cells via the NF-kB signalling pathway. Cell Biol Int. 2013;37:1267–75.
Heymann D, Rousselle AV. gp130 Cytokine family and bone cells. Cytokine. 2000;12:1455–68.
Tseng HC, Lee IT, Lin CC, et al. IL-1β promotes corneal epithelial cell migration by increasing MMP-9 expression through NF-κB- and AP-1-dependent pathways. PLoS ONE. 2013;8:e57955.
Lin CC, Kuo CT, Cheng CY, et al. IL-1β promotes A549 cell migration via MAPKs/AP-1- and NF-κB-dependent matrix metalloproteinase-9 expression. Cell Signal. 2009;21:1652–62.
Mountain DJH, Singh M, Menon B, et al. Interleukin-1β increases expression and activity of matrix metalloproteinase-2 in cardiac microvascular endothelial cells: role of PKCα/β1 and MAPKs”. Am J Physiol Cell Physiol. 2007;292:C867–75.
Kang SK, Shin IS, Ko MS, et al. Journey of mesenchymal stem cells for homing: strategies to enhance efficacy and safety of stem cell therapy. Stem Cells Int. 2012;2012:342968.
Ries C, Egea V, Karow M, et al. MMP-2, MT1-MMP, and TIMP-2 are essential for the invasive capacity of human mesenchymal stem cells: differential regulation by inflammatory cytokines. Blood. 2007;109:4055–63.
Lacey DC, Simmons PJ, Graves SE, et al. Proinflammatory cytokines inhibit osteogenic differentiation from stem cells: implications for bone repair during inflammation. Osteoarthr Cartilage. 2009;17:735–42.
Deshpande S, James AW, Blough J, et al. Reconciling the effects of inflammatory cytokines on mesenchymal cell osteogenic differentiation. J Surg Res. 2013;185:278–85.
Keller JF, Carrouel F, Staquet MJ, et al. Expression of NOD2 is increased in inflamed human dental pulps and lipoteichoic acid-stimulated odontoblast-like cells. Innate Immun. 2011;17:9–34.
Smith AJ. Pulpal responses to caries and dental repair. Caries Res. 2002;36:223–32.
Waterhouse PJ, Nunn JH, Whitworth JM. Prostaglandin E2 and treatment outcome in pulp therapy of primary molars with carious exposures. Int J Paediatr Dent. 2002;12:116–23.
Welin J, Wilkins JC, Beighton D, et al. Effect of acid shock on protein expression by biofilm cells of Streptococcus mutans. FEMS Microbiol Lett. 2003;227:287–93.
Ginsburg I. Role of lipoteichoic acid in infection and inflammation. Lancet Infect Dis. 2002;2:171–9.
Matsushima T, Ohbayashi E, Hosoda S, et al. Stimulation of IL-1b and IL-6 production in human dental pulp cells by peptidoglycans from carious lesion microorganisms. In: Shimono M, Maeda T, Suda H, Takahashi K, editors., et al., International Conference on Dentin/Pulp Complex 1995; 1995. Chiba, Japan: Quintessence Publishing; 1995. p. 310–2.
Rechenberg DK, Galicia JC, Peters OA. Biological markers for pulpal inflammation: a systematic review. PLoS ONE. 2016;11:e0167289.
Mumme M, Scotti C, Papadimitropoulos A, et al. Interleukın-1β modulates endochondral ossification by human adult bone marrow stromal cells. Eur Cell Mater. 2012;24:224–36.
Hess K, Ushmorow A, Fielder J, et al. TNF-α promotes osteogenic differentiation of human mesenchimal stem cells by triggering the NF-κB signaling pathway. Bone. 2009;45:267–376.
Cho TJ, Gerstenfeld LC, Einhorn TA. Differential temporal expression of members of the transforming growth factor β superfamily during murine fracture healing. J Bone Miner Res. 2002;17:513–20.
Wehling N, Palmer GD, Pilapil C, et al. Interleukin-1 and tumor necrosis factor-α inhibit chondrogenesis by human mesenchymal stem cells through NF-κB dependent pathways. Arthritis Rheum. 2009;60:801–12.
Redondo-Castro E, Cunningham C, Miller J, et al. Interleukin-1 primes human mesenchymal stem cells towards an anti-inflammatory and pro-trophic phenotype in vitro. Stem Cell Res Ther. 2017;8:79.
Grogan SP, Barbero A, Winkelmann V, et al. Visual histological grading system for the evaluation of in vitro-generated neocartilage. Tissue Eng. 2006;12:2141–9.
Bastidas-Coral AP, Bakker AD, Zandieh-Doulabi B, et al. Cytokines TNF-α, IL-6, IL-17F, and IL-4 differentially affect osteogenic differentiation of human adipose stem cells. Stem Cells Int. 2016;2016:1318256.
Friedenstein AJ, Petrakova KV, Kurolesova AI, et al. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6:230–47.
Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellulartherapy position statement. Cytotherapy. 2006;8:315–7.
Victoria-Escandell A, Ibañez-Cabellos JS, de Cutanda SB, et al. Cellular responses in human dental pulp stem cells treated with three endodontic materials. Stem Cells Int. 2017;2017:8920365.
Liu YK, Zhou ZY, Liu F. Transcriptome changes during TNF-a promoted osteogenic differentiation of dental pulp stem cells (DPSCs). Biochem Biophys Res Commun. 2016;476:426–30.
Hilkens P, Gervois P, Fanton Y, et al. Effect of isolation methodology an stem cell properties and multilineage differentiation potential of human dental puşp stem cells. Cell Tissue Res. 2012;353:65–78.
Tuncer Budanur D, Zibandeh N, Genç D, et al. Effect of CDMEM media containing Ectoine on human periodontal ligament mesenchymal stem cell survival and differentiation. Dent Traumatol. 2018;34:188–200.
**e Z, Tang S, Ye G, et al. Interleukin-6/interleukin-6 receptor complex promotes osteogenic differentiation of bone marrow-derived mesenchymal stem cells. Stem Cell Res Ther. 2018;9:13.
Tarle SA, Shi S, Kaigler D. Development of a serumfree system to expand dental-derived stem cells: PDLSCs and SHEDs. J Cell Physiol. 2011;226:66–73.
Steens J, Klein D. Current strategies to generate human mesenchymal stem cells in vitro. Stem Cells Int. 2018;2018(26):6726185.
ElSalhy M, Azizieh F, Raghupathy R. Cytokines as diagnostic markers of pulpal inflammation. Int Endod J. 2013;46:573–80.
Shanbhag AS, Jacobs JJ, Black J, et al. Cellular mediators secreted by interfacial membranes obtained at revision total hip arthroplasty. J Arthroplasty. 1995;10:498e506.
Kondo M, Yamaoka K, Sakata K, et al. Contribution of the interleukin-6/STAT-3 signaling pathway to chondrogenic differentiation of human mesenchymal stem cells. Arthritis Rheumatol. 2015;67:1250–60.
Fouad AF, Huang GT. Inflammation and immunological responses. In: Ingle J, Bakland LK, Baumgartner JC. Ingle’s Endodontics Hamilton 6th ed. BC Decker Inc; Ontario: Canada:2008. p:343–375.
Acknowledgements
None.
Funding
This study was supported by the X University Research Project BAPKO (Project number: SAG-C-DRP-111115–0508).
Author information
Authors and Affiliations
Contributions
S.S.K. contributed with the conception of the study, and the design and draft of the manuscript, and read and approved the final manuscript. T.A. contributed with the conception of the study, and the design and draft of the manuscript, and read and approved the final manuscript. H.S.Ö. contributed with the conception of the study, and read and approved the final manuscript. D.G. contributed with the conception of the study, and the design and draft of the manuscript, and read and approved the final manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics Committee of the X University Clinical Researches in Istanbul, Turkey approved the study protocol (22.05.15–1). Participation in the study was voluntary. All participants provided written informed consent to participate in this study.
The study was conducted in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sonmez Kaplan, S., Sazak Ovecoglu, H., Genc, D. et al. TNF-α, IL-1B and IL-6 affect the differentiation ability of dental pulp stem cells. BMC Oral Health 23, 555 (2023). https://doi.org/10.1186/s12903-023-03288-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12903-023-03288-1