Abstract
Background
Limited studies have investigated the relationship between Anti-Müllerian hormone (AMH) and metabolic syndrome (MetS), yielding inconclusive results. This study aimed to examine the relationship between AMH levels and MetS and its components in women from a general population.
Methods
This prospective study recruited 769 women. Generalized Estimating Equation (GEE) models analyzed longitudinal trends of MetS components. Cox proportional hazard models evaluated effect of age-specific AMH tertiles on MetS occurrence, adjusting for confounders.
Results
The GEE analysis indicated that women in the third tertile exhibited higher mean FPG compared to those in the first tertile of age-specific AMH (3 mg/dL; 95% CI: 0.40, 5.60; P = 0.024); however, this association became non-significant after adjustment. Notably, the second tertile showed a significant decrease in FPG mean changes over time (-0.69 mg/dL; 95% CI: -1.31, -0.07; P Interaction = 0.030). Women in the second and third tertiles of age-specific AMH demonstrated lower mean HDL-C compared to the first tertile (-2.96 mg/dL; 95% CI: -4.67, -1.26; P < 0.001 and -2.63 mg/dL; 95% CI: -4.31, -0.96; P = 0.002, respectively). The association between HDL-C changes and the second tertile remained significant after adjustment (-1.91 mg/dL; 95% CI: -3.68, -0.14; P = 0.034). No significant associations were observed between age-specific AMH tertiles and TG and SBP/DBP. Cox models revealed no significant differences in the hazard ratio of MetS between AMH tertiles after adjusting for confounders.
Conclusion
Despite minor variations in MetS components, AMH levels did not affect MetS risk in women from a general population.
Similar content being viewed by others
Introduction
Metabolic syndrome (MetS) is a cluster of metabolic abnormalities including central obesity, hypertension (HTN), hyperglycemia, and dyslipidemia, which frequently used as a proxy for predicting the risk of these potentially life-threating diseases, such as cardiovascular disease (CVD) and type 2 diabetes (T2D) [1]. The prevalence of MetS is increasing worldwide in both men and women [2]. However, several studies have demonstrated that postmenopausal women, particularly those with premature ovarian insufficiency (POI), are at an increased risk develo** MetS than premenopausal women [1, 3, 4]. The higher prevalence of MetS in postmenopausal women with undetectable or diminished ovarian reserve raises an intriguing hypothesis that there may be shared underlying factors contributing to MetS and ovarian reserve [5].
Anti-Müllerian hormone (AMH) is a type of dimeric glycoprotein which is typically produced by the granulosa cells of preantral and small antral follicles throughout the reproductive lifespan and serves as the most reliable indicator of ovarian reserve [6]. There is growing evidence that beyond reproductive implications, AMH may be involved in the pathogenesis of metabolic disorders [7]. As revealed by our earlier study, incorporating serum AMH concentrations into the Framingham Risk Score (FRS) and Pooled Cohort Equations (PCE) risk prediction tools improves the accuracy of predicting CVD risk. This highlights the significant potential of using this biomarker as a valuable tool to predict cardiometabolic risk in women [8].
There are limited studies directly investigating the relationship between AMH levels and MetS and its components in the general population; most research has focused on women with polycystic ovary syndrome (PCOS) [5, 7, 9,10,11,12,13,14,15,16,17,18,19]. It is well-documented that PCOS presents with higher serum concentrations of AMH and an increased risk of obesity and other metabolic disorders [9, 10, 17]. The inclusion of PCOS-related evidence in a general population study may lead to erroneous conclusions or inappropriate comparisons.
To address this gap in knowledge, we conducted a community-based prospective study with long-term follow-up to investigate the association between age-specific AMH tertiles and develo** MetS in a general population of women. We also aimed to compare the trend of components of MetS overtime in women with different levels of age-specific AMH. Finding from this study will provide novel insights into the role of AMH in the pathogenesis of metabolic disorders and further highlight the importance of early prevention and management of metabolic disturbances in women with PCOS.
Materials and methods
Study design and participants
This study was conducted using data collected from participants of the Tehran Lipid and Glucose Study (TLGS), a long-term prospective cohort established in 1998. The TLGS assesses various risk factors for non-communicable diseases, demographic variables, and reproductive histories through face-to-face interviews every three years in six follow-up visits. For this specific study, women aged 20 years or older who participated in the baseline and at least one follow-up visit and had regular and predictable menstrual cycles at the initiation of the study were included. Among the 1,015 women who participated in TLGS and had age-specific AMH records at baseline, 246 were excluded due to pre-existing MetS at baseline (n = 241), or having less than two follow-ups with information on MetS (n = 5). Therefore, the study analyzed 769 eligible women. Out of these, 429 women developed MetS during the study, while 340 women did not experience MetS by the end of the observation period.
Measurements
All study participants were interviewed to obtain medical, obstetrics, and family histories using pretested questionnaires. Clinical and anthropometric measurements were assessed by trained examiners at each follow-up.
The study conducted various biochemical measurements at baseline and during follow-up visits. Serum AMH levels were only tested at baseline, while all other measurements were taken at each visit. All blood samples were collected in the morning after a 12-h overnight fast and analyzed on the day of collection at the TLGS research laboratory. All sera were stored at –80 °C until the time of testing. The AMH concentration was measured using the two-site enzyme immunoassay (EIA) method with the Gen II kit from Beckman Coulter. Fasting plasma glucose (FPG), triglycerides (TG), and high-density lipoprotein cholesterol (HDL-C) were also measured using enzymatic colorimetric methods with related kits from Pars Azmon Inc., Iran. HDL-C was measured after apolipoprotein B-containing lipoproteins were removed with phosphotungistic acid. Assay performance was monitored using lipid control serum, Precinorm, and Precipath, and lipid standard was used to calibrate the Selectra 2 auto-analyzer for all laboratory analyses. The coefficients of variation (CVs) for AMH were calculated separately for intra-assay and inter-assay measurements, resulting in values of 1.9% and 2.0%, respectively. Similarly, the CVs for glucose, total and HDL-C, and TG were also determined for both intra-assay and inter-assay measurements, with glucose having an intra- and inter-assay CV of 2.2%, while HDL-C had intra-assay CVs of 0.5% and 2%, respectively. Lastly, TG had intra-assay and inter-assay CVs of 0.6% and 1.6%, respectively.
Term definition
Smoking status was classified into two categories, including ever smokers (current users and those who used to smoke in the past) and never smokers. For evaluating physical activity, a modified activity questionnaire (MAQ) was used, which is evaluated and validated in the Iranian population. According to the questionnaire, physical activity has been specified as low (MET < 600 min/wk), moderate (MET 600—1499 min/wk), and high (MET ≥ 1500 min/wk) levels [20]. A positive family history of diabetes was considered as having previously diagnosed diabetes in relatives.
Exposure
The study has categorized female participants into three groups based on their age-specific AMH levels. These groups correspond to tertiles of the population distribution, which divide subjects into three equal-sized groups based on a specific variable (in this case, age-specific AMH levels).
Outcome
The criteria for diagnosing MetS involve the presence of at least three of five key risk factors, which have been established based on the Joint Interim Statement [1]. These risk factors include abdominal obesity, as determined by a waist circumference of at least 95 cm, with specific cutoffs for Iranians [21], FPG levels equal to or greater than 100 mg/dL (or drug treatment), TG levels equal to or greater than 150 mg/dL (or drug treatment), low levels of HDL-C (less than 50 mg/dL in women, or drug treatment), and elevated BP, defined as systolic blood pressure (SBP) equal to or greater than 130 mm Hg, diastolic blood pressure (DBP) equal to or greater than 85 mm Hg, or antihypertensive drug treatment.
Statistical analysis
We described and compared the baseline characteristics of the participants in three tertiles of AMH. To determine normality assumptions in continuous variables, we used the Kolmogorov–Smirnov test. We used mean [standard deviation (SD)] and ANOVA tests for variables with normal distribution, and median [interquartile range (IQR)] and Kruskal–Wallis tests for those without normality assumptions. For categorical variables, we reported frequencies (%) and used the Chi-squared test or Fisher exact test to compare these variables in the three groups.
The age-related decline in AMH levels is well-established in the literature [22, 23]; this age-dependent variation in AMH concentrations precludes the use of a single reference value for the entire study population. Furthermore, several studies have documented a non-linear pattern of AMH decrease with advancing age in women [24,25,26]. For the purpose of the present study the age-specific AMH percentiles have been calculated using the normal-based methodology introduced by Altman and Chitty [27], and Royston and Wright [28]. For this calculation, the Fractional polynomial (FP) regression models were fitted separately to estimate the mean and SD of the log AMH values as functions of age. An exponential – normal (EN) 3-parameter model provided the most fitted results since 9.8% of the observations lie above the 90th percentile and 9.1% below the 10th percentile [26]. For the purpose of the present study, we classified our participants to three group according to their age-specific AMH tertiles, and compared their baseline characteristics.
To evaluate the Hazard Ratio (HR) of occurrence of MetS in various age-specific AMH tertiles, we used the Cox proportional hazard model. The proportional hazard assumption of Cox model were tested for age specific AMH tertiles and other variables in final model. We also investigated the secular longitudinal trends of five MetS components [WC (cm), FPG (mg/dl), HDL-C (mg/dl), SBP (mmHg), and DBP (mmHg)] in our follow-ups and the effects of factors on these trends using Generalized Estimating Equation models (GEE). The GEE modeling accounts for correlations within subjects through a working correlation matrix and enables investigators to estimate the effect size accurately, even in the presence of incomplete data which is common in cohort studies due to missing variables in some repeated measures. We reported the results of both unadjusted and adjusted models for Cox and GEE models by considering several potential confounders, including age, educational level, physical activity, smoking status, and family history of diabetes.
All statistical analyses were performed in STATA (version 12; STATA Inc., College Station, TX, USA), and p-values less than 0.05 were considered statistically significant.
Results
A total of 769 eligible women were followed up for a median of 16 years (IQR: 9–19). Table 1 presents the baseline characteristics of the study participants with and without MetS. Women who developed MetS had a higher median (IQR) age compared to those without MetS [36 (32–41) vs. 32 (28–37 years); p < 0.001]. Furthermore, women with MetS had a higher BMI compared to those without this syndrome [median (IQR) 27.23 (24.43–29.42) vs. 24.22 (22.01–26.83) kg/m2; p < 0.001]. Additionally, women who experienced MetS had a higher percentage of family history of diabetes compared to those without this syndrome (34% vs. 22.6%; p = 0.01). During follow-ups, women with MetS exhibited significantly higher levels of WC [median (IQR) 85 (79–90) vs. 79 (73–85) cm; p < 0.001], FPG [median (IQR) 87(82–92) vs. 85 (80–90) mg/ml p < 0.001], TG [median (IQR) 115 (88–147) vs. 85.5 (68–105) mg/dl; p < 0.001], SBP [median (IQR) 110 (102–118) vs. 105 (100–111) mmHg; p < 0.001], and DBP [median (IQR) 75 (70–80) vs. 71 (66.75–70) mmHg; p < 0.001] than healthy women. Conversely, they exhibited lower levels of HDL-C [median (IQR) 42 (35–49) vs. 46 (39.75–53) mg/dl; p < 0.001].
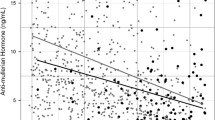
Table 2 presents the results of GEE models that estimated the effect of age-specific AMH tertiles on MetS components during follow-ups. The mean WC in women in the second and third tertiles of age-specific AMH did not significantly differ from those in the first tertile. Our study found that women in the third tertile of age-specific AMH had slightly higher mean FPG than those in the first tertile of age-specific AMH (3 mg/dl; 95% CI: 0.40, 5.60; P = 0.024); however, adjusting for confounders eliminate this association. For each follow-up visit, women who were in second and third age-specific AMH tertiles had lower mean change in FPG compared to those who were in the first teritle (-0.76 mg/dl; 95% CI: -1.36, -0.16; P Interaction = 0.013) and (-0.62 mg/dl; 95% CI: -1.21, -0.03; P Interaction = 0.040), respectively. However, this interaction effect remained statistically significant for the time and the second tertile of age-specific AMH (-0.69 mg/dl; 95% CI: -1.31, -0.07; P Interaction = 0.030) after adjusting for confounders. The mean TG over time did not significantly differ in women in the second and third tertiles of age-specific AMH compared to those in the first tertile, and this finding remained non-significant even after adjusting for confounders. Conversely, women in the second and third tertiles of age-specific AMH had lower means of serum levels of HDL-C than those in the first tertile (-2.96 mg/dL; 95% CI: -4.67, -1.26; P < 0.001 and -2.63 mg/dl; 95% CI: -4.31, -0.96; P = 0.002, respectively). However, after adjusting for confounders, this association remained significant only for the second tertile of age-specific AMH (-1.91 mg/dl; 95% CI: -3.68, -0.14; P = 0.034). The interaction between time and age-specific AMH tertile on the HDL-C was not significant. While both SBP and DBP exhibited upward trends, irrespective of age-specific AMH levels, there were no notable variances in the average SBP and DBP over time between women categorized in the second and third tertiles of age specific AMH compared to those in the first tertile. Furthermore, the interaction effect between time and age-specific AMH tertile on the mean alterations in BP was not statistically significant. Figures 1 A-F depict the temporal patterns of MetS components across the various tertiles of age-specific AMH.
A-F Trends in metabolic syndrome (MetS) components over time based on age-specific anti-mullerian hormone (AMH) tertiles. A Waist circumference (WC) trends over time based on age-specific AMH tertiles. B Fasting plasma glucose trends over time based on age-specific AMH tertiles. C Triglyceride trends over time based on age-specific AMH tertiles. D High-density lipoprotein cholesterol trends over time based on age-specific AMH tertiles. E Systolic blood pressure trends over time based on age-specific AMH tertiles. F Diastolic blood pressure trends over time based on age-specific AMH tertiles
In the unadjusted Cox models, HR for MetS did not display any significant differences among women in the second and third tertiles of age-specific AMH relative to those in the first tertile. Following adjustments made for potential confounding factors, the findings indicate that there was no discernible disparity in the HR for MetS between women grouped within these tertiles of age-specific AMH (Table 3). The proportionality hazard assumption was valid for AMH (P = 0.031); all the other adjusted variables in our Cox model have not violated from this assumption.
Discussion
Our study aimed to elucidate the relationship between MetS and AMH using data from a community-based prospective study with ~ 20 years follow-up. The results of our analysis revealed intriguing associations between individual components of MetS and age-specific AMH levels, highlighting the intricate interplay between hormonal imbalances and metabolic dysfunction. Notably, we did not find any significant association between the occurrence of MetS as an event and levels of age-specific AMH, even after adjusting for several potential confounders.
Numerous studies have examined the relationship between metabolic parameters and AMH in women, particularly those with PCOS. However, these studies have yielded conflicting findings. For instance, one cross-sectional study of reproductive-age women with PCOS found a positive association between serum AMH levels and HOMA IR levels [10]. Another cross-sectional analysis of 252 women aged 18–46 with PCOS revealed that AMH levels correlated positively with HDL-C cholesterol and negatively with fasting glucose, insulin resistance, BMI, SBP, and DBP. The study suggests that low AMH levels in young women with PCOS may predict a higher risk of MetS [7]. Conversely, a cross‑sectional study conducted in India on women diagnosed with PCOS aged 20–40 found no correlation between serum AMH levels and any component of MetS [13].
Limited research has explored the association between AMH and metabolic disturbances in the general population, yielding inconsistent findings [5, 15, 16, 29, 30]. For instance, a cross-sectional study conducted with 136 participants found that ovarian reserve function was significantly lower in individuals with MetS, particularly among women aged 20–29 [31]. Another cross-sectional analysis revealed lower AMH levels in women with T2D compared to those without T2D, specifically before age of 35 [15]. Similarly, a separate cross-sectional study involving non- PCOS women demonstrated an independent inverse relationship between insulin, fasting glucose, HOMA-IR, and AMH [16]. Additionally, a study examining women with diminished ovarian reserve and those with normal ovarian reserve discovered positive associations between low AMH levels and metabolic parameters such as HOMA-IR, CRP, TG, and LDL-C levels, while observing a negative correlation with HDL-C [5]. Conversely, a cross-sectional study comprising 291 women late reproductive age did not identify a significant correlation between MetS risk components and serum AMH levels [32]. However, prospective studies yielded inconsistent results. For example, our previous study indicated that women with lower ovarian reserve did not demonstrate distinct trends in adiposity and glucose metabolism parameters over their reproductive life span [29]. Furthermore, a prospective cohort study involving 3,293 women between the ages of 20 and 59 did not provide clear evidence of differences in AMH trajectories between women who developed T2D and those who did not [30]. The majority of available studies did not adjust their findings for essential confounders, which may be the reason for the controversy among studies. Additionally, a couple of studies have assessed the relationship between AMH and metabolic parameters in both PCOS and non-PCOS, separately. For instance, cross sectional data from eight US-based academic centers demonstrated intriguing associations among women with PCOS. It revealed that AMH displayed an inverse correlation with important indicators such as BMI, WC, fasting insulin, HOMA-IR, TG, and CRP. Additionally, it exhibited a direct relationship with higher levels of total cholesterol, LDL-C, and HDL-C. Similar pattern were observed in regularly-cycling women, where AMH varied inversely with WC, fasting insulin, and HOMA-IR. Notably, these associations between AMH and cardio-metabolic indices were predominantly influenced by BMI in both PCOS and non-PCOS individuals [12]. In another study involving 87 women diagnosed with PCOS and 53 healthy control subjects, no significant relationship were found between AMH levels and obesity, indices of IR, or variables related to MetS in both groups [33]. The variations in findings may arise from differences in the studied populations, research methodologies, measurement techniques, statistical analyses employed, and lack of adjustment for potential confounders.
A limited number of studies have assessed the association between WC and AMH with inconclusive results. Some studies have reported an inverse association between these measures of adiposity and AMH [12, 34], while others have observed no significant association [29, 35, 36]. In the context of our study, where we examined changes in WC over time across different age-specific AMH tertiles, we found that although there was a rising trend in WC among all women, regardless of their AMH tertile, there were no significant changes in WC when comparing different age-specific AMH tertiles. These findings suggest that AMH levels may not directly influence alterations in WC. The inconsistent findings among various studies may be attributed to differences in sample characteristics, measurement techniques, or confounding factors that were not accounted for. Further research is required to gain a better understanding of the relationship between WC, AMH, and potential underlying factors that contribute to the conflicting results seen in various studies.
Evidence also yielded inconsistent and inconclusive results regarding the association between AMH and glycemic parameters [5, 7, 15]. Our study detected a notable connection between age-specific AMH levels and fluctuations in FPG among women. Specifically, we observed that women in the highest tertile of age-specific AMH exhibited greater average in FPG compared to those in the lowest tertile of age-specific AMH. Although the study initially enrolled women with regular and predictable menstrual cycles, it did not exclude those with subclinical forms of PCOS characterized by elevated serum levels of AMH. This inclusion of participants at risk for metabolic disturbances due to PCOS remains a notable aspect of the study. However, after considering potential confounding factors through adjustment, this association lost statistical significance, suggesting that the initial relationship was likely influenced by these variables. Additionally, our analysis revealed an interaction effect (Time × age-specific AMH tertile), indicating that the impact of age-specific AMH tertiles on FPG diminishes over time. Therefore, despite the initial observation of a significant relationship between age-specific AMH levels and FPG fluctuations, our findings suggest that this association may be confounded by other factors and is subject to change over time. These results underscore the complexity of the relationship between AMH and glycemic parameters, highlighting the need for further research to elucidate the underlying mechanisms and identify potential confounders that might influence these associations.
In this study, we explored how age-specific AMH levels influenced the lipid profile in women. We found that the mean TG levels did not differ significantly across age-specific AMH tertiles, even though they increased over time. However, intriguing findings emerged regarding the effects of age-specific AMH on HDL-C levels. Women in the second and third age-specific AMH tertiles exhibited lower mean HDL-C compared to those in the first tertile. This association remained significant only for the second tertile of age-specific AMH and the passage of time after adjusting for confounding factors. The possible mechanism underlying this association may involve the effects of AMH on ovarian function and steroidogenesis. AMH is a marker of ovarian reserve and reflects the number of antral follicles in the ovaries [37]. AMH also inhibits the aromatization of androgens to estrogens, which may affect the lipid profile and cardiovascular risk in women [38]. Estrogens have been shown to increase HDL-C levels and protect against atherosclerosis [39]. Therefore, women with higher age-specific AMH levels may have lower estrogen levels and lower HDL-C levels compared to women with lower age-specific AMH levels. This may explain why women in the second tertile of age-specific AMH had lower mean HDL-C than those in the first tertile, even after adjusting for confounding factors. The disappearance of the significant association between the third tertile of age-specific AMH and HDL-C after controlling for potential confounders may imply that other factors, such as age, genetic variation, or environmental exposure, may also affect the relationship between AMH and HDL-C [40, 41]. Further studies are needed to elucidate the mechanisms and implications of this association.
High BP is another common feature of MetS that increases the risk of CVD and T2D. The association between BP and AMH remains inconclusive, with some studies reporting a reverse significant association [7, 34], while others find no association [42, 43] or a positive association [44]. Our findings suggest that regardless of AMH, there were no significant differences in the mean changes of SBP and DBP over time among women in the second and third tertiles of age-specific AMH. Moreover, the interaction between time and age-specific AMH tertile on the mean changes of BP was not significant.
Based on our results, although there were a few minor variations observed in the Mets component concerning age-specific AMH levels, our study has revealed no significant correlation between serum levels of this hormone and the occurrence of MetS. This finding remained unchanged even after accounting for potential confounding variables. This means that variations in age-specific AMH levels do not appear to play a substantial role in the development of MetS.
To the best of our knowledge, this study represents the first community-based prospective investigation into the risk of MetS in relation to serum concentrations of AMH among women of reproductive age in a general population. The study possesses several notable strengths. Firstly, it adopts a longitudinal community-based design, ensuring a comprehensive understanding of the subject matter. Additionally, the study has a lengthy follow-up duration with several interval follow up visits, enabling the observation of long-term trends. Moreover, the researchers accounted for significant confounding factors. Another strength of our study is that we used age-specific values for AMH rather than crude AMH to assess the association with MetS and its components. This approach can account for the age-related decline of AMH and provide more accurate estimates of ovarian reserve and lipid metabolism in women and employed age-specific values of AMH [26]. These approaches facilitated the identification of longitudinal associations between variables while effectively adjusting for confounding factors. Nonetheless, it is crucial to acknowledge certain limitations inherent in our study that should be taken into account when interpreting the results. We did not assess AMH levels on a specific day of the menstrual cycle. However, it is important to note that serum AMH concentrations remain consistent throughout the menstrual cycle, making it a valuable marker of fertility compared to other indicators. Another possible drawback was that we only took into account a single measurement of AMH for each case in our group. Taking multiple measurements of AMH could be considered as an alternative that enhances the precision of determining the ovarian reserve status for each individual [6]. Additionally, our results may have been influenced by unmeasured genetic and lifestyle factors due to lack of available data on these variables. Future studies should strive to incorporate genetic and lifestyle information to further elucidate the relationship between AMH and MetS.
Conclusions
Our study findings reveal that there were no significant differences in the hazard ratio of MetS among different age-specific AMH tertiles. Although we observed slight variations in the mean changes of specific MetS components, such as FPG and HDL-C, these differences did not exert a substantial impact on the overall HR of MetS. Consequently, further research is warranted to gain a more comprehensive understanding of the intricate association between AMH and metabolic health outcomes. By expanding our knowledge base in this area, future studies may help elucidate the clinical utility, if any, of incorporating AMH assessment into routine cardiometabolic risk screening protocols. Until then, healthcare professionals should continue to rely on well-established risk factors and evidence-based guidelines when evaluating and managing metabolic health in their patients.
Availability of data and materials
The datasets created during the present study can be obtained from the corresponding author upon making a reasonable request.
Abbreviations
- MetS:
-
Metabolic syndrome
- CVD:
-
Cardiovascular disease
- T2D:
-
Type 2 diabetes
- POI:
-
Premature ovarian insufficiency
- AMH:
-
Anti-Müllerian hormone
- FRS:
-
Framingham Risk Score
- PCE:
-
Pooled Cohort Equations
- PCOS:
-
Polycystic ovary syndrome
- TLGS:
-
Tehran Lipid and Glucose Study
- EIA:
-
Enzyme immunoassay
- FPG:
-
Fasting plasma glucose
- TG:
-
Triglycerides
- HDL-C:
-
High-density lipoprotein cholesterol
- CVs:
-
Coefficients of variation
- MAQ:
-
Modified activity questionnaire
- SD:
-
Standard deviation
- IQR:
-
Interquartile range
- HR:
-
Hazard Ratio
- WC:
-
Waist circumference
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- GEE:
-
Generalized Estimating Equation models
- HOMA-IR:
-
Homeostasis model assessment of insulin resistance
References
Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, et al. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation. 2009;120:1640–5.
Ford ES, Li C, Zhao G, Pearson WS, Capewell S. Trends in the prevalence of low risk factor burden for cardiovascular disease among United States adults. Circulation. 2009;120:1181–8.
Amiri M, Rahmati M, Farahmand M, Azizi F, Tehrani FR. Age at natural menopause in women with a history of chronic diseases–A population-based cohort study. Maturitas. 2022;158:16–24.
Stevenson J, Collins P, Hamoda H, Lambrinoudaki I, Maas A, et al. Cardiometabolic health in premature ovarian insufficiency. Climacteric. 2021;24:474–80.
Verit FF, Akyol H, Sakar MN. Low antimullerian hormone levels may be associated with cardiovascular risk markers in women with diminished ovarian reserve. Gynecol Endocrinol. 2016;32:302–5.
Ramezani Tehrani F, Bidhendi Yarandi R, Solaymani-Dodaran M, Tohidi M, Firouzi F, et al. Improving prediction of age at menopause using multiple anti-Müllerian hormone measurements: the Tehran Lipid-Glucose Study. J Clin Endocrinol Metab. 2020;105:1589–98.
Feldman RA, O’Neill K, Butts SF, Dokras A. Antimüllerian hormone levels and cardiometabolic risk in young women with polycystic ovary syndrome. Fertil Steril. 2017;107:276–81.
Amiri M, Ahmadi N, Hadaegh F, Mousavi M, Azizi F, et al. Does the addition of serum antimüllerian hormone concentrations to the Framingham Risk Score and Pooled Cohort Equations improve the prediction of cardiovascular disease? Menopause. 2023;30:406–13.
Bakeer E, Radwan R, El Mandoury A, El Rahman AA, Gad M, et al. Anti-Müllerian Hormone as a Diagnostic Marker in Egyptian Infertile Polycystic Ovary Syndrome Females: Correlations with Vitamin D, Total Testosterone, Dyslipidemia and Anthropometric Parameters. J Med Biochem. 2018;37:448–55.
Wiweko B, Indra I, Susanto C, Natadisastra M, Hestiantoro A. The correlation between serum AMH and HOMA-IR among PCOS phenotypes. BMC Res Notes. 2018;11:114.
Li X-J, Wang H, Lu D-Y, Yu T-T, Ullah K, et al. Anti-Müllerian Hormone Accelerates Pathological Process of Insulin Resistance in Polycystic Ovary Syndrome Patients. Horm Metab Res. 2021;53:504–11.
Rios JS, Greenwood EA, Pavone MEG, Cedars MI, Legro RS, et al. Associations between anti-mullerian hormone and cardiometabolic health in reproductive age women are explained by body mass index. J Clin Endocrinol Metab. 2020;105:e555–63.
Mitra S, Saharia GK, Jena SK. Correlation Between Serum AMH Levels and Cardiometabolic Indices in PCOS Women. Indian J Endocrinol Metab. 2021;25:545.
Bahadur A, Verma N, Mundhra R, Chawla L, Ajmani M, et al. Correlation of homeostatic model assessment-insulin resistance, anti-Mullerian hormone, and BMI in the characterization of polycystic ovary syndrome. Cureus. 2021;13:e16047.
Kim C, Karvonen-Gutierrez C, Kong S, Arends V, Steffes M, et al. Antimüllerian hormone among women with and without type 1 diabetes: the Epidemiology of Diabetes Interventions and Complications Study and the Michigan Bone Health and Metabolism Study. Fertil Steril. 2016;106:1446–52.
Park HT, Cho GJ, Ahn KH, Shin JH, Kim YT, et al. Association of insulin resistance with anti-Mullerian hormone levels in women without polycystic ovary syndrome (PCOS). Clin Endocrinol. 2010;72:26–31.
Piouka A, Farmakiotis D, Katsikis I, Macut D, Gerou S, et al. Anti-Mullerian hormone levels reflect severity of PCOS but are negatively influenced by obesity: relationship with increased luteinizing hormone levels. Am J Physiol Endocrinol Metab. 2009;296:E238–43.
Sahmay S, Mathyk BA, Sofiyeva N, Atakul N, Azemi A, et al. Serum AMH levels and insulin resistance in women with PCOS. Eur J Obstet Gynecol Reprod Biol. 2018; 224:159–64.
Fallahzadeh A, Ramezeni Tehrani F, Rezaee M, Mahboobifard F, Amiri M. Anti-Mullerian hormone and cardiometabolic status: a systematic review. Biomarkers. 2023;28:486–501.
Manley AF (1996) Physical activity and health: A report of the Surgeon General.
Azizi F, Khalili D, Aghajani H, Esteghamati A, Hosseinpanah F, et al. Appropriate waist circumference cut-off points among Iranian adults: the first report of the Iranian National Committee of Obesity. Arch Iran Med. 2010;3:243–44.
Depmann M, Eijkemans MJC, Broer SL, Tehrani FR, Solaymani-Dodaran M, et al. Does AMH relate to timing of menopause? Results of an Individual Patient Data meta- analysis. J Clin Endocrinol Metab. 2018;103(10):3593–600.
Tehrani FR, Firouzi F, Behboudi-Gandevani S. Investigating the Clinical Utility of the Anti-Mullerian Hormone Testing for the Prediction of Age at Menopause and Assessment of Functional Ovarian Reserve: A Practical Approach and Recent Updates. Aging Dis. 2022;13:458–67.
Lee JY, Jee BC, Lee JR, Kim CH, Park T, et al. Age-related distributions of anti-Müllerian hormone level and anti-Müllerian hormone models. Acta Obstet Gynecol Scand. 2012;91:970–5.
Seifer DB, Baker VL, Leader B. Age-specific serum anti-Müllerian hormone values for 17,120 women presenting to fertility centers within the United States. Fertil Steril. 2011;95:747–50.
Tehrani FR, Mansournia MA, Solaymani-Dodaran M, Azizi F. Age-specific serum anti-Müllerian hormone levels: estimates from a large population-based sample. Climacteric. 2014;17:591–7.
Altman D, Chitty L. Design and analysis of studies to derive charts of fetal size. Ultrasound Obstet Gynecol. 1993;3:378–84.
Royston P, Wright E. How to construct ‘normal ranges’ for fetal variables. Ultrasound in Obstetrics and Gynecology: The Official Journal of the International Society of Ultrasound Obstet Gynecol. 1998;11:30–8.
Amiri M, Ramezani Tehrani F, Rahmati M, Firouzi F, Azizi F. Do trends of adiposity and metabolic parameters vary in women with different ovarian reserve status? A population-based cohort study. Menopause. 2020;27:684–92.
Verdiesen RM, Onland-Moret NC, van Gils CH, Stellato RK, Spijkerman AM, et al. Anti-Müllerian hormone levels and risk of type 2 diabetes in women. Diabetologia. 2021;64:375–84.
Balkan F, Cetin N, Usluogullari CA, Unal OK, Usluogullari B. Evaluation of the ovarian reserve function in patients with metabolic syndrome in relation to healthy controls and different age groups. J Ovarian Res. 2014;7:1–6.
Kim S, Kim JJ, Kim M-J, Han KH, Lee JR, et al. Relationship between serum anti-Mullerian hormone with vitamin D and metabolic syndrome risk factors in late reproductive-age women. Gynecol Endocrinol. 2018;34:327–31.
Woo H-Y, Kim K-H, Rhee E-J, Park H, Lee M-K. Differences of the association of anti-Müllerian hormone with clinical or biochemical characteristics between women with and without polycystic ovary syndrome. Endocr J. 2012;59:781–90.
Bleil M, Adler N, Gregorich S, Sternfeld B, Rosen M, et al. Does accelerated reproductive aging underlie premenopausal risk for cardiovascular disease? Fertil Steril. 2012;98:S43–4.
Dolleman M, Verschuren W, Eijkemans M, Dollé M, Jansen E, et al. Reproductive and lifestyle determinants of anti-Müllerian hormone in a large population-based study. J Clin Endocrinol Metab. 2013;98:2106–15.
Cengiz H, Ekin M, Dagdeviren H, Yildiz Ş, Kaya C, et al. Comparison of serum anti-Müllerian hormone levels in normal weight and overweight–obese adolescent patients with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2014;180:46–50.
Tal R, Seifer DB, Tal R, Granger E, Wantman E, et al. AMH highly correlates with cumulative live birth rate in women with diminished ovarian reserve independent of age. J Clin Endocrinol Metab. 2021;106:2754–66.
Yin W-W, Huang C-C, Chen Y-R, Yu D-Q, ** M, et al. The effect of medication on serum anti-müllerian hormone (AMH) levels in women of reproductive age: A meta-analysis. BMC Endocr Disord. 2022;22:1–14.
Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med. 1999;340:1801–11.
Bandarian F, Daneshpour MS, Hedayati M, Naseri M, Azizi F. Identification of sequence variation in the apolipoprotein A2 gene and their relationship with serum high-density lipoprotein cholesterol levels. Iran Biomed J. 2016;20:84.
Parker J. Pathophysiological effects of contemporary lifestyle on evolutionary-conserved survival mechanisms in polycystic ovary syndrome. Life. 2023;13:1056.
Yarde F, Maas A, Franx A, Eijkemans M, Drost J, et al. Serum AMH levels in women with a history of preeclampsia suggest a role for vascular factors in ovarian aging. J Clin Endocrinol Metab. 2014;99:579–86.
De Kat AC, Verschuren WM, Eijkemans MJ, Broekmans FJ, Van Der Schouw YT. Anti-Müllerian hormone trajectories are associated with cardiovascular disease in women: results from the Doetinchem cohort study. Circulation. 2017;135:556–65.
De Kat A, Verschuren W, Eijkemans M, Van Der Schouw Y, Broekmans F. The association of low ovarian reserve with cardiovascular disease risk: a cross-sectional population-based study. Hum Reprod. 2016;31:1866–74.
Acknowledgements
The authors extend their sincere appreciation to the Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, for graciously approving this project.
Funding
None.
Author information
Authors and Affiliations
Contributions
M.A. contributed to the study's conception and design, execution, literature search, data collection, interpretation of data, critical discussion, and manuscript drafting. M.M. conducted the statistical analyses, interpreted the data, and reviewed the manuscript. M.N. and M.F. provided critical discussion and reviewed the manuscript. F.A. contributed to the study's design, execution, and critical discussion. F.R.T. contributed to the study's conception and design, interpreted the data, and drafted the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study received approval from the ethics committee of Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran (Approval ID: IR.SBMU.ENDOCRINE.REC.1401.058). Written informed consent was obtained from all study participants.
Consent for publication
Not applicable.
Competing interests
The authors have no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Amiri, M., Mousavi, M., Noroozzadeh, M. et al. Association between anti-mullerian hormone and metabolic syndrome: insights from a prospective community-based study. BMC Endocr Disord 24, 97 (2024). https://doi.org/10.1186/s12902-024-01627-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12902-024-01627-z