Abstract
Background
Diabetic foot ulcers (DFUs) have become a global health concern, which can lead to diabetic foot infection (DFI), lower leg amputation, and even mortality. Though the standard of care (SOC) practices have been recognized as the “gold standard” for DFU care, SOC alone may not be adequate to heal all DFUs and prevent their recurrence. The use of dermal matrix has emerged as an adjuvant treatment to enhance DFU healing. The current study aimed to evaluate the effectiveness and safety of dermal matrix application as an adjuvant treatment to the SOC.
Methods
The databases of PubMed, Embase and CENTRAL were independently searched by two authors, with the following key terms: “diabetic foot ulcer”, “acellular dermal matrix”, “wound healing”, and so on. Randomized controlled trials (RCTs) evaluated the efficacy and safety of dermal matrix in the treatment of DFUs were eligible for inclusion. The primary outcomes analyzed included time to complete healing and complete healing rate at the final follow-up, while secondary outcomes included wound area, ulcer recurrence rate, amputation risk and complication risk. Meta-analyses were performed using random-effect or fixed-effect models, based on the heterogeneity test.
Results
This study included a total of 15 RCTs with a total of 1524 subjects. Of these, 689 patients were treated with SOC alone, while 835 patients received SOC plus dermal matrix. Compared to the SOC group, significantly shorter time (MD = 2.84, 95%CI: 1.37 ~ 4.32, p < 0.001***) was required to achieve complete healing in dermal matrix group. Significantly higher complete healing rate (OR = 0.40, 95%CI: 0.33 ~ 0.49, p < 0.001***) and lower overall (RR = 1.83, 95%CI: 1.15 ~ 2.93, p = 0.011*) and major (RR = 2.64, 95%CI: 1.30 ~ 5.36, p = 0.007**) amputation risks were achieved in dermal matrix group compared to SOC group. No significant difference was found in the wound area, ulcer recurrence rate, and complication risk between the two groups.
Conclusions
The application of dermal matrix as an adjuvant therapy in conjunction with SOC effectively improved the healing process of DFUs and reduced the amputation risk when compared to SOC alone. Furthermore, dermal matrix application was well tolerated by the subjects with no added complication risk.
Similar content being viewed by others
Introduction
Diabetic foot ulcers (DFUs) have become a global health concern, with an estimated incidence of 19 to 35% in patients with diabetes mellitus [1]. It has been reported by the International Diabetes Federation that there will be 9.1–26.1 million patients develop DFUs annually [1]. Patients with DFUs are related with decreased quality of life (QoF) and increased risk of depression [2, 3]. Furthermore, diabetic foot infection (DFI) is more frequent in the DFU patients due to the incomplete skin and exposed bone, which may result in increased amputation risk to as high as 92% [4, 5]. It was reported that approximately 1 in 6 DFU patients will suffer from amputation, causing a mortality rate of about 47% within 5 years and a recurrence risk as high as 66% [6, 7].
DFUs treatment is associated with about 1/3 of the total diabetic care cost [8]. The primary goal of DFU treatment is to promote the re-epithelialisation of wound to reduce the complications risk associated with ulceration and to improve the patient’s QoF to a ‘pre-ulceration’ status. Besides glycemic control and revascularization, standard of care (SOC) treatment has been commonly selected as the conventional application for DFU wound management, which usually consists of the surgical sharp debridement, wound moist dressing, application of removable or irremovable off-loading device, and infection control [7, 9, 10]. The review of Everett et al. [11] summarized a total of 7 critical SOC practices, including surgical debridement, dressings promoting a moist wound environment, wound off-loading, vascular assessment, treatment of active infection, glycemic control, and ultidisciplinary care. Although these SOC practices are considered the “gold standard” for DFU care, the 20-week healing rate of DFU after SOC was less than 30% [12], and 40 and 65% of healed DFUs will recur within 1 year and 5 years, respectively [1]. Therefore, current SOC alone may not be sufficient to heal all DFUs and prevent their recurrence [13].
In recent years, a broad spectrum of novel treatments have been developed to improve diabetic wound healing. In areview by Snyder et al. [14], they identified a total if 76 commercially available skin substitutes used to treat chronic wounds. The majority of these substitutes do not contain cells and are derived from human placental membrane (the placenta’s inner layer), animal tissue, or donated human dermis allograft. These skin substitutes, whether allogeneic or xenogeneic graft, could provide the essential structure of extracellular matrix, signals for cellular migration, proliferation, angiogenesis, and endogenous matrix production and biochemical functions for enhancing wound healing [15, 16]. Many studies have demonstrated that these dermal matrices are effective when applied as adjuvant treatment to enhance DFUs healing [17,18,19]. However, high-level evidence to comprehensively illustrate the effectiveness and safety of SOC plus dermal matrix over SOC alone is still scarce.
Thus, the current systematic review will be conducted with the aim of evaluating the effectiveness and safety of dermal matrix application as an adjuvant treatment of SOC, basing on the available evidence from randomized controlled trials (RCTs).
Materials and methods
This study was carried out in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guideline [20], and the checklist is presented in Supplementary Appendix 1.
Data sources
The following three databases were independently searched by two authors: PubMed, Embase and CENTRAL. The searching was completed using a method of combination of subject and free terms, with the following key terms: “diabetic foot ulcer”, “acellular dermal matrix”, “cellular dermal matrix”, “wound healing”, and so on. No restriction on the publication countries/ regions and publication date, while the publication language was restricted on English. Additionally, the references lists of the included studies were reviewed, and the potential related studies were hand searched and screened for eligibility.
Inclusion and exclusion criteria
The retrieved records from the three databases were screened according to the following inclusion criteria: (1) patients: diagnosed with DFU; (2) intervention: biogenic skin substitutes for enhancing DFU healing, whether allogeneic or xenogeneic dermal matrix graft; (3) comparison: between SOC and dermal matrix; (4) outcomes: treatment outcomes of DFU, including ulcer healing rate, healing time, wound area, ulcer recurrence, amputation risk and complication risk; (5) studies: only prospectively designed RCTs were eligible.
Studies were excluded according to the following criteria: (1) patients with ulcers on foot caused by reasons other than diabetes, or patients with ulcers caused by diabetes on the lower leg; (2) patients treated with methods other than dermal matrix or SOC; (3) no available data on the effectiveness and safety outcomes; (4) studies designed as case series, cohort study, retrospective case-control study, systematic review/ meta analysis, literature review, and so on.
Study screening and data extracting
Two authors independently screened all electronic records retrieved from the databases, according to the inclusion and exclusion criteria to select eligible studies. At the beginning, the records were imported into the EndNote software version X9 to eliminate the duplicates. Then, the two authors reviewed the titles/ abstracts of the remaining non-duplicates, to remove clearly irrelevant studies. After then, the full-text of the remained studies was downloaded and reviewed to evaluate the eligibility for inclusion.
The data extraction process was also completed by two authors independently, to obtain the following items: (1) study characteristics: the first author’s name, publication year, corresponding country/ region, study period, and follow-up time; (2) subjects characteristics: patients number, dropped patients number, male percentage, mean age, mean BMI, diabetes type (type I or type II), length of diabetes history, glycosylated hemoglobin percentage (HbA1c%), ankle-brachial index (ABI), patients with diabetic polyneuropathy (DPN), DFU grade according to Wagner or other classifications, DFU site (planta, dorsal, or other sites), DFU size, and DFU age; (3) treatment details: treatment regimens in screening phase and treatment phase, screening criterion for randomization in screening phase, and dermal matrix product; (4) outcome evaluations: ulcer healing rate, healing time, wound area, ulcer recurrence, amputation risk and complication risk.
Quality assessment
The quality of the included RCTs was assessed using the Cochrane Collaboration tool for risk of bias assessment [21], which evaluates a total of 7 kinds of biases for RCTs as follows: (1) randomization sequence generation, (2) allocation concealment, (3) blinding of participants and personnel, (4) blinding of outcome assessment, (5) incomplete outcome data, (6) selective reporting and (7) other bias.
Statistical analysis
When results of per-protocol (PP) and intention-to-treat (ITT) analyses were both reported, the ITT principle was followed in the analyses process. Comparisons of continuous outcomes (including time to complete heal and wound area, at final follow-up) between SOC and dermal matrix groups, were expressed as mean difference (MD) and 95% confidence interval (95% CI). Single-rate meta analysis was performed to calculate the pooled healing rate and ulceration recurrence rate of dermal matrix group at the final follow-up. Comparisons of dichotomous outcomes between SOC and dermal matrix groups, were expressed as odds ratio (OR, complete healing rate) and risk ratio (RR, ulcer recurrence rate, amputation risk and complication risk) as well as their 95% CIs. Heterogeneity among studies was estimated by I2 statistics. If I2 ≥ 50%, it indicates significant heterogeneity and random-effect model was applied. Otherwise, a fixed-effect model was used in case of non-significant heterogeneity.
If significant heterogeneity was detected, sensitivity analysis was performed by omitting each individual study sequentially to assess the impact of each study on the results. For outcomes reported in more than 5 studies, funnel plot was plotted, and publication bias was assessed using Egger’s and Begg’s tests (p < 0.100 and p < 0.050 were considered to indicate significant publication bias respectively). If significant publication bias was detected, non-parametric trim-and-filling method was used to adjust the publication bias. Data analyses were performed using the R language version 4.2.1 (R Foundation for Statistical Computing, Vienna, Austria). All statistical tests were two-sided and P value of less than 0.05 was considered significant.
Results
Study selecting
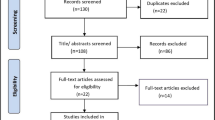
The flow chart of studies screening is shown in Fig. 1. From the initial search, 520 studies were identified, of which 132 were duplicates that wereimmediately excluded. A futher 334 records were excluded after screening the titles/ abstracts, leaving 54 articles for full-text review. As a result, a total of 15 [22,23,24,25,26,27,28,29,30,31,32,33,34,35,36] and 14 [22, 23, 25,26,27,28,29,30,31,32,33,34,35,36] RCTs were included in the qualitative and quantitative synthesis (meta-analysis), respectively.
Summary of the included studies
Table 1 summarizes the characteristics of the included studies. A total of 15 trials involving 1524 subjects were included in the analysis. The studies randomized a total of 689 patients to receive SOC alone and 835 patients to receive SOC plus dermal matrix. The male percentages were reported in 13 of the studies, ranging from 34.6 to 100.0%. The mean age was reported in 14 studies, ranging from 55.2 to 66.6 years. The mean BMI was available in 10 studies, ranging from 28.5 to 36.5 kg/m2. The follow-up periods were 4, 6, 12, 16, 21, 24, 28, and 42 weeks in 1 [22], 1 [36], 5 [25, 29,30,31, 34], 4 [23, 24, 27, 35], 1 [32], 1 [26], 1 [28] and 1 [33] studies, respectively. At the final follow-up, a total of 147 and 155 patients dropped out for follow-up.
Table 2 summarizes the basic information about the status of diabetes mellitus and DFUs at randomization. HbA1c% levels were reported in 12 studies, with a range of 7.11 to 10.2%. The mean ABI was reported in 4 studies, with a range of 0.7 to 1.2. A total of 12 studies reported the mean DFU size, with a range of 1.3 to 32.1 cm2. The treatment details of the included studies are listed in Table 3. Before the treatment phase, 8 studies [23,24,25, 27, 28, 31, 32, 34] included screening phase and applied SOC treatment (surgical debridement, wound dressings, wound off-loading, and infection management) to the enrolled patients lasting for 1 or 2 weeks. At the end of the screening phase, the including criterion for randomization was set as less than 20% [25, 27], 30% [23, 31, 32] or 40% [28] reduction of wound area in the ulcer site. Many different dermal matrix products were applied for wound repair, with the GraftJacket matrix (Wright Medical Technology, Inc., Arlington, TN, USA) being the most commonly researched product in 4 RCTs [22, 26, 29, 35].
Figure 2 shows the results of the quality assessment of the included RCTs. Due to the obviously different treatment process, the blinding of the patients was difficult. As a result, there is a high risk of bias in the item of “blinding of participants and personnel”. The item “blinding of outcome assessment” was also presented with high risk of bias in 4 of the studies. The other items were of relatively low risk of bias.
Effectiveness of the dermal matrix in DFU
The comparison of mean time to complete healing between SOC and dermal matrix is presented in Fig. 3. Six primary trials were pooled with random-effect model (I2 = 97%), and significantly shorter time was required to achieve complete healing in dermal matrix group compared to the SOC group (see the forest plot in Fig. 3A, MD = 2.84, 95%CI: 1.37 ~ 4.32, p < 0.001***). The funnel plot (Fig. 3B), Egger’s test (p = 0.143) and Begg’s test (p = 0.573) indicate that there is no significant publication bias. The forest plot of sensitivity analysis (Fig. 3C) showed there was no single study that significantly influenced the pooling result.
The pooling result for complete healing rate of dermal matrix group at final follow-up is shown in Fig. 4. Thirteen studies and 16 arms were pooled with random effects model (I2 = 86%), and the pooled healing rate of dermal matrix group was 0.70 (95CI: 0.61 ~ 0.78) (see the forest plot in Fig. 4A). The forest plot of sensitivity analysis (Fig. 4D) revealed that none of the studies had a significant impact on the pooled result.. However, the funnel plot (Fig. 4B), Egger’s test (p = 0.012) and Begg’s test (p = 0.529) indicated the presence of significant publication bias. Therefore, a trim and filling funnel plot was generated, which resulted in an adjusted healing rate of 0.56 (95CI: 0.47 ~ 0.66) for the dermal matrix group (Fig. 4C).
The comparison of the complete healing rate between SOC and dermal matrix is presented in Fig. 5. Thirteen studies and 16 arms were pooled with fixed-effect model (I2 = 33%), resulting in a significantly higher complete healing rate in dermal matrix group compared to the SOC group (see the forest plot in Fig. 5A, OR = 0.40, 95%CI: 0.33 ~ 0.49, p < 0.001***). The funnel plot (Fig. 5B), Egger’s test (p = 0.224) and Begg’s test (p = 0.242) did not show any significant publication bias. Sensitivity analysis was not conducted as there was no significant heterogeneity.
Figure 6 shows the comparison of wound area between SOC and dermal matrix, which pooled the data from three studies and four arms using random effects model (I2 = 98%). No significant difference between two groups was found (Fig. 6A, MD = 0.29, 95%CI: − 0.32 ~ 0.91, p = 0.352). The forest plot of sensitivity analysis (Fig. 6B) showed there was no arm caused significant influence on the pooling result.
Safety of the dermal matrix in DFU
The ulcer recurrence rate comparison between SOC and dermal matrix is presented in Fig. 7A-B. Five studies were pooled with fixed-effect model (I2 = 32%), and no significant difference in ulcer recurrence rate was observed between the two groups (Fig. 7A, RR = 1.32, 95%CI: 0.92 ~ 1.89, p = 0.138). The funnel plot (Fig. 7B), Egger’s test (p = 0.827) and Begg’s test (p = 1.000) did not show any significant publication bias. Sensitivity analysis was not conducted as there was no significant heterogeneity.
The pooling result for ulcer recurrence rate of dermal matrix group at final follow-up is shown in Fig. 7C-E. Five studies were pooled with random-effect model (I2 = 64%), resulting in a pooled ulcer recurrence rate of 0.11 (95CI: 0.05 ~ 0.17) for the dermal matrix group (see the forest plot in Fig. 7C). The forest plot of sensitivity analysis (Fig. 7E) showed that no study had a significant influence on the pooled result. The funnel plot (Fig. 7D), Egger’s test (p = 0.738) and Begg’s test (p = 1.000) did not indicate the presence of significant publication bias.
Figure 8A shows the forest plot comparing the overall amputation risk between SOC and dermal matrix groups using fixed-effect model (I2 = 0%), demonstrating that dermal matrix application could significantly lower the overall amputation risk (RR = 1.83, 95%CI: 1.15 ~ 2.93, p = 0.011*). After then, subgroup analyses were conducted for both major (Fig. 8B) and minor (Fig. 8C) amputation risks, showing that dermal matrix application could significantly lower the major amputation risk (RR = 2.64, 95%CI: 1.30 ~ 5.36, p = 0.007**), but had no significant impact on the minor amputation risk (RR = 1.02, 95%CI: 0.49 ~ 2.12, p = 0.959). Publication bias test and sensitivity analysis were not performed.
The comparison of complication rate between SOC and dermal matrix is presented in Fig. 9. Thirteen studies and 15 arms were pooled with fixed-effect model (I2 = 0%), and no significantly different complication rate was observed between two groups (Fig. 9A, RR = 1.06, 95%CI: 0.93 ~ 1.20, p = 0.409). The funnel plot (Fig. 9B), Egger’s test (p = 0.494) and Begg’s test (p = 0.622) did not indicate the presence of significant publication bias. Sensitivity analysis was not conducted as there was no significant heterogeneity.
Discussion
In this study, we performed a systematic review and meta-analysis based on high-level evidence from RCTs, and found that application of dermal matrix was associated with significantly shorter time to complete healing, increased healing rate, and reduced amputation risk, compared to SOC alone. However, there was no significant difference in wound area, ulcer recurrence and complication risk, between the two groups.
DFUs are always characterized by chronicity and recurrence, making them difficult to fully heal and potentially leading to minor or major limb amputations. The clinical challenges related with DFUs treatment have spawned multiple adjuvant techniques to improve the wound healing. Usually, an ulcer continued for more than 4 weeks is qualified as chronic wound, which can present additional challenges to complete the wound healing because of infection, biofilm formation, and underlying tissue desiccation that cause exacerbated conditions and disturbed healing process. Multiple biologic dressings have been applied in clinical researches in the setting of DFUs, showing promise treatment outcomes. Martin et al. [37] evaluated the outcomes of 17 consecutive patients with neuropathic diabetic foot wounds treated with an acellular matrix, which showed a 20-week healing rate of 82.4% with an average healing time of 8.9 ± 2.7 weeks. Lee et al. [38] compared the efficacy of applying a paste formulation of acellular dermal matrix (ADM) with conventional foam dressing in treating DFUs, reporting an increased healing rate (56.52% vs. 23.08%), increased ratio of healed area (74.17% ± 30.84% vs. 51.87% ± 32.81%) and deceased length of time to heal (13.54 ± 9.18 vs. 21.5 ± 11.98 days) in ADM group, at the 60-day primary outcome mark. Zelen et al. [25] compared the clinical outcomes of human reticular acellular dermis matrix (HR-ADM) versus SOC to facilitate wound closure in non-healing DFUs. At the final follow-up (12 weeks), DFUs of HR-ADM and SOC groups healed in 80 and 20% of the patients, with a mean healing time of 40 days and 77 days, respectively. There was no significantly increased adverse or serious adverse events between the two groups or any adverse events related to the graft. In another RCT by Hahn et al. [33], the clinical outcomes of a micronized dermal matrix (MDM) was compared with conventional negative-pressure wound therapy (NPWT) in the treatment of DFUs. As a result, all wounds treated with MDM showed healthy granulation tissue without noticeable complications during follow-up. The MDM group showed a higher healing rate compared to NPWT group, at 42 and 120 days, while similar healing rates were achieved between two groups at 6-month follow-up period. In 2017, a systematic review and meta-analysis conducted by Guo et al. [39] compared the efficacy and safety of ADM in DFU treatment, which showed that compared with the SOC alone, the ADM group was associated with higher complete healing rates at 12 and 16 weeks, and shorter mean time to complete wound healing. The adverse event rates in both groups were similar, indicating that the use of ADM did not increase the risk of adverse events.
In the current study, we included 15 RCTs involving 1524 patients, and demonstrated that dermal matrix is an effective and safe treatment option for enhancing DFU healing. The results of this study support previous studies documenting the successful application of dermal matrix therapy. Dermal matrix acts as a sterile tissue graft which can be applied directly to wound beds of DFUs and integrate with the surrounding host tissues to actively stimulate cell migration, angiogenesis, and epithelialization, resulting in accelerated wound healing [40]. Although the final follow-up wound area was similar between two treatment groups, the percentage area reduction (PAR) was demonstrated to be significantly increased in several studies [23, 26, 27, 31, 34]. However, the PAR was not pooled by meta-analysis due to the non-availability of the primary data (mean value and standard deviation of PAR).
This study, nevertheless, has several limitations that should be pointed out. Firstly, the dermal matrix products used in the studies varied among different manufacturers, which may introduce potential risk of bias. Secondly, due to the inconcealability of the treatment process with dermal matrix in the primary trials, an additional risk of bias may be caused by the unblinded application of dermal matrix to patients. Additionally, the studies reported outcomes at different follow-up times, making it difficult to pool the data. In this study, we selected the data at the final follow-up to conduct the analyses. Finally, most of the current available RCTs have relatively small sample sizes and short-term follow-up periods, which indicates that some more trials with larger sample size and longer follow-up period are required to provide some more convincing evidence.
Conclusions
The results of the current meta-analysis demonstrated that the application of dermal matrix as an adjuvant therapy to SOC can effectively enhance the healing process of DFUs and reduce the amputation risk when compared to SOC alone. Additionally, dermal matrix application was well tolerated by the subjects without added complication risk. However, some further well-designed prospective trials with larger sample sizes and longer follow-up periods are required to provide more convincing evidence.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- SOC:
-
Standard of care
- IDRT:
-
Integra dermal regeneration template
- NA:
-
Not available
- ADM:
-
Acellular dermal matrix
- CDM:
-
Cellular dermal matrix
- D-ADM:
-
DermACELL acellular dermal matrix
- GJ-ADM:
-
GraftJacket acellular dermal matrix
- Pre-D:
-
Prediabetes
- STSG:
-
Split-thickness skin grafting
- NPWT:
-
Negative-pressure wound therapy
- SIS:
-
Small intestine submucosa
- BMI:
-
Body mass index
- DFU:
-
Diabetic foot ulcer
- DFI:
-
Diabetic foot infection
- RCTs:
-
Randomized controlled trials
- MD:
-
Mean difference
- OR:
-
Odds ratio
- RR:
-
Risk ratio
- QoF:
-
Quality of life
- PRISMA:
-
Preferred reporting items for systematic reviews and meta-analyses
- DPN:
-
Diabetic polyneuropathy
- PP:
-
Per-protocol
- ITT:
-
Intention-to-treat
- 95% CI:
-
95% Confidence interval
- HR-ADM:
-
Human reticular acellular dermis matrix
- MDM:
-
Micronized dermal matrix
References
Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med. 2017;376:2367–75.
Nabuurs-Franssen MH, Huijberts MS, Nieuwenhuijzen Kruseman AC, Willems J, Schaper NC. Health-related quality of life of diabetic foot ulcer patients and their caregivers. Diabetologia. 2005;48:1906–10.
Ragnarson Tennvall G, Apelqvist J. Health-related quality of life in patients with diabetes mellitus and foot ulcers. J Diabetes Complicat. 2000;14:235–41.
Armstrong DG, Lavery LA, Harkless LB. Validation of a diabetic wound classification system: the contribution of depth, infection, and ischemia to risk of amputation. Diabetes Care. 1998;21:855–9.
Oyibo SO, Jude EB, Tarawneh I, Nguyen HC, Harkless LB, Boulton AJM. A comparison of two diabetic foot ulcer classification systems: the Wagner and the University of Texas wound classification systems. Diabetes Care. 2001;24:84–8.
Santema TB, Poyck PP, Ubbink DT. Skin grafting and tissue replacement for treating foot ulcers in people with diabetes. Cochrane Database Syst Rev. 2016;2:CD011255.
Singer AJ, Tassiopoulos A, Kirsner RS. Evaluation and Management of Lower-Extremity Ulcers. N Engl J Med. 2017;377:1559–67.
Driver VR, Fabbi M, Lavery LA, Gibbons G. The costs of diabetic foot: the economic case for the limb salvage team. J Vasc Surg. 2010;52:17S-22S.
Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet. 2005;366:1719–24.
Lev-Tov H, Li CS, Dahle S, Isseroff RR. Cellular versus acellular matrix devices in treatment of diabetic foot ulcers: study protocol for a comparative efficacy randomized controlled trial. Trials. 2013;14:8.
Everett E, Mathioudakis N. Update on management of diabetic foot ulcers. Ann N Y Acad Sci. 2018;1411:153–65.
Margolis DJ, Allen-Taylor L, Hoffstad O, Berlin JA. Healing diabetic neuropathic foot ulcers: are we getting better? Diabet Med. 2005;22:172–6.
Aldana PC, Khachemoune A. Diabetic foot ulcers: appraising standard of care and reviewing new trends in management. Am J Clin Dermatol. 2020;21:255–64.
Snyder D, Sullivan N, Margolis D, Schoelles K. Skin substitutes for treating chronic wounds [internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2020.
Climov M, Bayer LR, Moscoso AV, Matsumine H, Orgill DP. The role of dermal matrices in treating inflammatory and diabetic wounds. Plast Reconstr Surg. 2016;138:148S-157S.
Cho H, Blatchley MR, Duh EJ, Gerecht S. Acellular and cellular approaches to improve diabetic wound healing. Adv Drug Deliv Rev. 2019;146:267–88.
Reyzelman AM, Bazarov I. Human acellular dermal wound matrix for treatment of DFU: literature review and analysis. J Wound Care. 2015;24(128):129–34.
Deng W, Boey J, Chen B, et al. Platelet-rich plasma, bilayered acellular matrix grafting and negative pressure wound therapy in diabetic foot infection. J Wound Care. 2016;25:393–7.
Luthringer M, Mukherjee T, Arguello-Angarita M, Granick MS, Alvarez OM. Human-derived acellular dermal matrix grafts for treatment of diabetic foot ulcers: a systematic review and Meta-analysis. Wounds. 2020;32:57–65.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Brigido SA, Boc SF, Lopez RC. Effective management of major lower extremity wounds using an acellular regenerative tissue matrix: a pilot study. Orthopedics. 2004;27:s145–9.
Driver VR, Lavery LA, Reyzelman AM, et al. A clinical trial of Integra template for diabetic foot ulcer treatment. Wound Repair Regen. 2015;23:891–900.
Cazzell S, Moyer PM, Samsell B, Dorsch K, McLean J, Moore MA. A prospective, multicenter, single-arm clinical trial for treatment of complex diabetic foot ulcers with deep exposure using acellular dermal matrix. Adv Skin Wound Care. 2019;32:409–15.
Zelen CM, Orgill DP, Serena T, et al. A prospective, randomised, controlled, multicentre clinical trial examining healing rates, safety and cost to closure of an acellular reticular allogenic human dermis versus standard of care in the treatment of chronic diabetic foot ulcers. Int Wound J. 2017;14:307–15.
Cazzell S, Vayser D, Pham H, et al. A randomized clinical trial of a human acellular dermal matrix demonstrated superior healing rates for chronic diabetic foot ulcers over conventional care and an active acellular dermal matrix comparator. Wound Repair Regen. 2017;25:483–97.
Zelen CM, Orgill DP, Serena TE, et al. An aseptically processed, acellular, reticular, allogenic human dermis improves healing in diabetic foot ulcers: a prospective, randomised, controlled, multicentre follow-up trial. Int Wound J. 2018;15:731–9.
Tchanque-Fossuo CN, Dahle SE, Lev-Tov H, et al. Cellular versus acellular matrix devices in the treatment of diabetic foot ulcers: interim results of a comparative efficacy randomized controlled trial. J Tissue Eng Regen Med. 2019;13:1430–7.
Reyzelman A, Crews RT, Moore JC, et al. Clinical effectiveness of an acellular dermal regenerative tissue matrix compared to standard wound management in healing diabetic foot ulcers: a prospective, randomised, multicentre study. Int Wound J. 2009;6:196–208.
Hu Z, Zhu J, Cao X, et al. Composite skin grafting with human acellular dermal matrix scaffold for treatment of diabetic foot ulcers: a randomized controlled trial. J Am Coll Surg. 2016;222:1171–9.
Lantis JC, Snyder R, Reyzelman AM, et al. Fetal bovine acellular dermal matrix for the closure of diabetic foot ulcers: a prospective randomised controlled trial. J Wound Care. 2021;30:S18–27.
Veves A, Falanga V, Armstrong DG, Sabolinski ML, Apligraf Diabetic Foot Ulcer Study. Graftskin, a human skin equivalent, is effective in the management of noninfected neuropathic diabetic foot ulcers: a prospective randomized multicenter clinical trial. Diabetes Care. 2001;24:290–5.
Hahn HM, Lee DH, Lee IJ. Ready-to-use micronized human acellular dermal matrix to accelerate wound healing in diabetic foot ulcers: a prospective randomized pilot study. Adv Skin Wound Care. 2021;34:1–6.
Cazzell SM, Lange DL, Dickerson JE Jr, Slade HB. The Management of Diabetic Foot Ulcers with porcine small intestine submucosa tri-layer matrix: a randomized controlled trial. Adv Wound Care (New Rochelle). 2015;4:711–8.
Brigido SA. The use of an acellular dermal regenerative tissue matrix in the treatment of lower extremity wounds: a prospective 16-week pilot study. Int Wound J. 2006;3:181–7.
Campitiello F, Mancone M, Della Corte A, Guerniero R, Canonico S. To evaluate the efficacy of an acellular Flowable matrix in comparison with a wet dressing for the treatment of patients with diabetic foot ulcers: a randomized clinical trial. Updat Surg. 2017;69:523–9.
Martin BR, Sangalang M, Wu S, Armstrong DG. Outcomes of allogenic acellular matrix therapy in treatment of diabetic foot wounds: an initial experience. Int Wound J. 2005;2:161–5.
Lee M, Jun D, Choi H, Kim J, Shin D. Clinical efficacy of acellular dermal matrix paste in treating diabetic foot ulcers. Wounds. 2020;32:50–6.
Guo X, Mu D, Gao F. Efficacy and safety of acellular dermal matrix in diabetic foot ulcer treatment: a systematic review and meta-analysis. Int J Surg. 2017;40:1–7.
Macri L, Clark RA. Tissue engineering for cutaneous wounds: selecting the proper time and space for growth factors, cells and the extracellular matrix. Skin Pharmacol Physiol. 2009;22:83–93.
Acknowledgements
Not applicable.
Funding
This work was supported by grant from the Hebei Province Medical Science Research Project (No. 20241445). The sponsor or funder had no involvement in any part of this study. All authors confirmed the independence of researchers from funding sources.
Author information
Authors and Affiliations
Contributions
Lei Sui and Qiang **e: methodology, validation, formal analysis, investigation, data curation, writing-original draft, writing-reviewing and editing, and project administration. Lei Sui, Qiang **e, and Hong-tao Jiang: investigation, and data processing. Lei Sui: validation, writing-reviewing and editing. **ao-dong Li: project administration. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Checklist of the PRISMA for network meta-analysis.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sui, L., **e, Q., Jiang, Ht. et al. Effectiveness and safety of dermal matrix used for diabetic foot ulcer: a systematic review and meta-analysis of randomized controlled trials. BMC Endocr Disord 24, 23 (2024). https://doi.org/10.1186/s12902-024-01550-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12902-024-01550-3