Abstract
Background
High-translucency monolithic zirconia were developed to combine the esthetics of all ceramic restorations with the strength properties of zirconia. The purpose of this study was to compare the color stability of high-translucency monolithic zirconia ceramics with lithium disilicate luted using light-cure versus dual-cure resin cements following thermocyclic aging.
Methods
Forty specimens, each composed of 10 × 10 × 1 mm ceramic slice luted to dentin surface of an extracted tooth, were prepared and assigned into four groups (n = 10) as follows; LiDi/LC: lithium disilicate luted by light-cure resin cement; LiDi/DC: lithium disilicate luted by dual-cure resin cement; Zr/LC: zirconia luted by light-cure resin cement; and Zr/DC: zirconia luted by dual-cure resin cement. Color analysis of the specimens was performed before and after 3000 thermal cycles by means of spectrophotometry. The CIE L*a*b* values of the specimens were measured, and data were analyzed statistically at a significance value of p < 0.05.
Results
Thermocycling resulted in a significant change in color coordinates of specimens with an overall ΔE = 3.59 ± 1.60, but there was no statistically significant difference in the color change value among all tested groups (P = 0.756).
Conclusions
At 1 mm restoration thickness, the color stability of high-translucency monolithic lithium disilicate and zirconia ceramics were not significantly different irrespective of the cement type used.
Clinical implication Understanding the difference in color stability of dental ceramics may help in determining long-term esthetic result.
Similar content being viewed by others
Background
As the expected standards of the public in the area of dentistry have increased, so has the need for tooth-colored restorations with superior properties. Consequently, various tooth-colored restorations with superior esthetic and mechanical qualities have been developed. Lithium disilicate glass ceramics are commonly used nowadays due to their high strength, adequate bonding to tooth structure, easy construction procedure, and acceptable esthetic appearance [1]. Nevertheless, dental material manufacturers have developed high-translucency monolithic zirconium oxide restorations to combine the esthetics of all ceramic restorations with the superior strength properties of zirconia [2].
The optical and mechanical properties of highly translucent ceramic materials have been compared in few recent studies [2, 3]. Church et al. [2] reported in an in vitro study that a lithium disilicate material (IPS e.max CAD) has greater translucency than four other types of high-translucency monolithic zirconia. These were BruxZir shaded 16, InCoris TZIC, Lava Plus, and BruxZir HT, all being of the same thickness. Similarly, Yan et al. [3] found that the translucency of lithium disilicate is superior to those of both, 4-Y-PSZ (4 mol% yttria partially-stabilized zirconia) and 3-Y-PSZ, whereas it was insignificantly different from that of 5-Y-PSZ.
Color stabilities of monolithic ceramics and composite resin cements have also been reported. Putra et al. [4] have reported a minimal change in the percentage of light transmittance of BruxiZuir anterior solid zirconia, super-translucent Katana zirconia and ultra-translucent Katana zirconia, after a period of hydrothermal aging at 134 °C and 0.2 MPa. However, a change in color after artificial accelerated aging was reported for resin nano-ceramics, feldspathic ceramics, and leucite ceramics cemented with dual-cure resin cement to extracted teeth [5]. Moreover, the change in the color of the ceramic material was found to be directly related to the alteration in the color of the associated luting resin cement, where the thinner the restoration (0.5 mm), the greater was the reported color change when dual-cure or light-cure cements were used [6]. Mina et al. [7] studied the color stability after the accelerated aging of four different types of luting resin cements and reported a significant color change in Nexus 3 dual-cure cement, Rely-x ultimate dual-cure cement, and Nexus 3 light-cure cement, but not for Variolink esthetic light-cure cement. To our knowledge, the color stability of high-translucency ceramics has been insufficiently investigated in the literature. Therefore, the aim of this study was to determine and compare the color stability of high-translucency monolithic lithium disilicate and zirconia ceramics when luted with light-cure and dual-cure resin cements and subjected to thermocyclic aging. The specific objectives are:
-
1.
To determine the effect of thermocycling on the color coordinates of the luted ceramic material.
-
2.
To determine the effect of the ceramic type and/or cement type on the color coordinates of the luted ceramic material.
-
3.
To compare the color stability of luted lithium disilicate ceramic to that of luted zirconia ceramic.
The null hypothesis of the study was that the color stability of high-translucency monolithic lithium disilicate and high-translucency zirconia ceramics luted with light-cure or dual-cure resin cements are the same after thermocycling.
Material and methods
Specimens preparation
Forty extracted sound human premolars were chosen for this study after obtaining an ethical approval (#116-10-18). Teeth were extracted for orthodontics reasons. Immediately after extraction, remnants of soft tissue were removed using hand scaler. All teeth were assessed for visible fractures or discoloration. The dimensions of the occlusal surfaces of the crowns were measured using periodontal probe. Only sound teeth with 7–8 mm mesio-distal dimension and 8–9 mm bucco-lingual dimension were included in this study. Teeth roots were mounted in cylindrical mounts of self-cure acrylic resin (GC AMERICA INC). Subsequently, the teeth were stored in normal saline at room temperature. Occlusal enamel of all teeth was removed by occlusal reduction up to dentin using a diamond wheel on a high-speed handpiece (Diamond Metal Blade, ALLIED HIGH TECH PRODUCTS, INC). Forty (10 × 10 × 1 mm) ceramic slices were prepared from high-translucency monolithic lithium disilicate blocks (A1 shade) and high-translucency monolithic zirconia disc (A1 shade); twenty lithium disilicate slices (IPS e.max CAD HT, Ivoclar Vivadent) and twenty zirconia slices (Katana Zirconia STML, Kuraray Noritake Dental Inc). Ceramic blocks and disc were cut into slices using a diamond wheel of 5″ × 0.15″ × 0.5 (Diamond Metal Blade, ALLED HIGH TECH PRODUCTS, INC). Each slice was polished using silicon carbide grinding paper (macro-cut 180/p180 grit followed by micro-cut 1200/p2500 grit), then zirconia was sintered at 1550 °C for 2 h and Lithium disilicate was crystallized by firing at 770 °C for 5 min followed by firing at 850 °C for 10 min according to the manufacturer’s recommendations. The dimensions of the resultant slice were verified with a digital caliper.
The ceramic slices were luted to the dentin surface of extracted teeth using either light-cure resin cement (NX3 Nexus third generation light-cure, clear shade, Kerr) or dual-cure resin cement (NX3 Nexus third generation dual-cure, clear shade, Kerr). The materials used in this study and luting technique are described in Tables 1 and 2 respectively. A total of forty specimens were prepared and randomly assigned into four groups (n = 10 per group) as follows: group 1 (LiDi/LC): lithium disilicate luted by light-cure resin cement; group 2 (LiDi/DC): lithium disilicate luted by dual-cure resin cement; group 3 (Zr/LC): zirconia luted by light-cure resin cement; and group 4 (Zr/DC): zirconia luted by dual-cure resin cement. Specimens were stored in normal saline in a covered container at room temperature for 24 h before color measurement.
Thermocycling
All specimens were subjected to 3000 thermal cycles between 5 and 50 °C using a thermocycler machine (JULABO GmbH) with immersion time of 15 s in each bath.
Color analysis
Color measurements of the specimens were performed before and after thermocycling by means of spectrophotometry (X-Rite). The CIE L*a*b* values of the specimens were measured according to the color change formula: ΔE = [(ΔL)2 + (Δa)2 + (Δb)2]1/2, where ΔE = color change; Δ = lightness difference (L*) such that the greater the L*, the higher the brightness of the sample; Δa = the a* axis difference such that the higher the a*, the redder the sample and the lower the a*, the greener the sample; and Δb = the b* axis difference such that the higher the b*, the yellower the sample and the lower the b*, the bluer the sample. ΔL = L*F − L*I; Δa = a*F − a*I; Δb = b*F − b*I, where L*I, a*I, and b*I represent the initial color measurement and L*F, a*F, and b*F represent the final color measurement.
Statistical analysis
The Shapiro–Wilk test was used to test data for normal distribution. Parametric statistical tests were then performed. These were the paired t-test on individual L*, a*, and b* variables before and after thermocycling, two-way ANOVA on individual L*, a*, and b* variables for each test group, and one-way ANOVA to compare ΔE values for each study group. The confidence level was set as 95%. Data were expressed as mean ± standard deviation. Comparison was performed using SPSS version 20.0 for Windows. p < 0.05 was considered statistically significant.
Results
For each study group, the mean and standard deviations of the color coordinates before and after thermocycling, and of the change in color (ΔE), are presented in Table 3. The overall mean change in color (ΔE) found in this study was 3.59 ± 1.60. All groups showed a normal distribution of data with the Shapiro–Wilk test (p > 0.05) except for the color change in the Zr/LC group (p = 0.031).
Effect of thermocycling on color coordinates of luted ceramic material
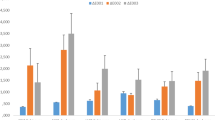
Thermocycling resulted in a significant change in the color coordinates of all tested specimens as determined by the paired t-test. Specimens became significantly brighter (p < 0.05), redder (p < 0.05), and more yellow (p < 0.05) after 3000 thermal cycles (Table 3; Fig. 1).
Effect of ceramic/cement type on color coordinates of luted ceramic material
A two-way analysis of variance for the L* value showed a main effect for the ceramic type (p < 0.05) so that brightness was significantly higher for zirconia (66.21 ± 2.45) than for lithium disilicate (58.99 ± 2.14). The main effect of cement type was non-significant (p > 0.05). However, the interaction effect was significant (p < 0.05) indicating that ceramic type effect was greater with dual-cure cement than with light-cure cement for zirconia and greater with light-cure than dual-cure for lithium disilicate. Similarly, analysis for the a* value revealed a main effect for the ceramic type (p < 0.05) suggesting that redness was significantly higher for lithium disilicate (− 0.72 ± 0.06) than for zirconia (− 1.52 ± 0.06). However, the main effect of cement type and ceramic/cement interaction on the a* value were non-significant (p > 0.05). The two-way ANOVA test for the b* value showed a main effect for the cement type (p < 0.05). Therefore, yellowness was significantly higher for dual-cure resin cement (5.2 ± 0.3) than for light-cure resin cement (4.2 ± 0.3). The main effect of ceramic type or ceramic/cement interaction on the b* value was non-significant (p > 0.05).
Comparison of color stability of luted ceramic material
The one-way ANOVA test showed that there was no statistically significant difference in the mean color change (ΔE) values between all tested groups (p = 0.756) (Table 3; Fig. 2).
Discussion
The current study utilized spectrophotometer to measure color coordinates of specimens. Color in dentistry can be evaluated using visual and/or instrumental methods. Although the visual method is subjective, it was the base for development of all color measuring instruments and it should complement their use. Instruments for color coordinate measurement include; spectrophotometer, colorimeters, and digital imaging systems. Spectrophotometer is considered one of the most accurate instruments for color measurement in dentistry. It measures the amount of light reflected from an object. This reflectance value is converted to shade tab equivalent. Colorimeter on the other hand is less accurate than spectrophotometer. It formulates color by filtering light in red, green, and blue areas of the visible spectrum. Additionally, digital cameras create a color image by acquiring red, green, and blue image information whereas software systems can compare shade in digital images with known reference shade [8].
The results of this study indicated that the color stabilities of the ceramic materials under the current investigation are comparable regardless of the type of luting cement used. Therefore, we failed to reject the null hypothesis. The current sample size was based on the reported mean and standard deviation of mean color change value among lithium disilicate, monolithic zirconia, and bilayer zirconia in Haralur et al. study [9] assuming alpha of 0.05 and a power of 80%.
Thermocycling resulted in a change in the color coordinates of all tested specimens. An increase in the L*, a*, and b* values indicates an increase in the brightness, redness, and yellowness of the specimen after cyclic aging, respectively. A change in the color of luted dental ceramics as a result of aging was reported in many previous studies [5,6,7, 10, 11]. In general, this can vary with aging conditions and can be attributed to an alteration in the color of the ceramic itself and/or the underlying cement. Ceramic material discoloration can be due to a loss of surface stain [12, 13], increased surface roughness, occurrence of surface cracks, change in ceramic translucency [13,14,15,16], and reduced ceramic thickness [5, 6]. On the other hand, resin cement discoloration can be manifested as a result of the degradation of unreacted polymers in the polymerization process. Therefore, the polymerization mode, time, cement shade, and composition all affect the cement color stability [7, 11]. Chaiyabutr et al. [17] reported that if a ceramic is less than 2 mm-thick, its optical color will be influenced by the underlying substrate color. In the current study, perhaps the high-translucency nature of the employed ceramics and the reduced thickness of 1 mm allowed for visualization of the color change of the underlying cement.
The overall mean color change value found in this study was 3.59 ± 1.6. The color difference is proposed to be perceptible when it can be detected by the human eye, and acceptable when it is tolerable [18]. Considering a threshold of perceptibility of ΔE = 1 and a threshold of acceptability of ΔE = 3.7 as concluded in a review by Khashayar et al. [19], the color change value in the current study is regarded perceptible but clinically acceptable for all specimens except for the lithium disilicate luted by the light-cure resin cement group (ΔE = 3.99 ± 1.83).
In our study, the color change values for specimens of IPS e.max CAD and Katana Zirconia STML were not significantly different (p = 0.756). Consistent with our findings, Subaci et al. [20] reported no significant difference in the color change values among three CAD-CAM monolithic ceramic materials. These were Vita Suprinity PC, IPS e.max CAD, and InCoris TZI C, all of the same thickness after 5000 thermocycles in a coffee solution. In contrast, several other studies demonstrated a significantly higher color change for zirconia than for lithium disilicate after artificial accelerated aging [9, 21]. In one investigation, in which lithium disilicate and zirconia underwent 3000 thermocycles between 5 °C and 55 °C in three discoloring solutions (coffee, green tea, and chlorohexidine gluconate), the ΔE values for zirconia were 5.60, 5.19, and 4.86 as compared to 1.78, 2.241, and 1.58 for lithium disilicate IPS Empress, respectively [9]. In another investigation, Kim et al. [21] reported that the color change for Katana monolithic zirconia was significantly higher than that of IPS e.max CAD following artificial aging in an autoclave at 134 °C under 0.2 MPa for 0, 1, 3, 5, or 10 h. Inconsistencies among findings in the literature on the color stability of ceramic materials can be attributed to variations in the specimen preparation and aging methods as well as to the diversity in material composition and optical properties. In high-translucency zirconia, such as the type used in this study and in Subaci’s study [20], the presence of at least 5.5 mol% of yttria increases the cubic phase content, which is responsible for the improved translucency and the lack of hydrothermal degradation under in vitro aging conditions when no load is applied [22]. This absence of low-grade thermal degradation, and its consequences of surface roughness and cracks, is speculated to improve the color stability of the highly translucent zirconia as compared to the low-translucency type.
In the present investigation, the brightness was significantly higher for zirconia samples than for lithium disilicate samples, due to ceramic type effect. The increased brightness of zirconia may be related to its higher refractive index, inducing more scattering of the light passing through, and to its lower translucency than lithium disilicate [23,24,25].
Additionally, the present study demonstrated that samples luted with dual-cure resin cement were significantly more yellow than samples luted using light-cure resin cement. The main contributor to this difference was the cement type. Consistent with our finding, several previous studies reported that dual-cure resin cement is more yellow than light-cure resin cement upon polymerization [11]. This was probably attributed to the degree of oxidation of the unreacted amine accelerators, causing their discoloration. The resultant discoloration was found to be greater in dual-cure than in light-cure resin cements [11, 26]. However, in the current study, both NX3 Nexus light-cure and dual-cure resin cements utilize an amine-free initiator system for better color stability. Nevertheless, the significant effect of the cement type on the b* value may indicate that these two cements degrade differently.
The results of the present study could have been enhanced by performing measurements of the substrate color coordinate and specimens transparency parameter, in addition to increasing the current sample size and number of thermal cycles. Further studies should be conducted on the optical and mechanical properties of the high-translucency ceramic materials to delineate their recommended clinical situations.
Conclusions
Within the limitations of this in vitro study, the following conclusions could be drawn:
-
Thermocycling resulted in a significant change in the color coordinates of all tested specimens.
-
The overall mean color change value found in this study was considered within the clinically acceptable level.
-
At 1 mm restoration thickness, the color stabilities of high translucency monolithic lithium disilicate and high-translucency zirconia ceramics were not significantly different, regardless of the cement type used.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Zarone F, Ferrari M, Mangano FG, et al. “Digitally oriented materials”: focus on lithium disilicate ceramics. Int J Dent. 2016;2016:9840594.
Church TD, Jessup JP, Guillory VL, et al. Translucency and strength of high-translucency monolithic zirconium oxide materials. Gen Dent. 2017;65:48–52.
Yan J, Kaizer MR, Zhang Y. Load-bearing capacity of lithium disilicate and ultra-translucent zirconia. J Mech Behav Biomed Mater. 2018;88:170–5.
Putra A, Chung KH, Flinn BD, et al. Effect of hydrothermal treatment on light transmission of translucent zirconias. J Prosthet Dent. 2017;118:422–9.
Karaokutan I, Yilmaz-Savas T, Aykent F, et al. Color stability of CAD/CAM fabricated inlays after accelerated artificial aging. J Prosthodont. 2016;25:472–7.
Silami FD, Tonani R, Alandia-Román CC, et al. Influence of different types of resin luting agents on color stability of ceramic laminate veneers subjected to accelerated artificial aging. Braz Dent J. 2016;27:95–100.
Mina NR, Baba NZ, Al-Harbi FA, et al. The influence of simulated aging on the color stability of composite resin cements. J Prosthet Dent. 2019;121:306–10.
Chu SJ, Trushkowsky RD, Paravina RD. Dental color matching instruments and systems. Review of clinical and research aspects. J Dent. 2010;38(Suppl 2):e2–16.
Haralur SB, Raqe S, Alqahtani N, et al. Effect of hydrothermal aging and beverages on color stability of lithium disilicate and zirconia based ceramics. Medicina. 2019;55:749.
Aljanobi G, Al-Sowygh ZH. The effect of thermocycling on the translucency and color stability of modified glass ceramic and multilayer zirconia materials. Cureus. 2020;12:e6968.
Rodrigues RB, Lima E, Roscoe MG, et al. Influence of resin cements on color stability of different ceramic systems. Braz Dent J. 2017;28:191–5.
Kurt M, Turhan BB. Effects of accelerated artificial aging on the translucency and color stability of monolithic ceramics with different surface treatments. J Prosthet Dent. 2019;12(712):e1-712.e8.
Volpato C, Cesar P, Bottıno M. Influence of accelerated aging on the color stability of dental zirconia. J Esthet Restor Dent. 2016;28:304–12.
Nakamura K, Harada A, Ono M, et al. Effect of low-temperature degradation on the mechanical and microstructural properties of tooth-colored 3Y-TZP ceramics. J Mech Behav Biomed Mater. 2016;53:301–11.
Shah K, Holloway JA, Denry IL. Effect of coloring with various metal oxides on the microstructure, color and flexural strength of 3Y-TZP. J Biomed Mater Res B Appl Biomater. 2008;87:329–37.
Cotes C, Arata A, Melo RM, et al. Effects of aging procedures on the topographic surface, structural stability, and mechanical strength of a ZrO2 -based dental ceramic. Dent Mater. 2014;30:e396–404.
Chaiyabutr Y, Kois JC, Lebeau D, et al. Effect of abutment tooth color, cement color, and ceramic thickness on the resulting optical color of a CAD/CAM glass-ceramic lithium disilicate-reinforced crown. J Prosthet Dent. 2011;105:83–90.
Vichi A, Louca C, Corciolani G, et al. Color related to ceramic and zirconia restorations: a review. Dent Mater. 2011;27:97–108.
Khashayar G, Bain PA, Salari S, et al. Perceptibility and acceptability thresholds for color differences in dentistry. J Dent. 2014;42:637–44.
Subaşı MG, Alp G, Johnston WM, et al. Effect of thickness on optical properties of monolithic CAD-CAM ceramics. J Dent. 2018;71:38–42.
Kim HK, Kim SH. Effect of hydrothermal aging on the optical properties of precolored dental monolithic zirconia ceramics. J Prosthet Dent. 2019;121:676–82.
Camposilvan E, Leone R, Gremillard L, et al. Aging resistance, mechanical properties and translucency of different Yttria-stabilized zirconia ceramics for monolithic dental crown applications. Dent Mater. 2018;34:879–90.
Harada K, Raigrodski AJ, Chung KH, et al. A comparative evaluation of the translucency of zirconias and lithium disilicate for monolithic restorations. J Prosthet Dent. 2016;116:257–63.
Kwon SJ, Lawson NC, McLaren EE, et al. Comparison of the mechanical properties of translucent zirconia and lithium disilicate. J Prosthet Dent. 2018;120:132–7.
Pecho OE, Ghinea R, Ionescu AM, et al. Color and translucency of zirconia ceramics, human dentine and bovine dentine. J Dent. 2012;40(Suppl 2):e34-40.
Kilinc E, Antonson SA, Hardigan PC, et al. Resin cement color stability and its influence on the final shade of all-ceramics. J Dent. 2011;39(Suppl 1):30–6.
Acknowledgements
This study was conducted at King Abdulaziz University Faculty of Dentistry (KAUFD), Jeddah, Saudi Arabia. Special thanks are owed to the Dental Research Laboratory for their technical support, to the biostatistician Huda Bashmail for her valid contribution to the statistical analysis and to Editage (www.editage.com) for English language editing.
Funding
This work was self-funded.
Author information
Authors and Affiliations
Contributions
LMA analyzed, interpreted data and was the major contributor in writing the manuscript; AM and TA Performed sample preparation, thermal cycling and color analysis; LA contributed in manuscript writing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This in-vitro study was conducted in 2019 at King Abdulaziz University Faculty of Dentistry (KAUFD), Jeddah, Saudi Arabia in line with the principles of the Declaration of Helsinki. The study protocol was approved by the Human Research Ethics Committee at the Faculty of Dentistry (Ethical approval # 116-10-18).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ashy, L.M., Al-Mutairi, A., Al-Otaibi, T. et al. The effect of thermocyclic aging on color stability of high translucency monolithic lithium disilicate and zirconia ceramics luted with different resin cements: an in vitro study. BMC Oral Health 21, 587 (2021). https://doi.org/10.1186/s12903-021-01963-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12903-021-01963-9