Abstract
Background
Postmenopausal osteoporosis (PMO) is the most common type of primary osteoporosis. ESR1 polymorphism rs2234693 and rs9340799 has been widely studied as a candidate gene associated with PMO, however, the findings were inconclusive. The present study aims to explore the relationship of ESR1 polymorphism rs2234693 and rs9340799 with PMO risk in a Chinese Han population.
Methods
PMO patients and healthy controls were recruited from gynecology department. DNA of all participants were extracted from the peripheral blood samples and genotyped by Mass Array method. A meta-analysis of case control studies was also conducted to further elucidate the relationship of polymorphism with PMO.
Results
Our results revealed that there were no associations of rs2234693 with PMO. However, GG genotype of rs9340799 was associated with a higher risk of PMO (OR = 1.51, 95%CI:1.08–4.34, p = 0.03), even adjusting for risk factors (OR = 1.83, 95%CI: 1.12–5.04, p = 0.04). Logistic regression analysis showed that dominant model was associated with a higher risk of PMO (OR = 2.07, 95%CI: 1.02–5.16, p = 0.02) after correcting the risk factors (OR = 2.14, 95%CI:1.12–5.64, p = 0.04); In addition, the Meta-analysis results revealed that both two polymorphisms were not associated with PMO.
Conclusions
In conclusion, ESR1 polymorphism rs9340799 was associated with PMO. However, well designed studies with larger sample sizes are required to further elucidate the associations.
Similar content being viewed by others
Background
Postmenopausal osteoporosis (PMO) caused by estrogen deficiency, is the most common type of primary osteoporosis which affects more than 40% of postmenopausal women [1,2,3]. As a typical senile disorder with decreasing bone-mineral density (BMD) and microstructural abnormality, PMO may lead to an increased risk for nonstress fractures [4]. Although, PMO is close related to estrogen levels following menopause, many factors, such as genetic elements had play important roles in the risk of osteoporosis [5, 6]. Among these factors, genetics are found to play a pivotal role in the occurrence of osteoporosis and have received highly attention [6]. Twin studies in adult Caucasian woman revealed that the heritability of BMD might be between 50 and 85% [7]. Genome-wide association study also reported that almost 400 single nucleotide polymorphisms (SNPs) distributed in more than 150 different loci, were associated with low BMD and osteoporosis [8].
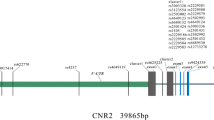
Estrogen activity is modulated through estrogen receptor α (ER-α) and β (ER-β) which are encoded by ESR1 on chromosome 6q25.1 and ESR2 on chromosome 14q23.2 respectively [9, 10]. Both ER-α and ER-β isoforms are expressed in osteoblasts, osteoclasts, and bone marrow stromal cells [11, 12]. However, ESR1 is the major mediator of estrogen action in bone and has been widely studied as a candidate gene associated with PMO [13]. Two polymorphisms rs2234693 and rs9340799 had been reported to close related with PMO, however, these findings were inconclusive [14,15,16]. Mondockova et al. had reported that the rs9340799 was significantly associated with BMD at the femoral neck [17], whereas, another study by tanriover et al. had showed that no relationship of the two genes with PMO [18]. In addition, another study by Tang et al. in a meta-analysis showed that the ESR1 rs2234693 T allele may increase the risk of hip fracture, but the rs9340799 polymorphism was not associated with hip fracture [19]. Thus, to draw a more precise association of ESR1 polymorphism (rs2234693 and rs9340799) with the risk of PMO, we sought to assess the impact of ESR1 polymorphism with PMO and determine a possible association in postmenopausal Chinese women in a case-control study.
Methods
A total of 380 unrelated postmenopausal women over 45 years old were recruited from the outpatient of **
Fasting blood samples of each participants were collected and stored at − 80 °C. A commercial kit (Qiagen, Hilden, Germany) was used to extracted the genomic DNA and the genetic polymorphism was identified by the Agena Mass ARRAY system (Agena/Sequenom Inc.) followed the manufacturer’s manual.
Meta-analysis
Electronic databases including PubMed, ISI Web of Science, National Knowledge Infrastructure (CNKI), and Wanfang Data were searched. MeSH and title/abstract were used for all eligible studies search. Studies included in our meta-analysis need to satisfy the following criteria: (1) studies that conducted in human subjects; (2) sufficient data provided for calculating the crude odds ratios (ORs) and 95% confidence intervals (95% CIs). Correspondingly, the exclusion criteria were those studies without detailed genotype data or reported with overlap** data.
Statistical analysis
All statistical analyses were performed by SPSS (version 18.0, SPSS Inc., Chicago, USA). Hardy–Weinberg equilibrium (HWE) was examined using the χ2 test. Demographics variables and genotype frequencies between groups were evaluated by student’s t test (for continuous variables) or Chi-squared test (for categorical variables). In addition, multiple logistic regression analyses were also performed to assess the association between osteoporosis as an outcome and risk factors including the ESR1 polymorphism. Confounding factors were also calculated, including age, gender, BMI, smoking status and drinking habit.
In Meta-analysis, STATA (version 12.0) was employed to make a pooled analysis. Heterogeneity was evaluated by I2 statistic. If I2 < 50%, the fixed effect model was used, otherwise, the random-effect model was adopted to calculate the pooled ORs. Pooled ORs and 95% CIs were calculated under the following genetic models: (1) allele, (2) recessive, (3) homozygous, (4) heterozygous, and (5) dominant. Publication bias was assessed by Begg’s funnel plots. If P-value < 0.05 was considerate to be with significant difference.
Results
Characteristics of the study participants
A total of 230 patients and 150 control participants were recruited for this study. All patients were Chinese with confirmed DXA for diagnosis of PMO. There was significant difference of BMD of total hip T-score between patients and control group (p < 0.01). No deviation from the HWE was observed (HWE = 0.97) in the control groups. In addition, no significant difference of smoking and drinking ratio were observed between patients and control group. The demographic and clinical characteristics of the participants were shown in Table 1.
Genetics association analysis
Genotype distribution of ESR1 gene polymorphisms (rs2234693 and rs9340799) are showed in Table 2. No significant differences were observed between patients and controls in the distribution of TT, TC, and CC genotypes of rs2234693 (p = 0.29). Whereas, there was a significant difference in the distribution of AA, AG and GG genotypes of rs9340799(p = 0.043).
To further elucidated the relationship of the polymorphism with PMO, a logistic regression analysis was also performed. We found that patients with GG allele of rs9340799 were significantly correlated with PMO morbidity (OR = 1.51, 95%CI:1.08–4.34, p = 0.03). After adjusting for age, BMI, smoking and drinking habits, there was still significant association of GG genotype (OR = 1.83, 95%CI: 1.12–5.04, p = 0.04) with PMO (Table 3), whereas no significant difference was observed under the AG genotype (p > 0.05); In addition, no significant association was observed in rs2234693 with PMO.
We also found a significant association of rs9340799 with PMO under the dominant model (OR = 2.07, 95%CI: 1.02–5.16, p = 0.02) even after adjusting for the risk factors (OR = 2.14, 95%CI:1.12–5.64, p = 0.04). Nevertheless, no significant association of rs2234693 polymorphism with PMO was observed under dominant and recessive genetic model (Table 4).
Meta-analysis results
A meta-analysis was also employed to further explore the relationship of ESR1 polymorphism with PMO. A total of 4 articles (including our results) were enrolled in the analysis (Fig. 1). The study characteristics were listed in Table 5. The random effect model was employed under the dominant, recessive, homozygous, heterozygous and allelic model for rs2234693. Whereas, for rs9340799, only dominant model was found with heterogeneity, thus the fixed model was used in the other four genetic models. The meta-analysis results showed that there were no association of rs2234693 and rs9340799 with PMO (p > 0.05). (Fig. 2 and Fig. 3). Publication bias evaluated by using Begg’s test show that no significant publication bias exists (p > 0.05) (Fig. 4).
Discussion
Osteoporosis is a multifactorial disease, characterized by loss of tissue microarchitecture and low BMD. It has been estimated that 30% of women and 12% of men were affected by osteoporosis [8, 22]. The most important adverse health outcome of osteoporosis is bone fractures. Women at postmenopausal stage are faced to extremely high risk of osteoporosis [23,24,25]. Several candidate genes, such as ESR1, the major mediator of estrogen action in bone, have been reported to be associated with BMD and osteoporosis [26,27,28]. Ioannidis et al. had revealed that ESR1 is a susceptibility gene for fractures [29]. However, the polymorphism of ESR1 with PMO was still inconclusive.
Studies to elucidate the ESR1 genetic contributions to PMO have continued for several decades. To a much lesser extent, the association between BMD and a polymorphism in the promoter region of ESR1, characterized by a variable number of studies and still not come to a unified conclusion. Mondockova et al. had found that rs9340799 polymorphism may contribute to decreased BMD in postmenopausal women in southern Slovakia [14]. Nevertheless, Wang et al. had showed that rs2234693 polymorphism but not rs9340799 was associated with PMO [23]. And Kurt et al. showed that both rs9340799 and rs2234693 polymorphism were contribute to the determination of bone mineral density in Turkish postmenopausal women [30]. In our study, we had found that the GG genotype and the dominant genetic model of rs9340799 were susceptible to PMO, whereas, no relationship was found in rs2234693. These results were partly in accordance with former studies.
Although our case-control study had got the positive conclusions of rs9340799 polymorphism with PMO, these results should be treated with caution. A meta-analysis was also conducted to further elucidate the relationship of disease and polymorphism. Our meta-analysis of pooled analysis had showed that either homozygote, heterozygote, recessive, allelic models or dominant of rs9340799 and rs2234693 were the risk factor of PMO. These may be attributed to a small sample size, different ethnic background and different examine methods. These data need to be replicated in a larger cohort, and functional studies will be necessary to investigate whether and how ESR1 gene polymorphism involved in the pathogenesis of PMO.
The rs2234693 and rs9340799 polymorphic sites are located in the promoter region of the first intron of ESR1 gene, and so far, their functional consequences are unknown [21]. Although case-control study had partly revealed the relationship of the polymorphism, its mechanism is still not clear. We speculate that introns may contain regulatory elements, and the mutation may cause methylation and finally influence the effect of ESR1.
The results of our study may help in identifying patients with potential PMO risk; however, several limitations should not be ignored. Firstly, as the quantity of the patients were not large enough, thus give rise to failure to achieve statistical significance of rs2234693. In addition, there was significant difference under BMI in the baseline characteristic, this may cause the bias to the results. Secondly, all participants enrolled were recruited from hospital which might result in potential selection bias. Thirdly, some potential confounding factors which may overestimate or underestimate the effect of gene polymorphism. Eventually, the meta-analysis was only enrolled 4 relative studies, this may give rise to publication bias and finally influence the overall results.
Conclusion
We had reported a significantly correlation of ESR1 genotype distribution with PMO in a Chinese Han population. This result was partly in accordance with the meat-analysis results. However, this is only a preliminary conclusion, a larger cohort study and functional studies will be necessary to investigate whether and how the polymorphism might involve in the pathogenesis of PMO.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due to potential for individual and organizational privacy to be compromised. Reasonable requests for parts of the data will be considered by the corresponding author.
Abbreviations
- PMO:
-
Postmenopausal osteoporosis
- BMD:
-
Bone-mineral density
- DXA:
-
Dual energy x-ray absorptiometry
- HRT:
-
Hormone replacement therapy
- BMI:
-
Body mass index
- HWE:
-
Hardy–Weinberg equilibrium
- 95% CI:
-
95% confidence interval
- SNP:
-
Single nucleotide polymorphisms
References
Black DM, Rosen CJ. Clinical practice. Postmenopausal Osteoporosis. N Engl J Med. 2016;374(3):254–62.
Liu H, Zhao H, Lin H, Li Z, Xue H, Zhang Y, Lu J. Relationship of COL9A1 and SOX9 genes with genetic susceptibility of postmenopausal osteoporosis. Calcif Tissue Int. 2019.
Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet. 2011;377(9773):1276–87.
Zhuang HF, Wang PW, Li YZ, Lin JK, Yao XD, Xu H. Analysis of related factors of brittle hip fracture in postmenopausal women with osteoporosis. Orthop Surg. 2020.
Cai X, Yi X, Zhang Y, Zhang D, Zhi L, Liu H. Genetic susceptibility of postmenopausal osteoporosis on sulfide quinone reductase-like gene. Osteoporos Int. 2018;29(9):2041–7.
Mitek T, Nagraba L, Deszczynski J, Stolarczyk M, Kuchar E, Stolarczyk A. Genetic predisposition for osteoporosis and fractures in postmenopausal women. Adv Exp Med Biol. 2019;1211:17–24.
Zhai G, Andrew T, Kato BS, Blake GM, Spector TD. Genetic and environmental determinants on bone loss in postmenopausal Caucasian women: a 14-year longitudinal twin study. Osteoporos Int. 2009;20(6):949–53.
Hidalgo-Bravo A, Parra-Torres AY, Casas-Avila L, Jimenez-Ortega RF, Ramirez-Salazar EG, Patino N, Rivera-Paredez B, Salmeron J, Valdes-Flores M, Velazquez-Cruz R. Association of RMND1/CCDC170-ESR1 single nucleotide polymorphisms with hip fracture and osteoporosis in postmenopausal women. Climacteric. 2019;22(1):97–104.
Pinkas J, Gujski M, Wierzbinska-Stepniak A, Owoc A, Bojar I. The polymorphism of estrogen receptor alpha is important for metabolic consequences associated with menopause. Endokrynol Pol. 2016;67(6):608–14.
Goulart AC, Zee RY, Pradhan A, Rexrode KM. Associations of the estrogen receptors 1 and 2 gene polymorphisms with the metabolic syndrome in women. Metab Syndr Relat Disord. 2009;7(2):111–7.
Wu F, Zhou D, Shen G, Cui Y, Lv Q, Wei F. Association of VDR and OPG gene polymorphism with osteoporosis risk in Chinese postmenopausal women. Climacteric. 2019;22(2):208–12.
Jia M, Dahlman-Wright K, Gustafsson JA. Estrogen receptor alpha and beta in health and disease. Best Pract Res Clin Endocrinol Metab. 2015;29(4):557–68.
Saoji R, Desai M, Das RS, Das TK, Khatkhatay MI. Estrogen receptor alpha and beta gene polymorphism in relation to bone mineral density and lipid profile in northeast Indian women. Gene. 2019;710:202–9.
Mondockova V, Adamkovicova M, Lukacova M, Grosskopf B, Babosova R, Galbavy D, Martiniakova M, Omelka R. The estrogen receptor 1 gene affects bone mineral density and osteoporosis treatment efficiency in Slovak postmenopausal women. BMC Med Genet. 2018;19(1):174.
Greendale GA, Chu J, Ferrell R, Randolph JF Jr, Johnston JM, Sowers MR. The association of bone mineral density with estrogen receptor gene polymorphisms. Am J Med. 2006;119(9 Suppl 1):S79–86.
Pontin PA, Nogara PRB, Fonseca FCP, Cesar Netto C, Carvalho KC, Soares Junior JM, Baracat EC, Fernandes TD, Maffulli N, Santos MCL, et al. ERalpha PvuII and XbaI polymorphisms in postmenopausal women with posterior tibial tendon dysfunction: a case control study. J Orthop Surg Res. 2018;13(1):316.
Rojano-Mejia D, Coral-Vazquez RM, Coronel A, Cortes-Espinosa L, del Carmen A-GM, Valencia-Villalvazo EY, Canto P. Relation of the estrogen receptor and vitamin D receptor polymorphisms with bone mineral density in postmenopausal Mexican-mestizo women. Gene. 2014;537(1):10–4.
Durusu Tanriover M, Bora Tatar G, Uluturk TD, Dayangac Erden D, Tanriover A, Kilicarslan A, Oz SG, Erdem Yurter H, Sozen T, Sain Guven G. Evaluation of the effects of vitamin D receptor and estrogen receptor 1 gene polymorphisms on bone mineral density in postmenopausal women. Clin Rheumatol. 2010;29(11):1285–93.
Tang L, Cheng GL, Xu ZH. Association between estrogen receptor alpha gene (ESR1) PvuII (C/T) and XbaI (a/G) polymorphisms and hip fracture risk: evidence from a meta-analysis. PLoS One. 2013;8(12):e82806.
Wang W, Cao J, Li YH. Association between estrogen receptor alpha gene (ERα) polymorphism and osteoporosis in postmenopausal women. J Henan Med College. 2017;29(1):11–5.
Farias-Cisneros E, Hidalgo-Bravo A, Miranda-Duarte A, Casas-Avila L, Rozental TD, Velazquez-Cruz R, Valdes-Flores M. COL1A1, CCDC170, and ESR1 single nucleotide polymorphisms associated with distal radius fracture in postmenopausal Mexican women. Climacteric. 2020;23(1):65–74.
Martin J, Viprey M, Castagne B, Merle B, Giroudon C, Chapurlat R, Schott AM. Interventions to improve osteoporosis care: a systematic review and meta-analysis. Osteoporos Int. 2020.
Wang C, Zhang Z, Zhang H, He JW, Gu JM, Hu WW, Hu YQ, Li M, Liu YJ, Fu WZ, et al. Susceptibility genes for osteoporotic fracture in postmenopausal Chinese women. J Bone Miner Res. 2012;27(12):2582–91.
Anastasilakis AD, Polyzos SA, Yavropoulou MP, Makras P. Combination and sequential treatment in women with postmenopausal osteoporosis. Expert Opin Pharmacother. 2020:1–14.
Bailey RL, Zou P, Wallace TC, McCabe GP, Craig BA, Jun S, Cauley JA, Weaver CM. Calcium supplement use is associated with less bone mineral density loss, but does not lessen the risk of bone fracture across the menopause transition: data from the study of Women's health across the nation. JBMR Plus. 2020;4(1):e10246.
Li S, Jiang H, Du N. Association between osteoprotegerin gene T950C polymorphism and osteoporosis risk in the Chinese population: evidence via meta-analysis. PLoS One. 2017;12(12):e0189825.
Bai WY, Wang L, Ying ZM, Hu B, Xu L, Zhang GQ, Cong PK, Zhu X, Zou W, Zheng HF. Identification of PIEZO1 polymorphisms for human bone mineral density. Bone. 2020;133:115247.
Kondo H, Fu**o H, Nagatomo F, Ishihara A. Influence of estrogen receptor alpha polymorphisms on bone density in response to habitual exercise in Japanese postmenopausal women. ScientificWorldJournal. 2014;2014:593927.
Ioannidis JP, Ralston SH, Bennett ST, Brandi ML, Grinberg D, Karassa FB, Langdahl B, van Meurs JB, Mosekilde L, Scollen S, et al. Differential genetic effects of ESR1 gene polymorphisms on osteoporosis outcomes. JAMA. 2004;292(17):2105–14.
Kurt O, Yilmaz-Aydogan H, Uyar M, Isbir T, Seyhan MF, Can A. Evaluation of ERalpha and VDR gene polymorphisms in relation to bone mineral density in Turkish postmenopausal women. Mol Biol Rep. 2012;39(6):6723–30.
Acknowledgements
The authors thank all the study participants for volunteering to participate in the study.
Funding
This work was supported financially by National Natural Science Foundation of China (No. 81691877) in the sample collection and examination.
Author information
Authors and Affiliations
Contributions
JS produced the idea to this study and collected the samples. JL and YF did the literature search, screened the potentially eligible studies and evaluated the data from each included study, they both contributed equally to this work. XH was responsible for making the final version of this paper. JY made the statistics. MC, XZ and YS critically revised this manuscript. The author(s) read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Ethical approval was obtained from the Institution Review Board of the **’an Hospital of Traditional Chinese Medicine. All participants in this study were provided with explanations via face-to-face interpretation and a written informed consent was obtained before inclusion.
Consent for publication
Not Applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shu, J., Li, J., Fu, Y. et al. Association of ESR1 polymorphism rs2234693 and rs9340799 with postmenopausal osteoporosis in a Chinese population. BMC Musculoskelet Disord 21, 346 (2020). https://doi.org/10.1186/s12891-020-03359-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12891-020-03359-2