Abstract
Introduction
Ventilator-associated pneumonia (VAP) presents a significant challenge in intensive care units (ICUs). Nebulized antibiotics, particularly colistin and tobramycin, are commonly prescribed for VAP patients. However, the appropriateness of using inhaled antibiotics for VAP remains a subject of debate among experts. This study aims to provide updated insights on the efficacy of adjunctive inhaled colistin and tobramycin through a comprehensive systematic review and meta-analysis.
Methods
A thorough search was conducted in MEDLINE, EMBASE, LILACS, COCHRANE Central, and clinical trials databases (www.clinicaltrials.gov) from inception to June 2023. Randomized controlled trials (RCTs) meeting specific inclusion criteria were selected for analysis. These criteria included mechanically ventilated patients diagnosed with VAP, intervention with inhaled Colistin and Tobramycin compared to intravenous antibiotics, and reported outcomes such as clinical cure, microbiological eradication, mortality, or adverse events.
Results
The initial search yielded 106 records, from which only seven RCTs fulfilled the predefined inclusion criteria. The meta-analysis revealed a higher likelihood of achieving both clinical and microbiological cure in the groups receiving tobramycin or colistin compared to the control group. The relative risk (RR) for clinical cure was 1.23 (95% CI: 1.04, 1.45), and for microbiological cure, it was 1.64 (95% CI: 1.31, 2.06). However, there were no significant differences in mortality or the probability of adverse events between the groups.
Conclusion
Adjunctive inhaled tobramycin or colistin may have a positive impact on the clinical and microbiological cure rates of VAP. However, the overall quality of evidence is low, indicating a high level of uncertainty. These findings underscore the need for further rigorous and well-designed studies to enhance the quality of evidence and provide more robust guidance for clinical decision-making in the management of VAP.
Similar content being viewed by others
Background
Ventilator-associated pneumonia (VAP) poses a significant challenge in intensive care units (ICUs) [1]. The rise in drug-resistant gram-negative pathogens, such as extended-spectrum beta-lactamase (ESBL)-producing and carbapenem-resistant Enterobacteriaceae, has been associated with treatment failures and increased mortality in ICU patients [2]. To address this issue, the use of adjunctive inhaled antibiotics has emerged as a viable strategy [3]. Inhaled antibiotics offer the advantage of achieving higher drug concentrations in the pulmonary epithelial cells, which is particularly beneficial for treating gram-negative infections with aminoglycosides [4]. Current clinical guidelines recomended to use inhaled antibiotics in addition to intravenous therapy, especially for patients infected with colistin/aminoglycoside-sensitive pathogens or those who have shown a poor response to intravenous antibiotics [5]. This approach improves treatment success rates while minimizing systemic antibiotic doses and associated toxicities.
Colistin and tobramycin are the most commonly prescribed nebulized antibiotics [6]. Previous systematic reviews have examined the efficacy of adjunctive inhaled antibiotic therapy for VAP treatment [7,8,9,10]. However, contrary to more recent studies, these systematic reviews did not find any advantages in utilizing adjunctive inhaled antibiotics, including in terms of clinical cure rates. Furthermore, differing opinions among experts have raised concerns regarding the appropriateness of prescribing inhaled antibiotics for patients with VAP [11]. This study presents a comprehensive systematic review and meta-analysis, offering updated insights on the effectiveness of adjunctive inhaled colistin and tobramycin.
Methods
Search strategy
We conducted a comprehensive search across multiple databases, including MEDLINE, EMBASE, LILACS, and COCHRANE Central, to identify relevant studies. Additionally, we explored clinical trial databases such as www.clinicaltrials.gov, www.base-search.net/, www.tripdatabase.com/, preprinted servers (MedRxiv, JMIR Preprints) and thesis and dissertations (Dart-Europe, EThOs, https://oatd.org/). The search spanned from the inception of the databases to June 2023. We also manually searched the reference lists of eligible studies for additional relevant articles. No language restrictions were applied. The detailed search strategy can be found in the Supplemental material.
Outcomes
This study aimed to evaluate the effectiveness of inhaled Colistin and Tobramycin on clinical cure (primary outcome) and in-hospital mortality, microbiological Cure, and incidence of adverse events (secondary outcomes). For this review, we adopted the definition of clinical and microbiological cure as provided by each individual study. Mortality was defined as death from any cause within 30 days of initiating the intervention.
Inclusion criteria
Randomized controlled trials meeting specific criteria were included in our analysis. The criteria encompassed the following: 1) the study population consisted of mechanically ventilated patients diagnosed with ventilator-associated pneumonia (VAP); 2) the intervention involved the use of inhaled Colistin and Tobramycin for the treatment of VAP, compared to intravenous antibiotics; and 3) the study reported at least one of the following outcomes: clinical cure, microbiological eradication, mortality, or adverse events. Articles that did not fulfill all of these criteria relating to the population, intervention, comparison, and outcome of interest were excluded. Furthermore, review conferences, letters, commentaries, non-randomized controlled trials, and animal experimental studies were also excluded.
Study selection and data extraction
Two independent reviewers performed the selection of studies and extraction of data. Screening of titles and abstracts was conducted based on the predefined inclusion criteria. Full-text articles were obtained for studies that met the inclusion criteria, while articles that did not meet these criteria were excluded. Disagreements between reviewers were resolved through consensus.
Risk of bias assessment
Two reviewers assessed the risk of bias (RoB) of the included studies with the Cochrane RoB tool [12]. Disagreements were resolved by consensus. The risk of publication bias among the studies was planned to be assessed by visual inspection of the funnel plot figure. To evaluate the quality of the included literature, and the GRADE tool (GDT) was used to evaluate the quality of the included outcomes [13].
Data synthesis and statistical methods
For dichotomous outcomes, we calculated relative risk (RR) with their 95% confidence interval (95%CI). Heterogeneity was assessed using the I2 statistics calculated from Cochran’s Q test. Since we recognise that the studies are based on multiple populations, we chose to use the random-effects model for the analysis, regardless of the I2 results. All statistical analysis was performed using Review Manager (RevMan 5.4).
Results
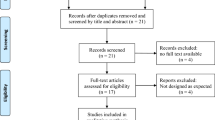
The initial search yielded 106 records, which were subsequently reduced to 31 after removing duplicates. Following the screening of titles and abstracts, 59 records were excluded, as shown in Fig. 1. Consequently, only seven randomized controlled trials (RCTs) that met the predefined inclusion criteria were included in the meta-analysis [14,15,16,17,18,19,20].
Comprehensive information regarding the included studies is provided in the Supplementary material. The studies range in design, with sample sizes varying from smaller studies like Hallal with 10 participants (5 treatment, 5 control) to larger studies such as Rattanaumpawan, which included 100 participants (51 treatment, 49 control) [14,15,16,17,18, 20]. In these studies, a broad range of patient ages were included. Each study employed specific diagnostic criteria for VAP and reported a diverse distribution of bacterial species responsible for infections. For example, Rattanaumpawan focused on patients with Gram-negative bacteria isolated from an endotracheal tube aspirate, including Acinetobacter (50%) and Pseudomonas (26%), among others [19]. The distribution of various bacterial species causing VAP was also diverse across the studies, with Pseudomonas aeruginosa, Acinetobacter spp., and Klebsiella spp. being some of the most reported pathogens. Specific interventions included the use of tobramycin at dosages like 40 mg every 8 h in the case of Brown, and colistin nebulized at 75 mg every 12 h for a duration of 9 to 12 days in Rattanaumpawan. These interventions were compared against control groups that received placebo treatments or systemic antibiotics without the aerosolized form. The duration of the interventions varied, with some studies specifying the exact number of days the aerosolized antibiotics were administered and others not. For instance, Le Conte employed tobramycin for 5 days, whereas Nassar did not provide specific duration details for the use of colistin [17, 18].
Table 1 presents the biases observed in these studies, indicating that three studies exhibited attrition bias, one had reporting bias, two had detection bias, and one had performance bias. The GRADE results, as depicted in Table 2, indicated a “very low quality” of evidence for both clinical cure and adverse events. Due to the limited number of studies, the interpretation of the results is constrained, and it is difficult to ascertain the risk of publication bias with sufficient confidence.
Meta-analysis of outcomes
Clinical cure
The meta-analysis included a total of seven RCTs, comprising 242 patients. The analysis revealed a higher probability of achieving clinical cure in the groups receiving tobramycin or colistin compared to the control group, with a relative risk (RR) of 1.23 and a 95% confidence interval (CI) of (1.04, 1.45). However, this evidence is rated as “very low quality,” as indicated in Fig. 2a.
Microbiological cure
Five RCTs, involving 279 patients [2,3,4,5,6], were included in the meta-analysis for the outcome of microbiological cure. The results demonstrated an effect of achieving microbiological cure in the groups receiving tobramycin or colistin compared to the control group, with an RR of 1.64 and a 95% CI of (1.31, 2.06). The quality of evidence supporting this finding is rated as “moderate,” as illustrated in Fig. 2b.
Adverse events
Three RCTs, including 152 patients [5, 6], were included in the analysis of adverse events. The results did not show any significant differences in the risk of adverse events between the groups, with an relative risk (RR) of 0.75 and a 95% CI of (0.75, 2.86). The evidence supporting this outcome is rated as “very low quality,” as shown in Fig. 2c.
Mortality
The meta-analysis comprised six RCTs, encompassing 333 patients [5]. The analysis did not reveal any significant differences in mortality between the groups receiving tobramycin or colistin compared to the control group, with a relative risk (RR) of 0.82 and a 95% CI of (0.59, 1.14). The quality of evidence supporting this finding is rated as “low,” as presented in Fig. 2d.
Discussion
The present systematic review and meta-analysis provide valuable insights into the efficacy and safety of adjunctive inhaled antibiotics for the treatment of ventilator-associated pneumonia (VAP). The findings suggest that the use of tobramycin or colistin as adjunctive inhaled antibiotics may have a positive impact on both the clinical and microbiological cure rates of VAP. The meta-analysis demonstrated a higher probability of clinical cure and a greater likelihood of achieving microbiological eradication in the groups receiving adjunctive inhaled antibiotics compared to the control group. However, it is important to note that the quality of evidence supporting these findings is rated as “very low” and “moderate,” indicating a significant degree of uncertainty. Therefore, further research is required to strengthen the evidence base and establish more definitive conclusions.
The results of our study align with previous evidence in the field [7, 21]. However, a notable difference between our study and previous systematic reviews is the inclusion of the studies conducted by Angermain and Hallal [14, 16]. These studies evaluated the effectiveness of tobramycin and reported positive results in terms of microbiological eradication and the probability of clinical cure, without observing a reduction in mortality. It is worth mentioning that due to their small sample sizes, the weight of these studies in the meta-analysis is relatively low compared to other studies, and thus our conclusions align with previous systematic reviews.
Our work has limitations. First, the small sample size (seven RCTs) restricts generalizability and raises concerns about chance findings. Additionally, potential biases identified in the included studies (attrition, reporting, detection, performance) could generate doubt on the reliability of the results. Furthermore, the low quality of evidence (“very low” for clinical cure and adverse events) highlights the need for further research before definitive conclusions can be drawn. The limited exploration of adverse events (only three studies reporting) restricts understanding of potential risks. The possibility of publication bias, given the restricted sample size, warrants caution in interpreting findings. Additionally, further investigation is needed to determine optimal dosing regimens and treatment durations for inhaled antibiotics. Furthermore, the impact of adjunctive inhaled antibiotics on specific patient subgroups and the potential development of antibiotic resistance requires further exploration. Our study has several strengths. Our search was exhaustive including gray literature and clinical trial registries. We follow the recommendations of the Cochrane collaboration and use GRADE to assess the quality of the evidence.
While this study sheds light on the potential benefits of adjunctive inhaled colistin and tobramycin for VAP, further research is crucial to optimize their use and refine clinical practice. Several key areas demand exploration: 1. Dosing and Treatment Duration: The optimal dosing regimen and treatment duration for inhaled antibiotics remain unclear. Future studies should explore a wider range of doses and durations to identify the most effective and safe approach, considering factors like patient characteristics and pathogen susceptibility. 2. Subpopulation Analysis: The present analysis did not delve into potential differences in treatment response across diverse patient subgroups (e.g., specific pathogens, underlying comorbidities). Future research should stratify analyses by relevant subgroups to provide more nuanced insights on which patients benefit most from this intervention. 3. Delivery System Optimization: The impact of delivery systems on drug bioavailability in the lungs was not addressed. Investigating and optimizing delivery systems could significantly improve treatment efficacy and potentially reduce required doses, enhancing safety and minimizing antibiotic resistance concerns. 4. Long-Term Outcomes and Resistance: The long-term effects of inhaled antibiotics on lung function, antibiotic resistance development, and other relevant outcomes require further investigation. Additionally, exploring the potential for emergence of resistant strains specific to inhaled antibiotics is crucial for informing sustainable resistance management strategies.
Conclusions
Tobramycin or colistin may have a positive impact on the clinical and microbiological cure rates of ventilator-associated pneumonia. The quality of evidence is low indicating a high degree of uncertainty. This highlights the importance of conducting more rigorous and well-designed studies to improve the quality of evidence and inform clinical decision-making.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Qi W, Murphy TE, Doyle MM, Ferrante LE. Association between daily average of mobility achieved during physical therapy sessions and hospital-acquired or ventilator-associated pneumonia among critically ill patients. J Intensive Care Med. 2023;38(5):418–24.
Rodriguez-Chavez LA, Esteban-Dionicio ML, Rodriguez-Mendoza CRE. Microbiological profile of bacteria causing ventilator-associated pneumonia in the intensive care unit of a high-complexity hospital. Rev Peru Med Exp Salud Publica. 2023;40(1):115–7.
Boisson M, Bougle A, Sole-Lleonart C, Dhanani J, Arvaniti K, Rello J, et al. Nebulized antibiotics for healthcare- and ventilator-associated pneumonia. Semin Respir Crit Care Med. 2022;43(2):255–70.
Zhu Y, Monsel A, Roberts JA, Pontikis K, Mimoz O, Rello J, et al. Nebulized colistin in ventilator-associated pneumonia and tracheobronchitis: historical background, pharmacokinetics and perspectives. Microorganisms. 2021;9(6):1154.
Torres A, Niederman MS, Chastre J, Ewig S, Fernandez-Vandellos P, Hanberger H, et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociacion Latinoamericana del Torax (ALAT). Eur Respir J. 2017;50(3):1700582.
Niederman MS. Adjunctive nebulized antibiotics: what is their place in ICU infections? Front Med (Lausanne). 2019;6:99.
Zhang X, Cui X, Jiang M, Huang S, Yang M. Nebulized colistin as the adjunctive treatment for ventilator-associated pneumonia: a systematic review and meta-analysis. J Crit Care. 2023;77:154315.
Tang R, Luo R, Wu B, Wang F, Song H, Chen X. Effectiveness and safety of adjunctive inhaled antibiotics for ventilator-associated pneumonia: a systematic review and meta-analysis of randomized controlled trials. J Crit Care. 2021;65:133–9.
Povoa FCC, Cardinal-Fernandez P, Maia IS, Reboredo MM, Pinheiro BV. Effect of antibiotics administered via the respiratory tract in the prevention of ventilator-associated pneumonia: a systematic review and meta-analysis. J Crit Care. 2018;43:240–5.
Russell CJ, Shiroishi MS, Siantz E, Wu BW, Patino CM. The use of inhaled antibiotic therapy in the treatment of ventilator-associated pneumonia and tracheobronchitis: a systematic review. BMC Pulm Med. 2016;16:40.
Palmer LB, Smaldone GC. The unfulfilled promise of inhaled therapy in ventilator-associated infections: where do we go from here? J Aerosol Med Pulm Drug Deliv. 2022;35(1):11–24.
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6.
Angermair S, Deja M, Thronicke A, Grehn C, Akbari N, Uhrig A, et al. A prospective phase IIA multicenter double-blinded randomized placebo-controlled clinical trial evaluating the efficacy and safety of inhaled Tobramycin in patients with ventilator-associated pneumonia (iToVAP). Anaesth Crit Care Pain Med. 2023;42(5):101249.
Brown RB, Kruse JA, Counts GW, Russell JA, Christou NV, Sands ML. Double-blind study of endotracheal tobramycin in the treatment of Gram-negative bacterial pneumonia. The endotracheal tobramycin study group. Antimicrob Agents Chemother. 1990;34(2):269–72.
Hallal A, Cohn SM, Namias N, Habib F, Baracco G, Manning RJ, et al. Aerosolized tobramycin in the treatment of ventilator-associated pneumonia: a pilot study. Surg Infect (Larchmt). 2007;8(1):73–82.
Le Conte P, Potel G, Clementi E, Legras A, Villers D, Bironneau E, et al. Administration of tobramycin aerosols in patients with nosocomial pneumonia: a preliminary study. Presse Med. 2000;29(2):76–8.
Nassar YS, Saber-Ayad M, Shash RY. Combined microbiological and clinical outcomes of short-term inhaled colistin adjunctive therapy in ventilator-associated pneumonia. Egypt J Chest Dis Tuberc. 2018;67(4):376–83.
Rattanaumpawan P, Lorsutthitham J, Ungprasert P, Angkasekwinai N, Thamlikitkul V. Randomized controlled trial of nebulized colistimethate sodium as adjunctive therapy of ventilator-associated pneumonia caused by Gram-negative bacteria. J Antimicrob Chemother. 2010;65(12):2645–9.
Stokker J, Karami M, Hoek R, Gommers D, van der Eerden M. Effect of adjunctive tobramycin inhalation versus placebo on early clinical response in the treatment of ventilator-associated pneumonia: the VAPORISE randomized-controlled trial. Intensive Care Med. 2020;46(3):546–8.
Xu F, He LL, Che LQ, Li W, Ying SM, Chen ZH, Shen HH. Aerosolized antibiotics for ventilator-associated pneumonia: a pairwise and Bayesian network meta-analysis. Crit Care. 2018;22(1):301.
Acknowledgements
None.
Funding
This study was supported by own funding of authors.
Author information
Authors and Affiliations
Contributions
J.A.B, DG. and A.F.Z. wrote the main manuscript text and DG. prepared Figures 1-2. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of University of Antioquia. The need for Informed consent was waived by an Institutional Review Board of University of Antioquia (2015–4690), following local regulations of resolution 8430/93, because this is a study which all information was extracted from the literature and do not use data from patients.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Buendía, J.A., Guerrero Patiño, D. & Zuluaga Salazar, A.F. Efficacy of adjunctive inhaled colistin and tobramycin for ventilator-associated pneumonia: systematic review and meta-analysis. BMC Pulm Med 24, 213 (2024). https://doi.org/10.1186/s12890-024-03032-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-024-03032-7