Abstract
Background
Masquelet membrane induction technology is one of the treatment strategies for large bone defect (LBD). However, the angiogenesis ability of induced membrane decreases with time and autologous bone grafting is associated with donor site morbidity. This study investigates if the PRP-FG-nHA/PA66 scaffold can be used as a spacer instead of PMMA to improve the angiogenesis ability of induced membrane and reduce the amount of autologous bone graft.
Methods
Platelet rich plasma (PRP) was prepared and PRP-FG-nHA/PA66 scaffold was synthesized and observed. The sustained release of VEGFA and porosity of the scaffold were analyzed. We established a femur LBD model in male SD rats. 55 rats were randomly divided into four groups depending on the spacer filled in the defect area. “Defect only” group (n = 10), “PMMA” group (n = 15), “PRP-nHA/PA66” group (n = 15) and “PRP-FG-nHA/PA66” group (n = 15 ). At 6 weeks, the spacers were removed and the defects were grafted. The induced membrane and bone were collected and stained. The bone formation was detected by micro-CT and the callus union was scored on a three point system.
Results
The PRP-FG-nHA/PA66 scaffold was porosity and could maintain a high concentration of VEGFA after 30 days of preparation. The induced membrane in PRP-FG-nHA/PA66 group was thinner than PMMA, but the vessel density was higher.The weight of autogenous bone grafted in PRP-FG-nHA/PA66 group was significantly smaller than that of PMMA group. In PRP-FG-nHA/PA66 group, the bone defect was morphologically repaired.
Conclusion
The study showed that PRP-FG-nHA/PA66 scaffold can significantly reduce the amount of autologous bone graft, and can achieve similar bone defect repair effect as PMMA. Our findings provide some reference and theoretical support for the treatment of large segmental bone defects in humans.
Similar content being viewed by others
Introduction
The repair and reconstruction of large bone defect (LBD) is one of the most challenging problems for orthopedic surgeons. LBD is often caused by severe trauma, debridement after infection, bone tumor resection, and other reasons [1]. The treatment of LBD is difficult, especially when combined with infection and poor soft tissue conditions. Blood vessels have many biological functions such as nutrient supply mechanism, molecular signaling transmission and stem cell transport. Angiogenesis is crucial for bone regeneration. Lack of angiogenesis is one of the reason of hindering bone development, regeneration and proper systemic functioning [2]. Studies have shown that the reason why many bone defects are difficult to heal is precisely because of the difficulty of local blood vessel formation [3]. Therefore, the treatment of bone defect requires not only filling the defect, but also recovering the local angiogenesis ability.
In recent years, Masquelet membrane induction technology has become one of the treatment strategies for LBD due to its ability to promote the blood vessel formation of induced membrane [4]. The Masquelet membrane induction technology was proposed by Masquelet et al. in 2000. This technique consists of two stages of surgery: First, after complete debridement, the defect is filled with polymethyl methacrylate (PMMA) cement as a spacer. Second, after 6–8 weeks of implantation, the PMMA spacers were removed and followed by autologous bone grafted. At present, this technology has achieved good results in the treatment of large bone defects [5]. However, the technology has many drawbacks. First, as a traditional membrane induction medium, PMMA cement has little bone induction activity. Second, PMMA has thermogenic effect during the solidification process, which is easy to cause thermal damage to the surrounding tissues [6]. Third, the angiogenesis ability of the membrane induced by PMMA gradually decreases after implantation [7]. Last, after the removement of PMMA, the space left always needs large amount of autologous bone which will cause donor site morbidity and secondary deformity. Therefore, it is important to improve the angiogenesis ability of induced membrane and reduce the amount of autograft bone grafted in the second stage.
Platelet rich plasma (PRP), an autologous concentration of platelets from autologous whole blood by secondary centrifugation, has rapidly evolved as a focal point of interest in the realm of orthopedic therapeutic [8]. The autologous derivation of PRP offers both economic advantages and obviates potential immunogenic complications commonly linked to exogenous recombinant growth factors. Such benefits not only reduce the risk of disease transmission but also facilitate its application in surgery [5). Quantitative parameters including bone volume (BV, mm3), bone volume density (BV/TV, %) and bone mineral density (g/cm3) for bone formation were obtained and statistically analyzed, the results were illustrated in Fig. 6. The PRP-FG-nHA/PA66 group and PMMA group showed significantly greater mineralized callus formation compared with PRP-nHA/PA66 group and defect only group, but no significant difference was found between the two groups in terms of the bone volume, bone volume density and bone mineral density.
Callus union was also scored based on the micro-CT scans. Defect only group had a mean score of 1.0 ± 0.71, with PRP-nHA/PA66 group having 1.8 ± 0.84, PRP-FG-nHA/PA66 group having 2.4 ± 1.14 and PMMA group having 2.8 ± 1.30. No significant difference was found between PRP-FG-nHA/PA66 group and PMMA group. Bridging of the defect, which was demonstrated by a score of two or greater, was observed in 1/5 (20%) of defect only group, 2/5 (40%) of PRP-nHA/PA66 group, 4/5 (80%) of PRP-FG-nHA/PA66 group, and 4/5 (80%) of PMMA group.
Discussion
In 1986, Masquelet accidentally discovered the induced membrane technology. At that time, the PMMA was used only as a spacer to fill the bone defect, and the induced membrane surrounding the PMMA was retained to avoid excessive bleeding. However, subsequent studies found that this membrane induced by PMMA had many benefits, the membrane not only promoted vascularization and corticalization of the graft but also prevented its resorption [23]. In 2010, Masquelet proposed the concept of induced membrane technology, and this technology opened up new prospects for the treatment of bone defects [24].
Masquelet induced membrane technology involves two stages of surgery. Radical debridement is critical in the first surgery, especially for cases such as infectious bone defects and osteomyelitis. Debridement can remove bacterial biofilms, necrotic tissue, scars, granulation tissue, and non-vital tissues present in the bone defect area. Study has shown that conservative debridement [25] is a major factor contributing to high recurrence rate of infection. Some studies even consider infectious bone diseases as malignant conditions requiring surgical debridement [26]. Because of the radical debridement in the first surgery, large bone defects are often formed in the second stage. The defect area may even exceed two-thirds of the bone length [27]. Although these defects can be repaired in the second stage, it requires massive bone grafting to fill the chamber. The main source of autograft is anterior or posterior iliac crest, and a large amount of cancellous autogenous iliac grafting may easily cause donor site diseases, including movement limitations, perceptual disturbances, deformity and pains [28, 29]. Therefore, massive autogenous bone graft is the major disadvantage of the Masquelet induced membrane technique [30]. In our study, we used PRP-FG-nHA/PA66 instead of PMMA as an spacer in the first stage, and then we observed the morphology of the induced membrane and weighed the amount of autologous bone required for the second stage of surgery. Our results showed that the PRP-FG-nHA/PA66, as a spacer, could not only significantly reduce the amount of autogenous bone graft in the second stage, but also repair the bone defect as PMMA. Our study indicated the induced membrane of PRP-FG-nHA/PA66 scaffold had higher efficacy in bone defect treatment than that of PMMA.
Schmitz JP et al. have found that if the defect length reaches one-tenth of the bone length, the defect will exceeded the critical size for self-repair [31]. The average femoral length of SD rats in our study was about 4 cm, therefore, we established a 5 mm large segmental bone defect in the right femurs of SD rat. In our previous study, we confirmed the effective of HUMSCs-PRP gel/nHA-PA66 scaffold to promote bone regeneration in the rat LBD repair [17]. However, the previous study also has many deficiencies. First, we found the immersion of PRP gel could only enable the PRP to cover the surface of the scaffold; Second, the HUMSCs are difficult to obtain and very expensive. Because of these deficiencies, we designed a new scaffold. We used lyophilization method to make the PRP gel fully attached to the surface of the scaffold and applied it to Masquelet induced membrane technology instead of PMMA as a spacer. Our results showed the PRP-FG-nHA/PA66 scaffold had many advantages. First, the PRP-FG-nHA/PA66 was prepared with a 1 mm diameter groove in the middle before implantation. This structure can make the spacers embedded in the defect area and reduce the damage to the induced membrane during the second operation to remove the spacer; Second, according to the literature, the porosity of human cancellous bone is 40–95% [31,32,33]. The average porosity of the PRP-FG-nHA/PA66 scaffold in our study was 58.7%, which was similar to normal cancellous bone, which met the basic requirements of scaffolds for large bone defects; Third, because of the application of fibrin as a “glue”, PRP gel could be attached not only to the outer surface, but also to the inner micropores surface of the nHA/PA66 scaffold. Our results indicated the freeze-drying step could strengthened the attachment of PRP gel. Finally, due to the thoroughly permeation and stable PRP attachment, the PRP-FG-nHA/PA66 scaffold achieved a longer growth factor release time than the PRP-nHA/PA66 scaffold, which was beneficial for the vascularization of the induced membrane. We could observed the induced membrane was rich in microvessels after 6 weeks of PRP-FG-nHA/PA66 scaffold implantation.
In Masquelet induced membrane technology, the neovascularization of the induced membrane is very important. Studies have shown that this membrane-like tissue is rich in various angiogenic factors, such as vascular endothelial growth factor A (VEGFA), transforming growth factor-β1 (TGF-β1), and so on [20]. The induced membrane has good angiogenesis ability, so it can improve the formation of microvessels and the perfusion the local blood in the bone defect area [34]. Even in the case of some ischemic diseases (such as radiotherapy, congenital pseudarthrosis formation, etc.), the induced membranes can effectively promote angiogenesis in the bone defect area [35, 36]. However, the angiogenesis ability, an important capacity of induced membrane, gradually decreases with time [37]. Studies have shown that the neovascularization of induced membrane decreased after 6 weeks of formation, and only about 60% of microvessels remained after 12 weeks [7]. Our study showed that the induced membrane around PMMA cement, although very thick after 6 weeks of implantation, was mainly composed of fibrous tissue, only a few microvessels existed in the inner layer. Insufficient angiogenesis capacity may not only prolong the treatment time, but even lead to early dissolution of the implanted autologous bone [38].
Although the angiogenic ability of induced membrane is critical for the treatment of Masquelet induced membrane technology, PMMA cement, as a traditional membrane induction medium, has many deficiencies. PMMA has little angiogenic ability and will release heat in the procession of solidification, which is easy to cause thermal damage to surrounding tissues [6]. In recent years, many studies have used biological scaffolds instead of PMMA bone cement as membrane induction media [].As an ideal scaffold material for bone defect filling, nano-hydroxyapatite/polyamide 66 (nHA/PA66) has excellent osteoconductive and osteoinductive activity. The scaffold has been widely used in a variety of clinical surgical procedures [40]. Because nHA/PA66 scaffold has many advantages compared to PMMA, we used nHA/PA66 as the scaffold of the composite material to fill the defect area.
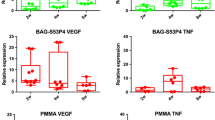
Previous study has shown that although many cytokines released after PRP activation are involved in the angiogenesis process, VEGFA has a dominant role among these cytokines [41]. The cytokines released after PRP activation, such as PDGF and TGF- β1, not only synergize with VEGFA, but also upregulate the expression of VEGFA and its receptors. Therefore, although many cytokines can be released after PRP activation, VEGFA is a key regulator of angiogenesis promoted by PRP. In this study, we used lyophilization method to combine PRP, FG with nHA/PA66 scaffold and analyzed the release of VEGFA. The result of ELISA showed that the PRP-FG-nHA/PA66 scaffold could release VEGFA for up to 30 days. PRP related cytokines make PRP-FG-nHA/PA66 scaffold has a good angiogenic ability.
To further demonstrate the angiogenic ability of PRP-FG-nHA/PA66 scaffold, we observed the microvessels formation of the induced membrane. At 6 weeks after implantation, neovascularization could be observed around both PMMA and PRP-FG-nHA/PA66 scaffold in the inner layer of the induced membrane. However, the vessel density around PRP-FG-nHA/PA66 scaffold was significantly greater than that in PMMA. The results of the histological observations are also consistent with what we observed in animal surgery. The results indicated that PRP-FG-nHA/PA66 scaffold we constructed had a good angiogenic ability. The high density of microvessels also indicated that the induced membrane formed by PRP-FG-nHA/PA66 scaffold had good capacity for the treatment of bone defects. These results might explain why PRP-FG-nHA/PA66, as a spacer, could significantly reduce the amount of bone graft in the second stage. There are also some limitations in our study. First, this study did not investigate the mechanism of PRP promoting the angiogenesis of induced membrane. Second, the PRP-related cytokines were not clarified. In the following study, we will optimize the preparation method of PRP and further investigate PRP-related cytokines. We hope the PRP-FG-nHA/PA66 scaffold can achieve better bone defect repair effect in future.
In conclusion, our study modified the Masquelet induced membrane technique for the repair of LBD, we used PRP-FG-nHA/PA66 scaffold instead of PMMA in the first-stage surgery. The study showed that PRP-FG-nHA/PA66 scaffold can significantly reduce the amount of second-stage cancellous autologous bone graft, and less autologous bone graft can achieve similar bone defect repair effect as PMMA. Our findings provide some reference and theoretical support for the treatment of large segmental bone defects in humans.
Data availability
The datasets generated during and analysed during the current study are not publicly available due to internal data storage restriction but are available from the corresponding author on reasonable request.
References
Wang J, Yin Q, Gu S, Wu Y, Rui Y. Induced membrane technique in the treatment of infectious bone defect: a clinical analysis. Orthop Traumatol Surg Res. 2019;105(3):535–9.
Filipowska J, Tomaszewski KA, Niedźwiedzki Ł, Walocha JA, Niedźwiedzki T. The role of vasculature in bone development, regeneration and proper systemic functioning. Angiogenesis. 2017;20(3):291–302.
Lee EJ, Jain M, Alimperti S. Bone microvasculature: stimulus for tissue function and regeneration. Tissue Eng Part B Rev. 2021;27(4):313–29.
Masquelet A, Kanakaris NK, Obert L, Stafford P, Giannoudis PV. Bone repair using the Masquelet technique. J Bone Joint Surg Am. 2019;101(11):1024–36.
Govaert GA, Ijpma FF, McNally, Gannamani S, Rachakonda KR, Tellakula Y, Takkalapally H, Maryada VR, Gurava Reddy AV. Combining non-vascularized fibula and cancellous graft in the masquelet technique: a promising approach to distal femur compound fracture management with large defects. Injury. 2023;55(2):111233.
Masquelet AC. Induced membrane technique: pearls and pitfalls. J Orthop Trauma. 2017;31(Suppl 5):S36–8.
Aho OM, Lehenkari P, Ristiniemi J, Lehtonen S, Risteli J, Leskelä HV. The mechanism of action of induced membranes in bone repair. J Bone Joint Surg Am. 2013;95(7):597–604.
Everts P, Onishi K, Jayaram P, Lana JF, Mautner K. Platelet-Rich plasma: New Performance understandings and therapeutic considerations in 2020. Int J Mol Sci. 2020;21(20):7794.
Zhu L, Li P, Qin Y, **ao B, Li J, Xu W, Yu B. Platelet-rich plasma in orthopedics: bridging innovation and clinical applications for bone repair. J Orthop Surg (Hong Kong). 2024;32(1):10225536231224952.
Cecerska-Heryć E, Goszka M, Serwin N, Roszak M, Grygorcewicz B, Heryć R, Dołęgowska B. Applications of the regenerative capacity of platelets in modern medicine. Cytokine Growth Factor Rev. 2022;64:84–94.
Etulain J. Platelets in wound healing and regenerative medicine. Platelets. 2018;29(6):556–68.
Van der Bijl I, Vlig M, Middelkoop E, de Korte D. Allogeneic platelet-rich plasma (PRP) is superior to platelets or plasma alone in stimulating fibroblast proliferation and migration, angiogenesis, and chemotaxis as relevant processes for wound healing. Transfusion. 2019;59(11):3492–500.
Oryan A, Alidadi S, Moshiri A. Platelet-rich plasma for bone healing and regeneration. Expert Opin Biol Ther. 2016;16(2):213–32.
Han J, Gao F, Li Y, Ma J, Sun W, Shi L, Wu X, Li T. The Use of Platelet-Rich Plasma for the Treatment of Osteonecrosis of the Femoral Head: A Systematic Review. Biomed Res Int. 2020; 2020: 1–11.
Clark RA. Fibrin glue for wound repair: facts and fancy. Thromb Haemost. 2003;90(6):1003–6.
Ortiz AC, Fideles SOM, Pomini KT, Reis CHB, Bueno CRS, Pereira ESBM, Rossi JO, Novais PC, Pilon JPG, Rosa Junior GM, Buchaim DV, Buchaim RL. Effects of Therapy with Fibrin glue combined with mesenchymal stem cells (MSCs) on bone regeneration: a systematic review. Cells. 2021;10(9):2323.
Liu W, Huang Y, Liu D, Zeng T, Wang J, Li A, Wang D, Wang X. The combination of platelet Rich plasma gel, human umbilical mesenchymal stem cells and Nanohydroxyapatite/polyamide 66 promotes angiogenesis and bone regeneration in large bone defect. Tissue Eng Regen Med. 2022;19(6):1321–36.
Wang H, Li Y, Zuo Y, Li J, Ma S, Cheng L. Biocompatibility and osteogenesis of biomimetic nano-hydroxyapatite/polyamide composite scaffolds for bone tissue engineering. Biomaterials. 2007;28:3338–48.
Qi XN, Mou ZL, Zhang J, Zhang ZQ. Preparation of chitosan/silk fibroin/hydroxyapatite porous scaffold and its characteristics in comparison to bi-component scaffolds. J Biomed Mater Res A. 2014;102(2):366–72.
Henrich D, Seebach C, Nau C, Basan S, Relja B, Wilhelm K, Schaible A, Frank J, Barker J, Marzi I. Establishment and characterization of the Masquelet induced membrane technique in a rat femur critical-sized defect model. J Tissue Eng Regen Med. 2016;10(10):E382–96.
Wang X, Ju F, Li A, Geng S, Sun J, Liu R, et al. Nell-1 gene modified mesenchymal stem cells on biomimetic porous nano-hydroxyapatite/polyamide 66 scaffolds effectively prevent nonunion in rats. J Biomaterials Tissue Eng. 2016;6(5):408–16.
DeBaun MR, Stahl AM, Daoud AI, Pan CC, Bishop JA, Gardner MJ, Yang YP. Preclinical induced membrane model to evaluate synthetic implants for healing critical bone defects without autograft. J Orthop Res. 2019;37(1):60–8.
Masquelet AC, Fitoussi F, Begue T, Muller GP. Reconstruction Des os longs par membrane Induite et autogreffe spongieuse. Ann Chir Plast Esthetique. 2000;45(3):346e353.
Masquelet AC, Begue T. The concept of induced membrane for reconstruction of long bone defects. Orthop Clin North Am. 2010;41(1):27–37. ; table of contents.
Lew DP, Waldvogel FA, Osteomyelitis. Lancet. 2004;364(9431):369–79.
Sanders J, Mauffrey C. Long bone osteomyelitis in adults: fundamental concepts and current techniques. Orthopedics. 2013;36(5):368–75.
Yu X, Wu H, Li J, **e Z. Antibiotic cement-coated locking plate as a temporary internal fixator for femoral osteomyelitis defects. Int Orthop. 2017;41(9):1851–7.
Starch-Jensen T, Deluiz D, Deb S, Bruun NH, Tinoco EMB. Harvesting of autogenous bone graft from the ascending mandibular ramus compared with the chin region: a systematic review and meta-analysis focusing on complications and donor site morbidity. J Oral Maxillofac Res. 2020;11:e1.
Yeap MC, Tu PH, Liu ZH, Hsieh PC, Liu YT, Lee CY, Lai HY, Chen CT, Huang YC, Wei KC, Wu CT, Chen CC. Long-term complications of Cranioplasty using stored autologous bone graft, three-Dimensional Polymethyl Methacrylate, or Titanium Mesh after Decompressive Craniectomy: a single-center experience after 596 procedures. World Neurosurg. 2019;128:e841–50.
Han W, Shen J, Wu H, Yu S, Fu J, **e Z. Induced membrane technique: advances in the management of bone defects. Int J Surg. 2017;42:110–6.
Schmitz JP, Hollinger JO. The critical size defect as an experimental model for craniomandibulofacial nonunions. Clin Orthop Relat Res. 1986;205:299–308.
Morgan EF, Unnikrisnan GU, Hussein AI. Bone mechanical properties in healthy and diseased states. Annu Rev Biomed Eng. 2018;20:119–43.
Chen J, Zhang D, Zhang T, Chen C, Song Y, Liu S, Su Y, Guo S. Effect of the vascularized bone components on the survival of vascularized composite allografts. J Surg Res. 2018;224:132–8.
Wang W, Zuo R, Long H, Wang Y, Zhang Y, Sun C, Luo G, Zhang Y, Li C, Zhou Y, Li J. Advances in the Masquelet technique: myeloid-derived suppressor cells promote angiogenesis in PMMA-induced membranes. Acta Biomater. 2020;108:223–36.
Matsuhashi M, Saito T, Noda T, Uehara T, Shimamura Y, Ozaki T. Treatment for postoperative infection of pathological femoral fracture after radiotherapy: two case reports and review of the literature. Arch Orthop Trauma Surg. 2021;141(7):1139–48.
Meselhy MA, Elhammady AS, Singer MS. Outcome of Induced membrane technique in treatment of failed previously operated congenital pseudarthrosis of the Tibia. Orthop Traumatol Surg Res. 2020;106(5):813–8.
Tang Q, ** H, Tong M, Zheng G, **e Z, Tang S, ** J, Shang P, Xu H, Shen L, Zhang Y, Liu H. Inhibition of Dll4/Notch1 pathway promotes angiogenesis of Masquelet’s induced membrane in rats. Exp Mol Med. 2018;50(4):1–15.
Auregan JC, Begue T. Induced membrane for treatment of critical sized bone defect: a review of experimental and clinical experiences. Int Orthop. 2014;38:1971–8.
Yu YH, Lee D, Hsu YH, Chou YC, Ueng SW, Chen CK, Liu SJ. A three-dimensional printed polycaprolactone Scaffold Combined with Co-axially Electrospun Vancomycin/Ceftazidime/Bone Morphological Protein-2 sheath-core nanofibers for the repair of segmental bone defects during the Masquelet Procedure. Int J Nanomed. 2020;15:913–25.
Li Q, Gao Q, Wang L, Liu L, Yang H, Song Y. Comparison of long-term Follow-Up of n-HA PA66 cage and PEEK cage of lumbar Interbody Fusion in Multi-level degenerative lumbar diseases: a stepwise propensity score matching analysis. Orthop Surg. 2024;16(1):17–28.
Melincovici CS, Boşca AB, Şuşman S, Mărginean M, Mihu C, Istrate M, Moldovan IM, Roman AL, Mihu CM. Vascular endothelial growth factor (VEGF) - key factor in normal and pathological angiogenesis. Rom J Morphol Embryol. 2018;59(2):455–67.
Acknowledgements
Not applicable.
Funding
This study was supported by Natural Science Foundation of Heilongjiang Province of China (Grant No. LH2022H037).
Author information
Authors and Affiliations
Contributions
XW performed most of the experiments, analyzed the data, and drafted the manuscript. WL, JW and MA were mainly involved in the literature search and manuscript writing. JW, TZ, YH and DL participated in the experiment. MA assisted in the polishing of the article. DW gave guidance and participated in the project design.
Corresponding author
Ethics declarations
Ethical statement
The animal studies were performed after receiving approval of the Institutional Animal Care and Use Committee (IACUC) in Harbin Medical University.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, X., Huang, Y., Liu, D. et al. The Masquelet induced membrane technique with PRP-FG-nHA/PA66 scaffold can heal a rat large femoral bone defect. BMC Musculoskelet Disord 25, 455 (2024). https://doi.org/10.1186/s12891-024-07567-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12891-024-07567-y