Abstract
Background
Intervertebral disc degeneration and sarcopenia are both age-related diseases without effective treatments. Their comorbidities may worsen the prognosis, and further studies on interaction and therapy are needed. The purpose of the study was to investigate the prevalence of sarcopenia in intervertebral disc degeneration, and to compare the characteristics of intervertebral disc degeneration with and without sarcopenia and effects of interferential current.
Methods
One hundred twenty disc degeneration patients were included from 2021 to 2022 in a single institute. Medical records, examination results and radiological reports were reviewed. Patients with sarcopenia were screened and grouped according to Asian Working Group for Sarcopenia 2019. VAS, ODI, SARC-F, SMI, gait speed (GS), grip strength, disc Pfirrmann grading, standard cross-sectional area (SCSA), degree of fatty infiltration (DFF), and nerve conduction velocity (NCV) were assessed before and after treatment.
Results
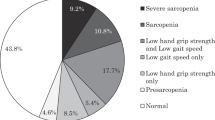
The prevalence of sarcopenia in intervertebral disc degeneration was 28.3%. The difference of VAS, ODI, disc Pfirrmann grading, SCSA, DFF and NCV between two groups were significant before intervention (P < 0.05), SCSA and DFF were related to the degree of disc degeneration. The improvement of SMI, GS, grip strength, VAS, SARC-F and ODI in intervertebral disc degeneration with sarcopenia group was significant after intervention, as well as SMI, GS, grip strength, VAS and ODI in those without sarcopenia (P < 0.05). The improvement of grip strength, GS, ODI and SARC-F in intervertebral disc degeneration with sarcopenia group were greater than the one without sarcopenia (P < 0.05), whereas there was no significance in improvement degree of other indicators between the two groups (P > 0.05).
Conclusion
The prevalence of sarcopenia was high in intervertebral disc degeneration, and paravertebral muscles degeneration correlated with the degree of disc degeneration. Compared to those without sarcopenia, intervertebral disc degeneration patients with sarcopenia have more severe pain, poorer mobility and neurological function. Interferential current is effective in intervertebral disc degeneration patients and sarcopenia patients.
Similar content being viewed by others
Introduction
Intervertebral disc degeneration (IDD) is a typical and frequently-occurring disease including disc herniation, and spondylolisthesis. It is considered to be a main contributor to lower back pain (LBP), numbness and lower-limb dysfunction, which has become a serious medical problem and imposing a financial and health burden to the individual and society for its high morbidity [1, 2]. Sarcopenia is an age-related disease that refers to the loss of muscle mass, strength and function, which may increase the risk of falls, fractures, and body dysfunction [3,4,5]. Currently, there are no approved therapies as the etiology of the two diseases are still not clearly defined. Previous studies found the prevalence of sarcopenia is 10–20% in the elderly and up to 25% in the lumbar spinal stenosis (LSS) [6,7,8]. The comorbidity of the two aggravates the condition and worsen the prognosis. The quality of life in lumbar disease patients with sarcopenia is not only affected, but also the risk of falls, the prevalence of osteoporosis, the rate of complications and re-admission increases, as well as a potentially prolonged hospitalization and even death [9,10,11]. Evidence suggests that IDD patients and patients with muscle atrophy or skeletal muscle dysfunction achieved good results after interferential currents (IFC) electrical stimulation [12,13,14,15,16]. It is a medium-frequency alternating current with amplitude-modulated in low frequency proposed by Austrian physicist Nemec in the 1950s [17], which can produce similar physiological effects to low-frequency current. As an applied electrical stimulation for the management of musculoskeletal diseases, it can reduce skin impedance to reach deep muscle tissue without increasing the patient's discomfort, and is effective in increasing neuromuscular excitability, relieving inflammation, eliminating edema, reducing nerve or muscle pain, promoting nerve regeneration, enhancing muscle strength, and improving physical mobility [18,19,20]. Additionally, interferential current stimulation is simple to use, safe, and has fewer adverse effects compared to other therapies. Despite the growing popularity of IFC therapy in various clinical settings, there have been few studies on its efficacy on intervertebral disc degeneration with sarcopenia patients. The aim of this study was to explore the effect of sarcopenia on IDD patients by comparing the characteristics of IDD patients with and without sarcopenia in the elderly population and their relationship. We also attempted to investigate the treatment effect of IFC on both patients.
Methods
Study design and participants
This was a retrospective study including consecutive patients who came to our hospital with low back pain and were ultimately diagnosed (both clinical and radiological) as IDD. All included patients received conservative treatment and IFC stimulation between October 2021 and October 2022 (Fig. 1). The information of patients and indicators are collected through the hospital information system. This study was approved by the Institutional Research and Medical Ethics Committee of Second **
All the IDD patients who met the criteria were measured with bioelectrical impedance analysis (BIA) to obtain the SMI, which was calculated by dividing appendicular skeletal muscle mass (AMM) by body height in meters squared (kg/m2). The patient's grip strength and gait speed were also recorded. Participants were grouped according to the criteria of Asian Working Group for Sarcopenia 2019 (AWGS2019) [21]: (1) IDD with sarcopenia (IWS) group: disc degeneration patients with sarcopenia (Males with SMI < 7.0 kg/m2, grip strength < 28 kg; females with SMI < 5.7 kg/m2, grip strength < 18 kg); (2) IDD (IWOS) group: disc degeneration patients without sarcopenia.
Therapeutic method
According to the medical records, all eligible patients received a consecutive two-week IFC therapy twice a day. Patients were asked to lay down in a prone position, with the low back area unclothed. The instruments (Bohua, BHE-200L, Japan) with four self-adhesive electrodes were used (9 × 5 cm) (ValuTrode®; Axelgaard, Fallbrook, CA). The electrodes of instrument were arranged in a cross-quadrupole arrangement (In the first channel, an electrode was positioned 3 cm to the right of L3's spinous process and 3 cm to the left of L5's; In the second channel, an electrode was positioned 3 cm to L3's left side and 3 cm to L5's right side. The following parameters were used: (1) a 4000 Hz carrier frequency; (2) a 65 Hz amplitude-modulated frequency; (3) a 1:1 swing pattern with a sweep frequency of 95 Hz. The intensity should be tolerated by the patient in terms of tingling, tremor, twitch and muscle contraction, which shall not exceed 70% of the maximum tolerable intensity with each stimulation lasting 20 min.
Analysis variables
SARC-F
A simple and inexpensive screening method proposed by Malmstrom et al. in 2013 [22]. It consists of five items: muscle strength (ability to lift 10 pounds), walking ability (ability to walk 1 km), rise from a chair, climb stairs (ability to climb 10 steps) and falls (number of falls in past year). 2 points for each of these items, 10 points in total. Of the first four items, a score of 2 is given if the patient found it difficult or impossible to complete, 1 for somewhat difficult and 0 for easily doable. In the last item, 0 points for 0 falls, 1 point for 1 ~ 3 falls and 2 points for ≥ 4 falls. Total score ≥ 4 indicates increased risk of sarcopenia.
VAS
Participants were asked to rate their pain on visual analogue scale. A 10 cm line is drawn across the top of the paper, with 0 at one end of the line indicating no pain and 10 at the other end indicating severe pain.
ODI
The severity of lumbar dysfunction was evaluated according to Oswestry dysfunction index score [23], which consists of 10 items: pain level, self-care, lifting, walking, sitting, standing, sleep, sexual life, social life and travel. Each item is scored from 0 to 5, with the actual score/50 × 100% as the final score. The higher the score, the more pronounced the functional impairment.
Functional measurements and radiological parameters
Grip strength
Grip strength was measured directly with a electronic hand dynamometer (CAMRY, MAX:90 kg, d = 100 g, EM101). During the test, the patient sits in a chair with the forearm resting on the armrest, then squeeze the grip dynamometer as tightly as possible within 3-5 s. The left and right hands are measured 3 times each, and the average reading of the 3 times is taken to represent the grip strength of the hand, and then the average grip strength of the left and right hands is taken to represent the patient's grip strength. The AWGS2019 recommended grip strength diagnostic thresholds are < 28 kg for men and < 18 kg for women [21].
Gait speed (GS)
6 m walk test was used to evaluate gait speed. During the test, the usual or comfortable pace is used as the standard, starting from the resting state, when the foot first touches the ground behind the line, the time taken to complete 6 m is recorded and the walking speed is obtained by calculating the ratio of distance to time, and measure at least 2 times, take the mean value as GS. A GS threshold of 1.0 m/s is recommended by the AWGS2019 standard [21].
Nerve conduction velocity (NCV)
NCV of the lower limbs was obtained through electrophysiological examination. The process was completed by professional technicians, and the NCV was obtained by calculating the ratio of distance (stimulation electrode to recording electrode) and time (electrical stimulation through stimulation electrode to recording electrode).
Standard cross-sectional area (SCSA)
The ImageJ (National Institutes of Health, Version 1.53, USA) was used to depict and calculate the cross-sectional area of the paravertebral muscles (psoas) in the L4/5 plane and the cross-sectional area of the corresponding disc, and the ratio of the two was SCSA [24].
Degree of fatty infiltration (DFF)
The ImageJ was used to depict and calculate fat infiltration area of the paravertebral muscles (psoas) and the area of the psoas in the L4/5. The ratio of the two is DFF [24].
SMI
Bioelectrical impedance analysis (Inbody, Inbody760, Korea) was used to obtain skeletal muscle index. During the process, the test is carried out by applying a weak current or voltage to the subject through surface electrodes, thereby detecting the electrical impedance and changes, and ultimately obtaining information about AMM. The ratio of AMM to height squared is the SMI. The test is performed on an empty stomach to reduce the interference of water with the results. According to AWGS2019, males < 7.0 kg/m2 and females < 5.7 kg/m2 can be diagnosed with low skeletal muscle mass measured by BIA [21].
Pfirrmann grading for evaluating lumbar disc degeneration [25]
Grade I: The disc's structure is homogenous, and it has a typical disc height and a bright, hyperintense white signal. Grade II: The disc structure is inhomogeneous, with a white signal that is hyperintense. The nucleus and anulus are clearly distinguished, and the disc height is typical, with or without horizontal gray bands. Grade III: The disc structure is inhomogeneous, with a gray signal intensity that is intermediate. The disc height is normal or slightly diminished, and the differentiation between nucleus and anulus is uncertain. Grade IV: The disc structure is inhomogeneous, with a signal intensity that is hypointense dark gray. The disc height is normal or considerably diminished, and the difference between nucleus and anulus is obliterated. Grade V: The disc structure is inhomogeneous, and the black signal intensity is hypointense. The disc space is compacted, and the difference between nucleus and anulus is gone. T2-weighted midsagittal rapid spin-echo images are used to grade.
Statistical analysis
SPSS 21.0 (IBM, Version 21.0, USA) was applied to analyze the data, and the measurement data were expressed as mean ± standard deviation (x ± s). Data normality and homogeneity of variance was evaluated by Kolmogorov–Smirnov test and Levene test, respectively, and all the outcomes had normal distribution. Between-group differences in baseline characteristics were evaluated by one way ANOVA (parametric data) and Kruskal–Wallis (non-parametric data). Categorical data are expressed as frequencies (percentages), with between-group differences being analyzed using Fisher’s exact test. Two independent sample t-test is used for the comparison between the two groups, and paired t-test is used for the comparison before and after treatment in a single group. Cohen’s d test was used to calculate the effect size (ES) and it was classified as small (0.0–0.2), moderate (0.3–0.5), or large (⩾0.6). Count data were expressed as number of cases, and the χ2 test was used for comparison between groups. Spearman's correlation analysis of SCSA, DFF and Pfirrmann grading was performed. The difference was considered statistically significant at P < 0.05.
Results
-
(1)
The prevalence of sarcopenia in IDD patients was 28.3%. The difference of VAS, ODI, disc Pfirrmann grading, SCSA, DFF and NCV between the two groups were statistically significant before intervention (P < 0.05), the SCSA and DFF of paraspinal muscles in both groups correlated with the degree of IDD (r = -0.216, r = 0.430; r = -0.152, r = 0.499; r = -0.137, r = 0.435; r = -0.236, r = 0.346) (Table 1; Fig. 2a, b);
-
(2)
The improvement of SMI, GS, grip strength, VAS, SARC-F and ODI in IWS group was statistically significant after intervention (p < 0.05) (Table 2), and the ones of SMI, GS, grip strength, VAS and ODI in IWOS group were also statistically significant after intervention (P < 0.05) (Table 2); while the differences of disc Pfirrmann grading, SCSA, DFF and NCV in both groups were not statistical significant (P > 0.05) (Table 2).
-
(3)
The improvements of grip strength, GS, ODI, and SARC-F were significantly greater in the IWS group than in the IWOS group after IFC therapy, and the differences were statistically significant (P < 0.05) (Table 3).
Discussion
Patients with IDD often suffer from low back pain and neurological deficit (e.g. numbness of legs, walking dysfunction, urinary and fecal disorders) which drastically affect their quality of life. Previous studies have suggested an association between degenerative spinal diseases and skeletal muscle, the average area of paravertebral muscles in lumbar disc herniation (LDH) patients is smaller than that of healthy people, and the reduction is more significantly in those whose unilateral symptoms are the main manifestation [26,27,28,29,30,31]. Eguchi et al. demonstrated that sarcopenia is associated with spinal deformities such as degenerative lumbar scoliosis and lumbar spinal stenosis in an elderly female patients [32]. Zotti et al. suggested that preoperative atrophy and reduction in cross-sectional area of the multifidus muscle are associated with postoperative complications after lumbar decompression surgery [33]. In our study, the prevalence of sarcopenia in IDD was 28.3%, much higher than the number (9%) in general population, and also consistent with previous studies suggesting 25% in LSS [34, 35].
Interaction between IDD and sarcopenia
Sarcopenia may be promoted by IDD
Our study indicated that sarcopenia was more common in IDD patients than normal, and that SCSA and DFF in paraspinal muscles were significantly worse in IWS patients compared to IWOS patients. In addition, the SCSA and DFF were correlated with the degree of disc degeneration. We deduced that IDD may promote or contribute to the occurrence and development of sarcopenia to some extent. On one hand, IDD patients often suffer from low back pain or/and leg pain due to the compression, irritation or inflammation of nerve root, leading to chronic pain and lower extremity dysfunction, and patients with long-term activity restriction are prone to sarcopenia [36, 37]. On the other hand, the process may be accompanied by secondary changes in the structure of the spine, which in the long term leads to decompensation of the skeletal muscles (mainly paraspinal muscles), increased pain and activity dysfunction [8, 38, 39].
Sarcopenia may worsen the symptoms of IDD patients
The progression of sarcopenia could result in a decrease in the muscle mass and strength, thus reduced stability of the discs or intervertebral joints, and therefore promotes degeneration. We found that in addition to poorer SMI, GS, and grip strength, IWS patients had worse SCAS, DFF, and NCV than IWOS patients (P < 0.05; Table 2). Meanwhile, the neurological function of sarcopenic patients may be impaired, thus worsen the symptoms of the IDD, which also reflected in our study that in addition to SARC-F, the VAS and ODI in IWS group were higher than IWOS group (P < 0.05; Table 2). The above explains why IWS patients exhibit more severe pain, poorer neurological function and worse activity status. Previous studies have found that degeneration of the paravertebral muscles may play an important role in the sagittal or coronal balance of spine, meaning that the biologic load on the discs increases when spinal stability decreases, eventually leading to IDD or even LDH, causing nerve root compression, low back pain, and lower extremity dysfunction [40,41,42]. Our study also confirmed that psoas degeneration was associated with the severity of disc degeneration, and sarcopenic patients with significant psoas degeneration have more severe pain, worse mobility and function (Table 1). Furthermore, it has been found that there is a decrease in the number of Muscle Stem Cells (MSCs) or Satellite Cells (SCs) in sarcopenia patients, which are essential for the regenerative and repair capacity of skeletal muscle [43, 44]. As a result, sarcopenic patients have poorer muscle mass, strength, and physical performance. This may also explain why clinical symptoms and complications are more pronounced in IDD patients who have sarcopenia. Although the role of stem cells in the genesis of sarcopenia is still under discussion [45], kee** a healthy satellite cell population or exogenously introducing a regenerative progenitor population to aging muscle has the potential to reverse the acquired impairments brought on by sarcopenia.
IFC is effective in both IWS and IWOS patients
A lot of IWS patients may not tolerate or even refuse exercise therapy because of pain, despite the fact that resistance and aerobic exercise are suggested as the best early treatment for sarcopenia. Additionally, “bad” emotion may also prevent patients from accepting higher-intensity exercise. Physiotherapy showed increasing prospects in the treatment of skeletal muscle disorders which recent studies pointed out [13, 46,47,48,49], and our results also revealed that IFC is not only effective in IDD patients, but also be effective in sarcopenia.
Interferential current therapy is widely used by different clinicians around the world and it is one of the electrotherapy techniques for the management of musculoskeletal disorders and improvement of somatic functions [49,50,51,52,53]. IFC is the application of an alternating medium frequency current (4000 Hz) amplitude modulated at a low frequency (0-250 Hz) [13, 54]. At this frequency, IFC is claimed to have better penetration to deeper tissue while overcoming the problem of skin impedance without causing discomfort [18, 55, 56]. When applied to specific tissues, IFC improves physical function and quality of life [13] by accelerating the uptake of inflammation around nerve roots and pain-related chemokines of the degenerative disc [57, 58]. On the basis of previous studies, our study confirmed the effectiveness of IFC in IWOS patients (Table 2), that is, the body function (GS, gait speed, ODI) and pain (VAS) of IWOS patients were significantly improved after IFC therapy.
Since the discovery of electricity, it has been known that electricity stimulates nerve fibers and induce muscle contractions during physiotherapy, and low and medium waveforms can be produced by varying the frequency of the electrical current. The effect of electrical stimulation on muscles enhances local metabolism, increases neuromuscular excitability and motor neurons, promotes the growth and germination of axons and neuromuscular junction regeneration [59], thus repairs and rebuilds the damaged or atrophied denervated skeletal muscles, and improves muscle quality and strength [15, 60,61,62]. Blickenstorfer and Kampe reported that the application of electrical stimulation to some skeletal muscles could induce brain plasticity of the pain-associated sensorimotor cortex. It changes the polarization state of nerve cell membrane by activating the region, altering neuronal excitability, generating action potential, causing muscle contraction and stimulating passive movements, thus enhances muscle strength, and mitigates the effects of skeletal muscle recession [63, 64]. Nonetheless, IWS group did not exhibit significant improvement in nerve conduction after IFC therapy in our research. Although the nerve structures may be healed, neurological function was not entirely restored. This may be due to the slower pace of nerve growth and the fact that the length of the IFC in the study was less than the period needed for nerve repair.
Despite no increase in neurological function, IFC was found significantly enhanced muscle function in our study. It is said that IFC induces involuntary contraction of skeletal muscle to promote its anabolism to increase fibronectin content in muscle and maintain or improve skeletal muscle mass and strength [46, 65,66,67,68,69,70]. An in-depth study found that it may reduce the concentration of calcium in mitochondria and calcium overload by increasing the activity of Na+-K+-ATPase and Ca2+-Mg2+-ATPase, thus stimulates oxidative phosphorylation, which plays a central role in reducing fatigue and improving muscle function [71, 72]. This conclusion was also supported by our investigation, where skeletal muscle index, grip strength, gait speed, ODI and SARC-F were improved significantly after intervention in IWS group (Table 2). At the same time, the skeletal muscle index, grip strength, gait speed, and ODI of the IWOS group also showed significant improvement after IFC therapy (Table 2). Among them, the skeletal muscle index intuitively reflects the mass of the skeletal muscles of the whole body, grip strength reflects skeletal muscle strength, while GS, ODI and SARC-F strongly reflect skeletal muscle-based physical functions. So the significant changes of the above indexes in the two groups after IFC therapy confirm the improvement of muscle function and the effectiveness of IFC therapy for sarcopenia. Interestingly, in IWOS patients, the SARC-F, which is an important scale evaluating physical function for screening sarcopenia, did not display statistical differences after the IFC therapy (Table 2). Therefore, the differences between before and after therapy of the two groups suggested that there was a close connection between sarcopenia and physical function, meaning that skeletal muscle function may play an important role in ensuring somatic function. Previous studies have made similar conclusions, explaining that deep muscles contribute to joint stability and help maintain posture; thus, dysfunction of deep muscles is significantly associated with increased risk of falls and decreased walking function [73,74,75].
A closer analysis revealed that the improvements of grip strength, GS, ODI, and SARC-F were significantly greater in IWS patients than IWOS patients after IFC therapy, and the differences were statistically significant (P < 0.05) (Table 3). It implied that IFC stimulation significantly improved skeletal muscle strength, function and body performance in sarcopenic patients although no differences were found in improving skeletal muscle quality, but this could still indicate that IFC has a certain effect on sarcopenia. Unfortunately, we were unable to find statistical differences in SCAS and DFF before and after IFC therapy between the two groups (Table 2), which may be caused by the fact that only the SCAS and DFF of the paravertebral muscles at the L4/5 plane were examined in this study. In addition, we cannot deny the impact of shorter IFC treatment cycles and follow-up times. All those may lead to the inability to observe significant statistical differences in SCAS and DFF of the two groups before and after IFC therapy. Therefore, the observation of the efficacy of these two measures requires longer periods and further studies. What’s more, the exact mechanism of action of IFC therapy is not known and the means to identify it remain to be developed.
Limitation
The research results reported here should be considered in the light of some limitations. Although there was no significant difference in age, gender, BMI, smoking, and osteoporosis between the two groups (Table 4), all the patients in our study are Asian, so the influence of race was exclueded. However, nutrition, inflammation and living habit such as diet and sleep, which have impact on sarcopenia could not be standardized. Apart from this, this retrospective study failed to indicate the optimal cycle and frequency of IFC for IDD with sarcopenia patients due to socio-economic factors and patient-specific circumstances (e.g., disparities in accessibility to healthcare, and educational and patient's compliance), although this may have an important impact on neurological recovery and skeletal muscle growth. Additionally, only L4/5 paravertebral muscles were examined in our study, and errors in the measurement process can not be avoided totally. Meanwhile, the long-term effects of IFC on sarcopenia or IDD remain unknown due to follow-up limitations. These are the sources of limitations and biases in the study. Therefore, in future studies, it may be necessary to further investigate the long-term effects of interferential currents, conduct randomized controlled trials, or explore other potential interventions for disc degeneration and sarcopenia.
Conclusion
The prevalence of sarcopenia was high in IDD patients, and the degree of paravertebral muscles degeneration was correlated with the degree of IDD. Compared with IWOS patients, IWS patients have more severe pain, poorer mobility, and worse neurological function. After IFC therapy, both IWS and IWOS patients had effective symptom relief. The improvement of skeletal muscle strength and function in IWS patients was significantly greater. Therefore, IFC stimulation is effective in elderly IDD patients with or without sarcopenia.
Availability of data and materials
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- IDD:
-
Intervertebral disc degeneration
- LBP:
-
Lower back pain
- LSS:
-
Lumbar spinal stenosis
- IFC:
-
Interferential currents
- IWS:
-
Intervertebral disc degeneration with sarcopenia
- IWOS:
-
Intervertebral disc degeneration without sarcopenia
- MRI:
-
Magnetic resonance imaging
- VAS:
-
Visual analogue scale
- ODI:
-
Oswestry dysfunction index
- SMI:
-
Skeletal muscle index
- GS:
-
Gait speed
- NCV:
-
Nerve conduction velocity
- BIA:
-
Bioelectrical impedance analysis
- AMM:
-
Appendicular skeletal muscle mass
- AWGS2019:
-
Asian Working Group for Sarcopenia 2019
- SCSA:
-
Standard cross-sectional area
- DFF:
-
Degree of fatty infiltration
- LDH:
-
Lumbar disc herniation
- MSCs:
-
Muscle Stem Cells
- SCs:
-
Satelite Cells
References
Zhang Y, Yang J, Zhou X, Wang N, Li Z, Zhou Y, Feng J, Shen D, Zhao W. Knockdown of miR-222 inhibits inflammation and the apoptosis of LPS-stimulated human intervertebral disc nucleus pulposus cells. Int J Mol Med. 2019;44(4):1357–65. https://doi.org/10.3892/ijmm.2019.4314. Epub 2019 Aug 16.
Chang Y, Yang M, Ke S, Zhang Y, Xu G, Li Z. Effect of platelet-rich plasma on intervertebral disc degeneration in vivo and in vitro: a critical review. Oxid Med Cell Longev. 2020;2020(21):8893819. https://doi.org/10.1155/2020/8893819.
Walston JD. Sarcopenia in older adults. Curr Opin Rheumatol. 2012;24(6):623–7. https://doi.org/10.1097/BOR.0b013e328358d59b.
Tay L, Ding YY, Leung BP, Ismail NH, Yeo A, Yew S, Tay KS, Tan CH, Chong MS. Sex-specific differences in risk factors for sarcopenia amongst community-dwelling older adults. Age (Dordr). 2015;37(6):121. https://doi.org/10.1007/s11357-015-9860-3.
Silva RF, Figueiredo MDLF, Darder JJT, Santos AMRD, Tyrrell MAR. Sarcopenia screening in elderly in primary health care: nurse knowledge and practices. Rev Bras Enferm. 2020;73(suppl 3):e20200421. https://doi.org/10.1590/0034-7167-2020-0421. English, Portuguese.
Fatoye F, Gebrye T, Odeyemi I. Real-world incidence and prevalence of low back pain using routinely collected data. Rheumatol Int. 2019;39(4):619–26. https://doi.org/10.1007/s00296-019-04273-0.
Muraki S, Oka H, Akune T, Mabuchi A, En-Yo Y, Yoshida M, Saika A, Suzuki T, Yoshida H, Ishibashi H, Yamamoto S, Nakamura K, Kawaguchi H, Yoshimura N. Prevalence of radiographic lumbar spondylosis and its association with low back pain in elderly subjects of population-based cohorts: the ROAD study. Ann Rheum Dis. 2009;68(9):1401–6. https://doi.org/10.1136/ard.2007.087296.
Wu WT, Lee TM, Han DS, Chang KV. The prevalence of sarcopenia and its impact on clinical outcomes in lumbar degenerative spine disease-a systematic review and meta-analysis. J Clin Med. 2021;10(4):773. https://doi.org/10.3390/jcm10040773.
Rangel EL, Rios-Diaz AJ, Uyeda JW, Castillo-Angeles M, Cooper Z, Olufajo OA, Salim A, Sodickson AD. Sarcopenia increases risk of long-term mortality in elderly patients undergoing emergency abdominal surgery. J Trauma Acute Care Surg. 2017;83(6):1179–86. https://doi.org/10.1097/TA.0000000000001657.
Hajibandeh S, Hajibandeh S, Jarvis R, Bhogal T, Dalmia S. Meta-analysis of the effect of sarcopenia in predicting postoperative mortality in emergency and elective abdominal surgery. Surgeon. 2019;17(6):370–80. https://doi.org/10.1016/j.surge.2018.09.003.
Hsu J, Krishnan A, Lin CT, Shah PD, Broderick SR, Higgins RSD, Merlo CA, Bush EL. Sarcopenia of the psoas muscles is associated with poor outcomes following lung transplantation. Ann Thorac Surg. 2019;107(4):1082–8. https://doi.org/10.1016/j.athoracsur.2018.10.006.
Dias LV, Cordeiro MA, de Schmidt Sales R, Dos Santos MMBR, Korelo RIG, Vojciechowski AS, de Mace do ACB. Immediate analgesic effect of transcutaneous electrical nerve stimulation (TENS) and interferential current (IFC) on chronic low back pain: Randomised placebo-controlled trial. J Bodyw Mov Ther. 2021;27:181–90. https://doi.org/10.1016/j.jbmt.2021.03.005.
Fuentes JP, Armijo Olivo S, Magee DJ, Gross DP. Effectiveness of interferential current therapy in the management of musculoskeletal pain: a systematic review and meta-analysis. Phys Ther. 2010;90(9):1219–38. https://doi.org/10.2522/ptj.20090335.
Wang XJ, He NN, Ji WB, Yu L, Zhang P. Effect of penetration electroacupuncture combined with intermediate frequency electrotherapy, facial acupoint massage, and cervical reduction on facial nerve function and curative effect of senile refractory facial paralysis. J Healthc Eng. 2021;2021:3776006. https://doi.org/10.1155/2021/3776006.
Endo A, Yakabi A, Kubo A. Effect of interferential current on deep abdominal muscle thickness. J Phys Ther Sci. 2022;34(4):306–10. https://doi.org/10.1589/jpts.34.306.
Tanaka M, Hirayama Y, Fujita N, Fu**o H. Comparison of premodulated interferential and pulsed current electrical stimulation in prevention of deep muscle atrophy in rats. J Mol Histol. 2013;44(2):203–11. https://doi.org/10.1007/s10735-012-9473-4.
Nemec H. Interferential therapy: a new approach in physical medicine. Br J Physiother. 1959;12:9–12.
Beatti A, Rayner A, Chipchase L, Souvlis T. Penetration and spread of interferential current in cutaneous, subcutaneous and muscle tissues. Physiotherapy. 2011;97(4):319–26. https://doi.org/10.1016/j.physio.2011.01.008.
Rajfur J, Pasternok M, Rajfur K, Walewicz K, Fras B, Bolach B, Dymarek R, Rosinczuk J, Halski T, Taradaj J. Efficacy of selected electrical therapies on chronic low back pain: a comparative clinical pilot study. Med Sci Monit. 2017;23:85–100. https://doi.org/10.12659/msm.899461.
Johnson MI, Tabasam G. A single-blind investigation into the hypoalgesic effects of different swing patterns of interferential currents on cold-induced pain in healthy volunteers. Arch Phys Med Rehabil. 2003;84(3):350–7. https://doi.org/10.1053/apmr.2003.50005.
Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, Jang HC, Kang L, Kim M, Kim S, Kojima T, Kuzuya M, Lee JSW, Lee SY, Lee WJ, Lee Y, Liang CK, Lim JY, Lim WS, Peng LN, Sugimoto K, Tanaka T, Won CW, Yamada M, Zhang T, Akishita M, Arai H. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020;21(3):300-307.e2. https://doi.org/10.1016/j.jamda.2019.12.012.
Malmstrom TK, Morley JE. SARC-F: a simple questionnaire to rapidly diagnose sarcopenia. J Am Med Dir Assoc. 2013;14(8):531–2. https://doi.org/10.1016/j.jamda.2013.05.018.
Fairbank JC, Pynsent PB. The Oswestry disability index. Spine (Phila Pa 1976). 2000;25(22):2940–52. https://doi.org/10.1097/00007632-200011150-00017. discussion 2952.
Tabaraee E, Ahn J, Bohl DD, et al. Quantification of multifidus atrophy and fatty infiltration following a minimally invasive microdiscectomy. Int J Spine Surg. 2015;9:25. https://doi.org/10.14444/2025.
Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976). 2001;26(17):1873–8. https://doi.org/10.1097/00007632-200109010-00011.
Yaltırık K, Güdü BO, Işık Y, Altunok Ç, Tipi U, Atalay B. Volumetric muscle measurements indicate significant muscle degeneration in single-level disc herniation patients. World Neurosurg. 2018;116:e500–4. https://doi.org/10.1016/j.wneu.2018.05.019.
Chen WY, Wang K, Yuan WA, Zhan HS. Relationship between lumbosacral multifidus muscle and lumbar disc herniation. Zhongguo Gu Shang. 2016;29(6):581–4 Chinese PMID: 27534095.
Park MS, Moon SH, Kim TH, Oh J, Lee SJ, Chang HG, Shin JH. Paraspinal muscles of patients with lumbar diseases. J Neurol Surg A Cent Eur Neurosurg. 2018;79(4):323–9. https://doi.org/10.1055/s-0038-1639332.
Stanuszek A, Jędrzejek A, Gancarczyk-Urlik E, Kołodziej I, Pisarska-Adamczyk M, Milczarek O, Trompeta J, Chrobak W. Preoperative paraspinal and psoas major muscle atrophy and paraspinal muscle fatty degeneration as factors influencing the results of surgical treatment of lumbar disc disease. Arch Orthop Trauma Surg. 2022;142(7):1375–84. https://doi.org/10.1007/s00402-021-03754-x.
Dangaria TR, Naesh O. Changes in cross-sectional area of psoas major muscle in unilateral sciatica caused by disc herniation. Spine (Phila Pa 1976). 1998;23(8):928–31. https://doi.org/10.1097/00007632-199804150-00016.
Hiyama A, Katoh H, Sakai D, Sato M, Tanaka M, Nukaga T, Watanabe M. Correlation analysis of sagittal alignment and skeletal muscle mass in patients with spinal degenerative disease. Sci Rep. 2018;8(1):15492. https://doi.org/10.1038/s41598-018-33867-0.
Eguchi Y, Suzuki M, Yamanaka H, Tamai H, Kobayashi T, Orita S, Yamauchi K, Suzuki M, Inage K, Fujimoto K, Kanamoto H, Abe K, Aoki Y, Toyone T, Ozawa T, Takahashi K, Ohtori S. Associations between sarcopenia and degenerative lumbar scoliosis in older women. Scoliosis Spinal Disord. 2017;12:9. https://doi.org/10.1186/s13013-017-0116-0.
Zotti MGT, Boas FV, Clifton T, Piche M, Yoon WW, Freeman BJC. Does pre-operative magnetic resonance imaging of the lumbar multifidus muscle predict clinical outcomes following lumbar spinal decompression for symptomatic spinal stenosis? Eur Spine J. 2017;26(10):2589–97. https://doi.org/10.1007/s00586-017-4986-x.
Papadopoulou SK, Tsintavis P, Potsaki P, Papandreou D. Differences in the prevalence of sarcopenia in community-dwelling, nursing home and hospitalized individuals. A systematic review and meta-analysis. J Nutr Health Aging. 2020;24(1):83–90. https://doi.org/10.1007/s12603-019-1267-x.
Cruz-Jentoft AJ, Landi F, Schneider SM, Zúñiga C, Arai H, Boirie Y, Chen LK, Fielding RA, Martin FC, Michel JP, Sieber C, Stout JR, Studenski SA, Vellas B, Woo J, Zamboni M, Cederholm T. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing. 2014;43(6):748–59. https://doi.org/10.1007/s12603-019-1267-x.
Wiedmer P, Jung T, Castro JP, Pomatto LCD, Sun PY, Davies KJA, Grune T. Sarcopenia - molecular mechanisms and open questions. Ageing Res Rev. 2021;65:101200. https://doi.org/10.1016/j.arr.2020.101200.
Chang KV, Chen YC, Wu WT, Shen HJ, Huang KC, Chu HP, Han DS. Expression of telomeric repeat-containing rna decreases in sarcopenia and increases after exercise and nutrition intervention. Nutrients. 2020;12(12):3766. https://doi.org/10.3390/nu12123766.
Gibbons D, Ahern DP, Curley AE, Kepler CK, Butler JS. Impact of sarcopenia on degenerative lumbar spondylosis. Clin Spine Surg. 2021;34(2):43–50. https://doi.org/10.1097/BSD.0000000000001047.
Hiyama A, Katoh H, Sakai D, Tanaka M, Sato M, Watanabe M. The correlation analysis between sagittal alignment and cross-sectional area of paraspinal muscle in patients with lumbar spinal stenosis and degenerative spondylolisthesis. BMC Musculoskelet Disord. 2019;20(1):352. https://doi.org/10.1186/s12891-019-2733-7.
Panjabi M, Abumi K, Duranceau J, Oxland T. Spinal stability and intersegmental muscle forces. A biomechanical model. Spine (Phila Pa 1976). 1989;14(2):194–200. https://doi.org/10.1097/00007632-198902000-00008.
Bergmark A. Stability of the lumbar spine. A study in mechanical engineering. Acta Orthop Scand Suppl. 1989;230:1–54. https://doi.org/10.3109/17453678909154177.
Ranger TA, Cicuttini FM, Jensen TS, Peiris WL, Hussain SM, Fairley J, Urquhart DM. Are the size and composition of the paraspinal muscles associated with low back pain? A systematic review. Spine J. 2017;17(11):1729–48. https://doi.org/10.1016/j.spinee.2017.07.002.
Sousa-Victor P, Muñoz-Cánoves P. Regenerative decline of stem cells in sarcopenia. Mol Aspects Med. 2016;50:109–17. https://doi.org/10.1016/j.mam.2016.02.002.
Muñoz-Cánoves P, Neves J, Sousa-Victor P. Understanding muscle regenerative decline with aging: new approaches to bring back youthfulness to aged stem cells. FEBS J. 2020;287(3):406–16. https://doi.org/10.1111/febs.15182.
Snijders T, Parise G. Role of muscle stem cells in sarcopenia. Curr Opin Clin Nutr Metab Care. 2017;20(3):186–90. https://doi.org/10.1097/MCO.0000000000000360.
Sillen MJ, Franssen FM, Gosker HR, Wouters EF, Spruit MA. Metabolic and structural changes in lower-limb skeletal muscle following neuromuscular electrical stimulation: a systematic review. PLoS ONE. 2013;8(9):e69391. https://doi.org/10.1371/journal.pone.0069391.
Gerovasili V, Stefanidis K, Vitzilaios K, Karatzanos E, Politis P, Koroneos A, Chatzimichail A, Routsi C, Roussos C, Nanas S. Electrical muscle stimulation preserves the muscle mass of critically ill patients: a randomized study. Crit Care. 2009;13(5):R161. https://doi.org/10.1186/cc8123.
Bellew JW, Sanders K, Schuman K, Barton M. Muscle force production with low and medium frequency burst modulated biphasic pulsed currents. Physiother Theory Pract. 2014;30(2):105–9. https://doi.org/10.3109/09593985.2013.823582.
Dirks ML, Wall BT, van Loon LJC. Interventional strategies to combat muscle disuse atrophy in humans: focus on neuromuscular electrical stimulation and dietary protein. J Appl Physiol (1985). 2018;125(3):850–61. https://doi.org/10.1152/japplphysiol.00985.2016.
Lindsay DM, Dearness J, McGinley CC. Electrotherapy usage trends in private physiotherapy practice in Alberta. Physiother Can. 1995;47(1):30–4. PMID: 10140421. Available from: https://pubmed.ncbi.nlm.nih.gov/10140421/.
Flynn DM. Chronic musculoskeletal pain: nonpharmacologic noninvasive treatments. Am Fam Physician. 2020;102(8):465–77 PMID: 33064421.
Suh HR, Han HC, Cho HY. Immediate therapeutic effect of interferential current therapy on spasticity, balance, and gait function in chronic stroke patients: a randomized control trial. Clin Rehabil. 2014;28(9):885–91. https://doi.org/10.1177/0269215514523798.
Moore JS, Gibson PR, Burgell RE. Neuromodulation via interferential electrical stimulation as a novel therapy in gastrointestinal motility disorders. J Neurogastroenterol Motil. 2018;24(1):19–29. https://doi.org/10.5056/jnm17071.
Goats GC. Interferential current therapy. Br J Sports Med. 1990;24(2):87–92. https://doi.org/10.1136/bjsm.24.2.87.
Poitras S, Brosseau L. Evidence-informed management of chronic low back pain with transcutaneous electrical nerve stimulation, interferential current, electrical muscle stimulation, ultrasound, and thermotherapy. Spine J. 2008;8(1):226–33. https://doi.org/10.1016/j.spinee.2007.10.022.
Ng RT, Lee WS, Ang HL, Teo KM, Yik YI, Lai NM. Transcutaneous electrical stimulation (TES) for treatment of constipation in children. Cochrane Database Syst Rev. 2016;7(7):CD010873. https://doi.org/10.1002/14651858.CD010873.pub2.
Koca I, Boyaci A, Tutoglu A, Ucar M, Kocaturk O. Assessment of the effectiveness of interferential current therapy and TENS in the management of carpal tunnel syndrome: a randomized controlled study. Rheumatol Int. 2014;34(12):1639–45. https://doi.org/10.1007/s00296-014-3005-3.
Suriya-amarit D, Gaogasigam C, Siriphorn A, Boonyong S. Effect of interferential current stimulation in management of hemiplegic shoulder pain. Arch Phys Med Rehabil. 2014;95(8):1441–6. https://doi.org/10.1016/j.apmr.2014.04.002.
Brierley SM, Linden DR. Neuroplasticity and dysfunction after gastrointestinal inflammation. Nat Rev Gastroenterol Hepatol. 2014;11(10):611–27. https://doi.org/10.1038/nrgastro.2014.103.
Kang HW, Kim HJ, Kim WY, Min WK, Min TJ, Lee YS, Kim JH. Effects of cranial electrotherapy stimulation on preoperative anxiety and blood pressure during anesthetic induction in patients with essential hypertension. J Int Med Res. 2020;48(8):300060520939370. https://doi.org/10.1177/0300060520939370.
Ferrari G, Colucci A, Barbariga M, Ruggeri A, Rama P. High frequency electrotherapy for the treatment of meibomian gland dysfunction. Cornea. 2019;38(11):1424–9. https://doi.org/10.1097/ICO.0000000000002063.
Song D, Ma Y, Zhang L, Ma Q. Intermediate frequency electrotherapy stimulation to the medial femoris muscle for functional recovery of knee joint after anterior cruciate ligament reconstruction. Pak J Med Sci. 2022;38(3Part-I):652–6. https://doi.org/10.12669/pjms.38.3.5298.
Kampe KK, Jones RA, Auer DP. Frequency dependence of the functional MRI response after electrical median nerve stimulation. Hum Brain Mapp. 2000;9(2):106–14. https://doi.org/10.1002/(sici)1097-0193(200002)9:2%3c106::aid-hbm5%3e3.0.co;2-y.
Blickenstorfer A, Kleiser R, Keller T, Keisker B, Meyer M, Riener R, Kollias S. Cortical and subcortical correlates of functional electrical stimulation of wrist extensor and flexor muscles revealed by fMRI. Hum Brain Mapp. 2009;30(3):963–75. https://doi.org/10.1002/hbm.20559.
Geney-Castro DE, Vanegas-Muñóz J, Plata-Contreras J, Salinas-Duran F. Medial femoral cutaneous nerve conduction study with distal recording: A novel technique. Muscle Nerve. 2020;61(3):383–6. https://doi.org/10.1002/mus.26788.
Frazer LL, Santschi EM, Fischer KJ. Stimulation of subchondral bone cyst healing by placement of a transcondylar screw in the equine medial femoral condyle. Vet Surg. 2019;48(7):1194–203. https://doi.org/10.1111/vsu.13247.
Koopman R, van Loon LJ. Aging, exercise, and muscle protein metabolism. J Appl Physiol (1985). 2009;106(6):2040–8. https://doi.org/10.1152/japplphysiol.91551.2008.
Bamman MM, Clarke MS, Feeback DL, Talmadge RJ, Stevens BR, Lieberman SA, Greenisen MC. Impact of resistance exercise during bed rest on skeletal muscle sarcopenia and myosin isoform distribution. J Appl Physiol (1985). 1998;84(1):157–63. https://doi.org/10.1152/jappl.1998.84.1.157.
Oates BR, Glover EI, West DW, Fry JL, Tarnopolsky MA, Phillips SM. Low-volume resistance exercise attenuates the decline in strength and muscle mass associated with immobilization. Muscle Nerve. 2010;42(4):539–46. https://doi.org/10.1002/mus.21721.
Alkner BA, Norrbrand L, Tesch PA. Neuromuscular adaptations following 90 days bed rest with or without resistance exercise. Aerosp Med Hum Perform. 2016;87(7):610–7. https://doi.org/10.3357/AMHP.4383.2016.
Larsen AE, Tunstall RJ, Carey KA, Nicholas G, Kambadur R, Crowe TC, Cameron-Smith D. Actions of short-term fasting on human skeletal muscle myogenic and atrogenic gene expression. Ann Nutr Metab. 2006;50(5):476–81. https://doi.org/10.1159/000095354.
Alway SE, Siu PM. Nuclear apoptosis contributes to sarcopenia. Exerc Sport Sci Rev. 2008;36(2):51–7. https://doi.org/10.1097/JES.0b013e318168e9dc.
Ikezoe T, Mori N, Nakamura M, Ichihashi N. Atrophy of the lower limbs in elderly women: is it related to walking ability? Eur J Appl Physiol. 2011;111(6):989–95. https://doi.org/10.1007/s00421-010-1728-8.
França FR, Burke TN, Hanada ES, Marques AP. Segmental stabilization and muscular strengthening in chronic low back pain: a comparative study. Clinics (Sao Paulo). 2010;65(10):1013–7. https://doi.org/10.1590/s1807-59322010001000015.
Pizzigalli L, Filippini A, Ahmaidi S, Jullien H, Rainoldi A. Prevention of falling risk in elderly people: the relevance of muscular strength and symmetry of lower limbs in postural stability. J Strength Cond Res. 2011;25(2):567–74. https://doi.org/10.1519/JSC.0b013e3181d32213.
Acknowledgements
The authors thank the Second **angya Hospital of Central South University for providing patients information.
Funding
This study was supported by Fundamental Research Funds for the Central Universities of Central South University Foundation (Grant No.2022ZZTS0260) and Hunan Provincial Innovation Foundation For Postgraduate (Grant No. CX20220342).
Author information
Authors and Affiliations
Contributions
Hui Yuan: Writing-original draft; Lini Dong: Screening and study participants; Ou Zhang:Provided facilities/equipment and consultation; **aoxiao Wang: Measurement and Data collection; Zejun Chen: Resources and Investigation; Yunchao Li: Methodology and Data statistics; Haoyu He: Data Curation and Validation; Guohua Lϋ: Supervision and Project management; Lei Kuang and **g Li: Instruction and Revision. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was performed in line with the principles of the Declaration of Helsinki. The study was approved by the Institutional Research and Medical Ethics Committee of Second **angya Hospital of Central South University (Ethical review number: 744/2021), and patients’ written informed consent have been obtained.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yuan, H., Dong, L., Zhang, O. et al. A comparison of interferential current efficacy in elderly intervertebral disc degeneration patients with or without sarcopenia: a retrospective study. BMC Musculoskelet Disord 25, 214 (2024). https://doi.org/10.1186/s12891-024-07337-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12891-024-07337-w