Abstract
Background
Owing to the introduction of highly active antiretroviral therapy (HAART), the trajectory of mortality and morbidity associated with human immunodeficiency virus (HIV) infection has significantly decreased in developed countries. However, this remains a formidable public health challenge for people living with HIV in resource-poor settings. This study was undertaken to determine the pooled person-time incidence rate of mortality, analyze the trend, and identify predictors of survival among HIV-infected adults receiving HAART.
Methods
Quantitative studies were searched in PubMed, Embase, Scopus, Google Scholar, African Journals Online, and Web of Science. The Joana Briggs Institute critical appraisal tool was used to assess the quality of the included articles. The data were analyzed using the random-effects Dersimonian-Laird model.
Results
Data abstracted from 35 articles involving 39,988 subjects were analyzed. The pooled person-time incidence rate of mortality (all-cause) was 4.25 ([95% uncertainty interval (UI), 3.65 to 4.85]) per 100 person-years of observations. Predictors of mortality were patients aged ≥ 45 years (hazard ratio (HR), 1.70 [95% UI,1.10 to 2.63]), being female (HR, 0.82 [95% UI, 0.70 to 0.96]), history of substance use (HR, 3.10 [95% UI, 1.31 to 7.32]), HIV positive status non disclosure (HR, 3.10 [95% UI,1.31 to 7.32]), cluster of differentiation 4 + T cell - count < 200 cells/mm3 (HR, 3.23 [95% UI, [2.29 to 4.75]), anemia (HR, 2.63 [95% UI, 1.32 to 5.22]), World Health Organisation classified HIV clinical stages III and IV (HR, 3.02 [95% UI, 2.29 to 3.99]), undernutrition (HR, 2.24 [95% UI, 1.61 to 3.12]), opportunistic infections (HR, 1.89 [95% UI, 1.23 to 2.91]), tuberculosis coinfection (HR, 3.34 [95% UI, 2.33 to 4.81]),bedridden or ambulatory (HR,3.30 [95% UI, 2.29 to 4.75]), poor treatment adherence (HR, 3.37 [95% UI,1.83 to 6.22]), and antiretroviral drug toxicity (HR, 2.60 [95% UI, 1.82 to 3.71]).
Conclusion
Despite the early introduction of HAART in Ethiopia, since 2003, the mortality rate has remained high. Therefore, guideline-directed intervention of identified risk factors should be in place to improve overall prognosis and increase quality-adjusted life years.
Similar content being viewed by others
Introduction
Globally, despite an overall decline in the reported prevalence, the human immune deficiency virus (HIV) continues to afflict more than 39 million people (37.5 million were 15 years of age or older) in 2022, and an estimated 1.3 million incident cases (1.2 million were 15 years of age or older) and 630,000 deaths from HIV-related illnesses were registered in the same year [1].
Africa is home to an estimated 25.6 million HIV-infected people and 60% of global acquired immunodeficiency syndrome (AIDS) deaths [2]. Due to the introduction and scaling-up of HAART, HIV-related mortality has steadily declined over the past two decades in developed countries; however, the problem remains important in low-resource settings, including Ethiopia [2, 3]. In sub-Saharan Africa (SSA), which is home to an estimated 67% of the global HIV-infected population and 76% of global AIDS deaths, the proportion of early mortality among adults accessing HAART was very high; between 6 and 26% of patients died [4], which ascertains that treatment of HIV is still a challenge in resource-poor settings (5.55 deaths per 100 person-years of observation (PYO) compared to resource-rich settings (2 deaths per 100 PYO) [5].
In Ethiopia, an estimated 603,537 people were living with HIV (570,511 were 15 years of age or older), and annual AIDS deaths were estimated at 9,984 (approximately 86% were 15 years of age or older), according to the Ethiopian Public Health Institute HIV estimates and projections for the year 2023 [6]. With the introduction of HAART in resource-limited settings in the early 2000s, Ethiopia was among the first African countries to introduce HAART in 2003 in selected health facilities. With the free HAART program in early 2005, a significant number of deaths have been averted due to the concerted efforts of the government and its partners, and HAART coverage for 15 years of age or older has reached 82% in 2022, and the country is striving to attain the 95-95-95 global goal by 2030 [7].
The provision of HAART is necessary, but not sufficient to increase survival among patients receiving treatment, and evidence suggests that timely diagnosis, assessment of eligibility, and provision of treatment free of charge are associated with a lower risk of mortality [5]. Furthermore, evidence from various studies in low settings suggests that advanced HIV/AIDS at presentation [4, 5, 8,9,10,11], low quality of health service care [4], undernutrition [8, 9, 12,13,14,15], anemia [8, 9, 12, 16], sex [8, 9, 13, 17, 18], tuberculosis (TB)-HIV co-infection at enrollment [19, 20], and poor HAART adherence [21, 22] were predictors of mortality among adults receiving HAART.
Although there has been no nationally representative summary data, estimates from individual studies conducted in health facilities providing chronic HIV care and treatment services in Ethiopia revealed a person-time incidence rate of mortality between 0.28 deaths per 100 persons per year [23] in Suhul Hospital in the Tigray region and 22.9 deaths per 100 persons per year in DebreMarkos Referral Hospital in the Amhara region [24]. To better understand the success of the HIV program in Ethiopia and inform policymakers, we aimed answers to the following questions: (1) What is the pooled person-time incidence rate of mortality among HIV-infected adult patients initiating HAART in Ethiopia? (2) What are the predictors of mortality among adult HIV-infected patients initiating HAART in Ethiopia? (3) What is the trend of death over time in adult patients initiating HAART in Ethiopia?
Methods and materials
Study protocol registration and reporting
A full study protocol, written based on the Preferred reporting items for systematic review and meta-analysis protocols 2015 [25], submitted to the Prospective Register of Systematic Reviews and registered with registration number CRD42023481380. We reported the systematic review and meta-analysis results using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 checklist [26] (Additional file 1).
Eligibility criteria
Population/type of participants
Persons living with HIV (PLHIV) aged 15 or older and initiating HAART in Ethiopia were considered.
Condition/domain
Articles that described the outcome of the interest based on PLHIV survival and predictors of mortality after initiating HAART were considered.
Context/settings
Follow up studies (retrospective/prospective) conducted in Ethiopia and published in the English language from inception to August 31, 2023, were included. Otherwise, articles without full-text access; articles that did not contain required information on the outcomes of interest; studies published in non-open access journals; findings from personal opinions; articles reporting outside the scope of the outcome of interest; qualitative study design; case reports; case series; letters to editors; and unpublished data were excluded.
Information sources and search strategy
A double-blinded search was carried out by two authors (BZW and YSA) from March 1, 2023, to August 31, 2023, in the Excerpta Medica database, PubMed, Web of Science, African Journals Online, Google Scholar, and Scopus. Furthermore, the reference lists of final articles included in the quantitative synthesis were scanned to ensure literature saturation. Literature search strategies were developed using medical subject headings and text words related to the outcomes of interest. The search terms employed include: “ mortality”, “death”, “survival”, “HIV/AIDS”, “Human immune deficiency virus”, “acquired immune deficiency syndrome”, “ART”, “antiretroviral therapy”, “HAART”, “highly active antiretroviral therapy”, “prevalence,” “proportion”, “incidence”, “associated factors”, “predictors”, “determinants”, “adults”, adolescents, and “Ethiopia” (S1 Table).
Study selection procedures
Articles were exported to the reference management software, EndNote X7, where duplicate studies were then eliminated. Two authors (AH and GAK) independently screened the titles and abstracts. The screened articles were then subjected to a full article review by two independent authors (AG and AK). Pre-specified criteria for inclusion in the review were followed to determine which records were relevant and should be included. Where more information was required to answer queries regarding eligibility, the remaining authors were involved. Disagreements about whether a study should be included were resolved by discussion. Moreover, the reasons for excluding the articles were recorded at each step.
Data extraction
Two authors (ASB and BZW), working independently, excerpted the relevant data from the studies using a standardized Microsoft Excel spreadsheet. For data extraction, Joana Briggs Institute data collection formats suitable for meta-analysis were employed [27]. The data extraction format captured data on the following main components: information about data extraction from reports (name of data extractors, date of data extraction, and study identification number), study authors, year of publication of the article, study methods (study design, statistical analysis), study settings (regions, and specific areas from which study participants recruited), population characteristics (sex, age), information related to the pre-specified outcome domain, measurement tool or instrument, and information related to the results for each study included in the quantitative analysis (number of participants included in the analysis, and the non-response rate). In the case of disagreements between the two data extractors, a third author (AH) was involved in adjudicating unresolved disagreements through discussion and re-checking of the original articles.
Methodological quality assessment
Two authors (YSA and GAK) evaluated the original studies using the Joanna Briggs Institute critical appraisal checklist designed for cohort studies which included 11 constructs. The response options were labeled as ‘yes’, 'no', and 'unclear question'. The total score was computed by counting the number of 'yes' answers in each row. Articles with critical appraisal scores of 7 and above were included in the systematic review and meta-analysis (S2 Table).
Outcome and effect measures
The primary outcome of interest was the person-time incidence rate of mortality. The pooled incidence density was computed as the number of deaths divided by the total number of years of observation multiplied by 100. The secondary outcome was predictors of mortality, and the hazard ratio was the summary effect measure employed. We categorized the predictor variables as follows: residence (rural vs. urban), age (< 45 vs. ≥ 45), sex (male vs.female), substance use (yes vs.no), HIV-positive status disclosure (yes vs. no), HAART adherence (poor vs. good and fair), cotrimoxazole preventive therapy (yes vs. no), tuberculosis preventive therapy (yes vs. no), hemoglobin (< 10 g/dl vs. ≥ 10 g/dl), opportunistic infections (OIs) (yes vs. no), body mass index (BMI) (< 18.5 vs. ≥ 18.5), the World Health Organisation (WHO) classified HIV clinical stages (III and IV vs.I and III), the cluster of differentiation(CD)4 + T lymphocyte count (< 200 vs. ≥ 200), TB-HIV co-infection (yes vs. no), functional status (working vs. ambulatory/bedridden), and antiretroviral drug (ARV) toxicity (yes vs.no).
Data synthesis
Extracted data were imported from Microsoft Excel 2010 into Stata 16 MP version for analysis. The presence and extent of variability among studies (inconsistency or heterogeneity) were evaluated graphically (present when the uncertainty interval for the results of individual studies generally depicted in forest plots using the horizontal lines have poor overlap) and more formally, using statistical methods (the Cochrane chi-squared test, included in the forest plots, the threshold for statistical significance was set at P ≤ 0.1; Higgins and Thompson’s I2 statistics: 0% to 40%: may not be important; 30% to 60% may represent moderate heterogeneity; 50% to 90%: may represent substantial heterogeneity; 75% to 100%: considerable heterogeneity) [28]. We employed the random-effect meta-analysis model to estimate Der Simonian and Laird’s pooled effect, as considerable statistical heterogeneity was observed (Higgins and Thompson’s I2 statistics was ≥ 50% and P.value was ≤ 0.1). Subgroup analyses (based on sample size, and HAART eligibility as covariates), meta-regression (based on year of publication, and sample size as covariates), and sensitivity analyses were performed. To evaluate the presence of small study effects, publication bias was explored through statistical methods (Egger test: significant at P ≤ 0.05) and funnel plots [29]. Variables with P ≤ 0.05 were deemed statistically significant predictors of mortality, and the strength of the association was presented by HR with a corresponding 95% uncertainty interval (UI).
Results
Search and study selection
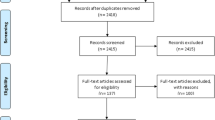
The database search identified 8377 articles. After 6978 duplicate records were removed, the remaining 1399 were screened based on their title and abstracts, with 1457 being removed as unrelated to the study domain. Fourty-six full-text articles were evaluated against eligibility criteria, and 17 of them were removed (different outcome, n = 2, inconsistent results, n = 3, unpublished reports, n = 3, pre-HAART, n = 4, poor quality, n = 2, and age < 15 years old, n = 3). Furthermore, through citation searching, six articles were retrieved. Finally, 35 articles were eligible for quantitative analysis (Fig. 1).
Study characteristics
A total of 35 eligible studies [30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64], with 39,988 participants were included. The study sample size ranged from 272 [34] to 11,013 [60] individuals. An estimated 61% (n = 24,316) of participants were females. All epidemiological studies were cohort [30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64]. The participants contributed 91,866.861 PYO, and 4,050 deaths were recorded. Mean or median survival time was reported in 13 of the 35 studies [31, 33, 34, 40, 41, 43, 46, 47, 53, 55, 58, 59, 64]. There were two subnational studies as part of the Advanced Clinical Monitoring for HIV/AIDS in patients with HIV infection [52, 60]. Six of the studies were conducted in the Amhara region [30, 42, 44, 51, 54, 59], four in the Oromia region [32,33,34, 55]; three in Addis Ababa city administration [38, 43, 53]; 12 in the Southern Nations, Nationalities, and Peoples' Region (SNNPR) [31, 35, 36, 39,40,41, 45, 46, 57, 62,63,64], three in Tigray [37, 49, 50], three in Harari region [47, 48, 61], one each in Afar [56] and Somalia [58] regions. The overall proportion of mortality was 10.13%, and estimates from individual studies suggest the cumulative incidence of mortality ranged from 4.43% [33] to 29.68% [30]. About 69% (n = 24) of the studies were published after the implementation of the WHO’s universal test and treatment strategy [30, 33, 35, 37,38,39,40,41,42,43,44, 47, 48, 51, 52, 54,55,56,57, 59, 60, 62,63,64] (Table 1).
Mortality
The pooled person-time incidence rate of mortality among adult patients initiating HAART in Ethiopia was 4.25 deaths ([95% UI, 3.65,4.85]; I2 = 95.6%) per 100 PYO (Fig. 2).
Subgroup (subset) meta-analysis
To identify the source of statistical heterogeneity, we undertook a subgroup random-effect meta-analysis for subsets of sample size partitioned into < 1000 and ≥ 1000 participants and HAART eligibility split into before and after the WHO’s universal test and treatment policy. The pooled person-time incidence rate of mortality was 4.57 deaths per 100 PYO (Fig. 3). Moreover, the pooled person-time incidence rate of mortality before the implementation of the WHO’s universal test and treatment policy was higher (4.50 deaths per 100 PYO) (Fig. 4).
Meta-regression
We further performed meta-regression analyses to explore the cause of heterogeneity, using the sample size and year of publication as covariates at 5% statistical significance. As illustrated in Table 2, these covariates were not found to be the cause of statistical heterogeneity.
Sensitivity meta-analysis
A leave-out-one sensitivity analysis was conducted to assess the impact of each study on the pooled incidence density of mortality while gradually excluding each study. The results showed that the combined effects did not change significantly as a result of the excluded study (Fig. 5).
Publication bias
To determine whether there is a possibility of publication bias or small-study effects, we looked at the distribution of studies about the summary effect sizes graphically using funnel plots. Thus, on inspection, the funnel plot showed there is no prominent asymmetrical distribution (Fig. 6).
Funnel plots of publication biases. The x-axis shows the effect size, and the standard errors of the effect sizes were plotted on the y-axis. The dashed lines represent the 95% confidence interval. The dots show the distribution of individual studies. Studies with smaller sample sizes are scattered at the bottom of the funnel, and vice versa
Furthermore, the formal Egger linear regression test was not statistically significant (P = 0.080) corroborating the absence of evidence of small study effects (Table 3).
Trends in estimated death rates
As shown in Fig. 7, an overall significant decline in mortality from 8.76 deaths per 100 PYO (in 2010) to 5.73 deaths per 100 PYO (in 2023) was observed.
Predictors of mortality among patients initiating highly active antiretroviral therapy
Table 4 summarizes the pooled hazard ratio of 13 variables (these can be categorized into demographic-related, clinical-related, laboratory-related, and behavioral-related variables) which were found to be predictors of survival among patients receiving HAART in Ethiopia. These were, patients aged ≥ 45 years (HR, 1.70 [95% UI,1.10 to 2.63]), being female (HR, 0.82 [95% UI, 0.70 to 0.96]), history of substance use (HR, 3.10 [95% UI, 1.31 to 7.32]), HIV positive status non disclosure (HR, 3.10 [95% UI,1.31 to 7.32]), cluster of differentiation 4 + T- count < 200 cells/mm3 (HR, 3.23 [95% UI, [2.29 to 4.75]), anemia (HR, 2.63 [95% UI, 1.32 to 5.22]), World Health Organisation classified HIV clinical stages III and IV (HR, 3.02 [95% UI, 2.29 to 3.99]), undernutrition (HR, 2.24 [95% UI, 1.61 to 3.12]), opportunistic infections (HR, 1.89 [95% UI, 1.23 to 2.91]), tuberculosis coinfection (HR, 3.34 [95% UI, 2.33 to 4.81]),bedridden or ambulatory (HR,3.30 [95% UI, 2.29 to 4.75]), poor adherence (HR, 3.37 [95% UI,1.83 to 6.22]), and antiretroviral drug toxicity (HR, 2.60 [95% UI, 1.82 to 3.71]).
Discussion
The findings of this systematic review and meta-analysis revealed that 4,050 deaths were registered among adult patients initiating HAART in Ethiopia, corresponding to a cumulative incidence of 10.13% and pooled mortality incidence density of 4.25 per 100 PYO. Our finding was by far higher than the rate of mortality reported from India (3.12 deaths per 100 PYO) [71] and a multiregional study in Africa and Asia (2.7 deaths per 100 PYO) [72]. However, the current pooled estimate was lower than the individual study estimates from studies conducted in rural settings of South Africa (7.5 deaths per 100 PYO) [73], India (8.1 deaths per 100 PYO) [74], and Uganda (12 deaths per 100 PYO) [75]. The duration of follow-up of the cohorts, year of publication, geographic and cultural barriers, study sizes, and level of engagement in the implementation of HIV programs might partly explain the discrepancy in survival.
Moreover, subgroup analysis revealed a steady decrease in the mortality incidence rate (from 4.15 deaths per 100 PYO before the universal test and treatment strategy to 4.50 deaths per 100 PYO after) following the full-scale implementation of the WHO’s universal test and treatment policy in the country. This is congruent with the strategic approach implemented in Uganda, where a baseline six-month mortality rate of 3.3% decreased by 1.6% after the universal test and treatment policy [76] and a threefold decrease in mortality in Cameroon [77]
Regarding prognostic factors, age, and sex were the two important demographic predictors of mortality identified in this review. First, increased age (45 years or older) was correlated with increased hazards (70% higher) of mortality compared with individuals aged < 45 years. The current findings are congruent with those of studies conducted in other countries [78, 79]. This may be because immunosenescence leads to increased susceptibility to infections in the elderly population and decreased ability to eradicate OIs [80], which in turn decreases the survival of persons receiving HAART. This study also revealed that female patients had an 18% lower mortality risk than male patients. This is in line with a study in Cameroon, where men had twice the risk of mortality compared to females [81], a systematic review by Gupta et al. [82], which found that men were more likely to die early in the course of treatment, and a study in rural settings of Uganda, found that females had a 45% lower risk of mortality [83]. Nevertheless, research reports by Woldegeorgis et al. [84], and Nicastri et al. [85] found little evidence for sex differences, which requires a powerful study. Although an in-depth examination is needed, these differences could be attributed to disparities in socioeconomic status, healthcare-seeking behaviors, treatment adherence, biological differences, and risky sexual behaviors.
Patients who had reported a history of substance use while taking HAART had a 62% higher risk of mortality compared to patients who did not. Research findings consistent the with current findings were reported in Vietnam [86], in which substance use increased the risk of non-AIDS deaths among patients on HAART. Another study indicated that a history of smoking (twice), and alcohol use (25 to 35% higher) were correlated with a decreased life expectancy despite effective HAART [87]. This is because substance consumption, particularly alcohol consumption, interferes with HAART adherence and adverse drug reactions, culminating in decreased HAART effectiveness and, therefore, the survival of patients on treatment.
HIV-positive status disclosure significantly and positively affects HAART adherence through social support and self-efficacy, which in turn contributes to increased quality-adjusted life years of PLHIVs on HAART [88,89,90]. A systematic review by Yehualashet et al. [91] indicated that the pooled national estimate of HIV-positive status disclosure among adult PLHIVs was lower in Ethiopia than in develo** countries. This study found that PLHIVs with non-disclosure status had threefold higher mortality hazards than those who disclosed their status. This finding is consistent with that of a study conducted in China [89].
Decreased immunity was another factor related to the increased risk of mortality identified in this study. In line with studies conducted elsewhere [10, 12, 17, 92,93,94,95], the mortality hazard was 3.23 times higher among patients whose baseline CD4 + T-cell count was < 200 cells/mm3 than among patients whose baseline CD4 + T-cell count was greater. Although symptoms of HIV can appear at any time during HIV infection, the spectrum of the more severe and life-threatening complications of HIV infection, such as disseminated tuberculosis, which is indicative of a severe defect in cell-mediated immunity in poor settings, occurs as the CD4 + T-cell count declines, more importantly in patients with CD4 + T-cell counts < 200/μL, which decreases the survival of patients receiving HAART.
Anemia was another predictor of mortality in this systematic review. Patients whose baseline hemoglobin level was < 10 g per deciliter had 2.63 times higher hazards of mortality compared to patients who had a hemoglobin level of ≥ 10 g per deciliter In line with our findings, a study in HIV-infected patients from across Europe indicated that anemia is a strong independent marker of clinical prognosis [96]. The results supporting our findings were also reported in Senegal [12], Zambia [8], and Tanzania [94]. Anemia is common throughout HIV infection, and the causes are likely to be multifactorial and may be the direct result of HIV infection harboring underlying opportunistic neoplasms such as lymphoma, OIs such as systemic fungal, and mycobacterium infections, bleeding (gastrointestinal malignancy/severe infection), and poor dietary intake (vitamins such as cobalamin and folate, iron, and general nutritional deficiencies). In addition, antiretroviral and other medication toxicities are associated with bone marrow suppression, further challenging the survival of HIV-infected patients receiving HAART. According to a systematic review by Negesse et al. [97] three of ten HIV-infected adult patients on HAART had anemia in Ethiopia, which underscores anemia is a formidable challenge in HIV - infected Ethiopian patients.
Patients with advanced HIV clinical stages (III/IV) at presentation to chronic HIV care and treatment are three times more likely to die than those with mild or asymptomatic HIV clinical stages (I/II). Findings from studies conducted in a rural center in the Far-North province of Cameroon [81], Uganda [98], four sub-Saharan African countries (Côte d'Ivoire, Malawi, South Africa, and Zimbabwe) [99], and Jamaica, analysis of national surveillance data [100] revealed increased hazards of mortality in these patients compared with the general population. This is because life-threatening OIs and malignancies occur at the advanced WHO HIV clinical stage and remain the major drivers of HIV-related mortality and morbidity in PLHIVs.
Undernutrition was another predictor of mortality in this study. Undernourished patients (BMI < 18.5 kg per m2) had 2.24 times higher hazards of mortality compared to patients whose baseline BMI was 18.5 kg per m2 or greater. The effects of HIV on nutrition have been well studied, and a recent meta-analysis revealed that the prevalence of undernutrition among adults initiating HAART was 23.74% in SSA, with the highest (60%) and lowest (8.3%) burdens in Ethiopia and Kenya, respectively [101]. Findings in agreement with our study have been reported elsewhere in Africa [8, 13, 14, 94] and Haiti [102]. The possible justification emanates from the synergistic effect of both HIV and undernutrition; HIV causes poor appetite secondary to chronic inflammation, and enteropathy interferes with nutrient absorption from the gastrointestinal tract [103]. Undernutrition, in turn, accelerates the progression of the clinical stages of HIV because of the direct effect of undernutrition on immunity, all of which negatively affect the survival of HIV-infected children [104, 105].
We found 89% higher hazards of mortality among patients who exhibited OIs than among those who did not. Similarly, research supporting the current finding was reported from India, which stated that patients with any OI before the start of HAART were 2.3 times more likely to die in comparison to patients without any OIs [71]. In Ethiopia, ART is initiated for all HIV-infected patients as rapidly as possible, irrespective of their immunological status; up to 50% [106] of patients present for care and treatment at late clinical stages, with acquired immune deficiency syndrome-defining OIs. Furthermore, a recent meta-analysis revealed that the burden of OIs was high with a pooled prevalence of 43.97% among adult patients receiving HAART [107].
The study found that patients with TB co-infection at the start of HAART were three times more likely to die compared to those without TB at baseline [71]. Moreover, another study found that TB significantly predicts early mortality in adults on HAART in low and middle-income countries [82]. The increase in plasma HIV ribonucleic acid levels during active TB, a leading cause of death worldwide in HIV infection, maybe a possible explanation.
HIV-infected adult patients whose baseline functional status was bedridden or ambulatory had three times higher hazards of mortality compared to patients whose functional status was working. In agreement with our findings, bedridden patients had twice higher hazards of mortality in Kenya [108], threefold in Nepal [109], and in India [74]. This is because late-stage HIV patients have already developed severe forms of OIs and neoplasms, which are responsible for deteriorated quality of life and restricted daily activities, all of which affect their survival despite HAART.
Medication Adherence was another predictor of mortality identified in this review. Patients who had poor or fair adherence to HAART were three times more likely to die compared to patients who had good adherence. Findings in line with our study were reported from India [71, 110], and Canada [111]. A high level of sustained medication adherence is required to suppress viral replication, improve immunological outcomes, decrease OIs, minimize hospitalization and inpatient death, decrease the risk of develo** ARV drug resistance, and reduce the risk of transmitting HIV [84, 107, 112].
Last, patients who experienced ARV drug toxicity after initiating HAART had 2.6-fold higher hazards of mortality compared to those who did not. In agreement with the current findings, a systematic review by Mouton et al. [113] found a hospital mortality proportion of 2.5% to 16% among HIV-infected adults on HAART in SSA. According to a report from research conducted in seven teaching hospitals in Ethiopia, 22% of HIV-positive patients experience mild to life-threatening ARV drug toxicity [114]. ARV drug toxicity is one of the major causes of non-adherence after initiating HAART, resulting in treatment failure and hospitalization.
Strengths and limitations of the study
To our knowledge, this systematic review and meta-analysis is the first to estimate the pooled person-time incidence rate of mortality, describe the trends in death rates over time by comparing strategies before and after the universal test and treatment, and investigate potential risk factors associated with death in HIV-infected adults receiving HAART in Ethiopia. Methodologically, the study was adequate as sufficient primary studies were found and a large number of study sizes with fairly sufficient follow-up time were required for outcomes to occur, all of which increased the precision of the study and the true estimate of the mortality rate in adults initiating HAART in Ethiopia. This study had some limitations. First, some statistical heterogeneity was observed; therefore, interpretation of the results in the context is required.
Conclusion and recommendations
In conclusion, despite almost 20 years of HAART initiation in Ethiopia, the mortality rate remains high. Therefore, patients must be counseled and monitored for enhanced medication adherence, ARV toxicity, and non-AIDS-related predictors of mortality like substance use. Furthermore, screening efforts are essential in the early detection and management of tuberculosis and other OIs, earlier initiation of HAART, and due attention to patients presenting with symptomatic HIV, and anemia, nutritional interventions for undernourished adults, and encouraging partner notification.
Implications of findings
Our findings have important implications for the provision of comprehensive HIV care and treatment for adults with HIV infection in Ethiopia. Furthermore, with strong conviction, the findings of this meta-analysis contribute to the provision of evidence that can be utilized by researchers, policymakers, clinicians, and other stakeholders in resource-poor settings.
Availability of data and materials
All data supporting the findings of this study are available within the paper and its Supplementary Information.
Abbreviations
- AIDS:
-
Acquired immune deficiency syndrome
- ARV:
-
Antiretroviral therapy
- CD:
-
Cluster of differentiation
- HAART:
-
Highly active antiretroviral therapy
- HIV:
-
Human immune deficiency virus
- HR:
-
Hazard ratios
- OIs:
-
Opportunistic infections
- PLHIV:
-
Persons living with the human immune deficiency virus
- PYO:
-
Person-years of observations
- SNNPR:
-
The Southern Nations Nationalities Peoples region
- SSA:
-
The sub-Saharan African countries
- TB:
-
Tuberculosis
- UI:
-
Uncertainty interval
- WHO:
-
The World Health Organization
References
The Joint United Nations Programme on HIV/AIDS (UNAIDS). Global HIV statistics. Fact sheet 2023 epidemiological estimates. https://www.unaids.org/en/resources/fact-sheet.
HIV statistics, globally and by WHO region, 2023. Epidemiological fact sheet. https://cdn.who.int/media/docs/default-source/hq-hiv-hepatitis-and-stis-library/j0294-who-hiv-epi-factsheet-v7.pdf.
Cao G, et al. Prevalence of anemia among people living with HIV: a systematic review and meta-analysis. EClinicalMedicine. 2022;44:101283.
Lawn SD, et al. Early mortality among adults accessing antiretroviral treatment programs in sub-Saharan Africa. AIDS. 2008;22(15):1897–908.
Braitstein P, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet (London, England). 2006;367(9513):817–24.
The Ethiopian Public Health Institute (EPHI). HIV estimates and projections for the years 2022 and 2023. https://ephi.gov.et/wp-content/uploads/2021/02/HIV-Estimates-and-Projection-for-the-year-2022-and-2023.pdf.
The Federal Ministry of Health of Ethiopia (FMOH). National guidelines for comprehensive HIV prevention, care, and treatment. 2023.
Stringer JS, et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. JAMA. 2006;296(7):782–93.
Toure S, et al. Rapid scaling-up of antiretroviral therapy in 10,000 adults in Cote d’Ivoire: two-year outcomes and determinants. AIDS (London, England). 2008;22(7):873.
Lawn SD, et al. Early mortality among adults accessing a community-based antiretroviral service in South Africa: implications for programme design. AIDS. 2005;19(18):2141–8.
De Iaco G, et al. Factors predicting early death in a cohort of patients with HIV/AIDS in Burkina Faso: the need for earlier diagnosis and antiretroviral treatment. In: Abstract Book 4th IAS Conference on HIV Pathogenesis, Treatment and Prevention. International AIDS Society; 2007.
Etard J-F, et al. Mortality and causes of death in adults receiving highly active antiretroviral therapy in Senegal: a 7-year cohort study. AIDS. 2006;20(8):1181–9.
Ferradini L, et al. Scaling up of highly active antiretroviral therapy in a rural district of Malawi: an effectiveness assessment. lancet. 2006;367(9519):1335–42.
Zachariah R, et al. Risk factors for high early mortality in patients on antiretroviral treatment in a rural district of Malawi. AIDS. 2006;20(18):2355–60.
Moh R, et al. Incidence and determinants of mortality and morbidity following early antiretroviral therapy initiation in HIV-infected adults in West Africa. AIDS. 2007;21(18):2483–91.
Laurent C, et al. Long-term benefits of highly active antiretroviral therapy in Senegalese HIV-1-infected adults. J Acquir Immune Defic Syndr. 2005;38(1):14–7.
Coetzee D, et al. Outcomes after two years of providing antiretroviral treatment in Khayelitsha, South Africa. Aids. 2004;18(6):887–95.
Lawn SD, et al. Determinants of mortality and non-death losses from an antiretroviral treatment service in South Africa: implications for program evaluation. Clin Infect Dis. 2006;43(6):770–6.
Lawn SD, et al. Burden of tuberculosis in an antiretroviral treatment program in sub-Saharan Africa: impact on treatment outcomes and implications for tuberculosis control. AIDS. 2006;20(12):1605–12.
Moore D, et al. Prevalence, incidence and mortality associated with tuberculosis in HIV-infected patients initiating antiretroviral therapy in rural Uganda. AIDS. 2007;21(6):713–9.
Nachega JB, et al. Adherence to highly active antiretroviral therapy assessed by pharmacy claims predicts survival in HIV-infected South African adults. J Acquir Immune Defic Syndr. 2006;43(1):78–84.
Lawn SD, et al. Early mortality among adults accessing antiretroviral treatment programs in sub-Saharan Africa. AIDS (London, England). 2008;22(15):1897–908.
Misgina KH, et al. Predictors of mortality among adult people living with HIV/AIDS on antiretroviral therapy at Suhul Hospital, Tigrai, Northern Ethiopia: a retrospective follow-up study. J Health Popul Nutr. 2019;38(1):37.
Tadele A, Shumey A, Hiruy N. Survival and predictors of mortality among adult patients on highly active antiretroviral therapy at debre-markos referral Hospital, North West Ethiopia; a retrospective cohort study. J AIDS Clin Res. 2014;5:2.
Shamseer L, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015:349. https://doi.org/10.1136/bmj.g7647.
Page MJ, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Aromataris E, Munn Z. Chapter 1: JBI Systematic Reviews. In: Aromataris E, editor. JBI Manual for Evidence Synthesis. 2020.
Higgins JP, et al. Cochrane handbook for systematic reviews of interventions. John Wiley & Sons; 2019.
Egger M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
Gebremichael SG. AIDS-duration predictors of HIV/AIDS patients on antiretroviral therapy at Debre Berhan referral hospital, north-central Ethiopia. MOJ Public Health. 2020;9(4):99–105.
Tsegaye E, Worku A. Assessment of antiretroviral treatment outcome in public hospitals, South Nations Nationalities and Peoples, Region Ethiopia. Ethiop J Health Dev. 2011;25(2):102–9.
Hambisa MT, Ali A, Dessie Y. Determinants of mortality among HIV positives after initiating antiretroviral therapy inwestern ethiopia: a hospital-based retrospective cohort study. ISRN AIDS. 2013;2013:1–8.
Abebe TW, et al. Determinants of survival among adults on antiretroviral therapy in Adama Hospital Medical College, Oromia Regional state, Ethiopia. J HIV AIDS (ISSN 2380–5536 ). 2016;2(1).
Alemu AW, Sebastian MS. Determinants of survival in adult HIV patients on antiretroviral therapy in Oromiyaa, Ethiopia. Glob Health Action. 2010:3.
Hailemariam S, et al. Determinants of survival in HIV patients: a retrospective study of Dilla University Hospital HIV cohort. Int J Virol AIDS. 2016;3(2):2–6.
Girum T, et al. The effect of “universal test and treat” program on HIV treatment outcomes and patient survival among a cohort of adults taking antiretroviral treatment (ART) in low-income settings of Gurage zone, South Ethiopia. AIDS Res Ther. 2020;17(1):19.
Belay H, et al. Effect of late HIV diagnosis on HIV-related mortality among adults in general hospitals of Central Zone Tigray, northern Ethiopia: a retrospective cohort study. HIV AIDS (Auckl). 2017;9:187–92.
Tesfaye B, et al. Effect of the test and treat strategy on mortality among HIV-positive adult clients on antiretroviral treatment in public Hospitals of Addis Ababa. Ethiopia HIV AIDS (Auckl). 2021;13:349–60.
Kebede A, et al. Epidemiology of survival pattern and its predictors among HIV positive patients on highly active antiretroviral therapy in Southern Ethiopia public health facilities: a retrospective cohort study. AIDS Res Ther. 2020;17(1):49.
Wondimu W, Dube L, Kabeta T. Factors affecting survival rates among adult TB/HIV co-infected patients in Mizan Tepi University teaching hospital, South West Ethiopia. HIV AIDS (Auckl). 2020;12:157–64.
Barata TY, et al. Incidence of mortality and its predictors among adult human immune virus infected patients on antiretroviral therapy in Wolaita Sodo University comprehensive specialized Hospital, Southern Ethiopia: a retrospective follow-up study. HIV AIDS (Auckl). 2023;15:361–75.
Teshale AB, Tsegaye AT, Wolde HF. Incidence of mortality and its predictors among HIV positive adults on antiretroviral therapy in University of Gondar comprehensive specialized Hospital, Northwest Ethiopia. HIV AIDS (Auckl). 2021;13:31–9.
Tesfayohannes S, et al. Mortality and its predictors among adult human immune-deficiency virus-infected patients attending their antiretroviral treatment at health centers, Addis Ababa, Ethiopia: multicenter retrospective cohort study. AIDS Res Treat. 2022;2022:6128718.
Ahunie MA, Ebrahim EA. Mortality predictors of HIV-infected patients on antiretroviral therapy in Debre Tabor General Hospital and Woreta Health Center, South Gondar Zone, Northwest Ethiopia. Asian Pacific J Trop Dis. 2017;7(2):99–105.
Mulissa Z, Jerene D, Lindtjorn B. Patients present earlier and survival has improved, but pre-ART attrition is high in a six-year HIV cohort data from Ethiopia. PLoS one. 2010;5(10):e13268.
Setegn T, et al. Predictors of mortality among adult antiretroviral therapy users in Southeastern Ethiopia: retrospective cohort study. AIDS Res Treat. 2015;2015:148769.
Birhanu A, Dingeta T, Tolera M. Predictors of mortality among adult HIV-infected patients taking Antiretroviral Therapy (ART) in Harari Hospitals, Ethiopia. HIV AIDS (Auckl). 2021;13:727–36.
Eticha EM, Gemeda AB. Predictors of mortality among adult patients enrolled on Antiretroviral Therapy in Hiwotfana specialized University Hospital, Eastern Ethiopia: retrospective cohort study. J HIV Clin Sci Res. 2018;5(1):007–11.
Biadgilign S, Reda AA, Digaffe T. Predictors of mortality among HIV infected patients taking antiretroviral treatment in Ethiopia: a retrospective cohort study. AIDS Res Ther. 2012;9(12):15.
Tadesse K, Haile F, Hiruy N. Predictors of mortality among patients enrolled on antiretroviral therapy in Aksum hospital, northern Ethiopia: a retrospective cohort study. PLoS One. 2014;9(1):e87392.
Workie KL, Birhan TY, Angaw DA. Predictors of mortality rate among adult HIV-positive patients on antiretroviral therapy in Metema Hospital, Northwest Ethiopia: a retrospective follow-up study. AIDS Res Ther. 2021;18(1):27.
Fekade D, et al. Predictors of survival among adult Ethiopian patients in the national ART program at seven University teaching Hospitals: a prospective cohort study. Ethiop J Health Sci. 2017;27(Suppl 1):63–71.
Mengesha S, Belayihun B, Kumie A. Predictors of survival in HIV-infected patient after initiation of HAART in Zewditu Memorial Hospital, Addis Ababa, Ethiopia. Int Sch Res Notices. 2014;2014:250913.
Birhanu H, Alle A, Birhanu MY. Rate and predictors of mortality among adults on antiretroviral therapy at Debre Markos Referral Hospital, North West Ethiopia. HIV AIDS (Auckl). 2021;13:251–9.
Seyoum D, et al. Risk factors for mortality among adult HIV/AIDS patients following antiretroviral therapy in Southwestern Ethiopia: an assessment through survival models. Int J Environ Res Public Health. 2017;14(3):296.
Salih AM, Yazie TS, Gulente TM. Survival analysis and predictors of mortality among adult HIV/AIDS patients initiated antiretroviral therapy from 2010 to 2015 in Dubti General Hospital, Afar, Ethiopia: a retrospective cohort study. Heliyon. 2023;9(1):e12840.
Yohannes T, Moges S, Laelago T. Survival analysis of adult human immune virus infected patients under antiretroviral treatment at Wachamo University. Int J Intern Med Geriatr. 2019;1(1):21–32.
Damtew B, Mengistie B, Alemayehu T. Survival and determinants of mortality in adult HIV/Aids patients initiating antiretroviral therapy in Somali Region, Eastern Ethiopia. Pan Afr Med J. 2015;22:138.
Nigussie F, et al. Survival and predictors of mortality among adult HIV/AIDS patients initiating highly active antiretroviral therapy in Debre-Berhan Referral Hospital, Amhara, Ethiopia: a retrospective study. HIV AIDS (Auckl). 2020;12:757–68.
Getaneh Y, et al. Survival and predictors of mortality among adults initiating highly active antiretroviral therapy in Ethiopia: a retrospective cohort study (2007–2019). Biomed Res Int. 2022;2022:5884845.
Digaffe T, Seyoum B, Oljirra L. Survival, and predictors of mortality among adults on antiretroviral therapy in selected public Hospitals in Harar, Eastern Ethiopia. J Trop Dis. 2014;02(05).
Abuto W, et al. Survival and predictors of mortality among HIV positive adult patients on highly active antiretroviral therapy in public Hospitals of Kambata Tambaro Zone, Southern Ethiopia: a retrospective cohort study. HIV AIDS (Auckl). 2021;13:271–81.
Tachbele E, Ameni G. Survival and predictors of mortality among human immunodeficiency virus patients on anti-retroviral treatment at **ka Hospital, South Omo, Ethiopia: a six years retrospective cohort study. Epidemiol Health. 2016;38:e2016049.
Sapa WB, Negassi NT, and A.H. Chofore, survival pattern and its determinants among adult HIV-infected patients after initiation of HAART in Dilla Hospital Ethiopia. J Clin Exp Immunol. 2016;1(1):2–6.
Demissie MW. Statistical modeling for the survival of HIV/AIDS patients treated with Highly Active Anti-Retroviral Therapy (HAART): a case study at Dilchora Hospital, Dire Dawa, Ethiopia. J Biom Biostat. 2018;9(5):1–10.
Lelisho ME, et al. Survival rate and predictors of mortality among TB/HIV co-infected adult patients: retrospective cohort study. Sci Rep. 2022;12(1):18360.
Refera H, Wencheko E. Survival of HIV-TB co-infected adult patients under ART in Ambo Referral Hospital, Ethiopia. Ethiop J Health Dev. 2013;27(2):88–93.
Deres G, et al. Survival time and associated factors among adults living with hiv after initiation of HAART in South Gondar, Northwest Ethiopia: a retrospective cohort. J Multidiscip Healthc. 2021;14:1463–74.
Siraj M, Gedamu S, Tegegne B. Predictors of survival time among HIV-infected adults after initiating anti-retroviral therapy in Kombolcha Town: a 5-year retrospective cohort study. HIV AIDS (Auckl). 2022;14:181–94.
Kebebew K, Wencheko E. Survival analysis of HIV-infected patients under antiretroviral treatment at The Armed Forces General Teaching Hospital, Addis Ababa, Ethiopia. Ethiop J Health Dev. 2012;26(3):186–92.
Joseph N, et al. Prognostic factors of mortality among adult patients on antiretroviral therapy in India: a hospital-based retrospective cohort study. BioMed Res Int. 2019;2019:1419604.
Collaboration, A.T.i.L.I.C. and A.C. Collaboration, Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367(9513):817–24.
MacPherson P, et al. Mortality and loss to follow-up among HAART initiators in rural South Africa. Trans R Soc Trop Med Hyg. 2009;103(6):588–93.
Bajpai R, et al. Effects of antiretroviral therapy on the survival of human immunodeficiency virus-positive adult patients in Andhra Pradesh, India: a retrospective cohort study, 2007–2013. J Prev Med Public Health. 2016;49(6):394.
Abaasa AM, et al. Good adherence to HAART and improved survival in a community HIV/AIDS treatment and care program: the experience of The AIDS Support Organization (TASO), Kampala Uganda. BMC Health Serv Res. 2008;8:1–10.
Mugenyi L, et al. Effect of universal test and treatment on retention and mortality among people living with HIV-infection in Uganda: an interrupted time series analysis. PLoS One. 2022;17(5):e0268226.
Bekolo CE, et al. Universal test and treat in Cameroon: a comparative retrospective analysis of mortality and loss to follow-up before and after a strategic change in approach to HIV care. Pan Afr Med J. 2023;45:191.
Mugusi FM, et al. Factors associated with mortality in HIV-infected and uninfected patients with pulmonary tuberculosis. BMC Public Health. 2009;9(1):1–8.
Domingos MP, Caiaffa WT, Colosimo EA. Mortality, TB/HIV co-infection, and treatment dropout: predictors of tuberculosis prognosis in Recife, Pernambuco State, Brazil. Cadernos Saude Publica. 2008;24:887–96.
Stervbo U, et al. Effects of aging on human leukocytes (part I): immunophenoty** of innate immune cells. Age (Dordr). 2015;37(5):92.
Sieleunou I, et al. Determinants of survival in AIDS patients on antiretroviral therapy in a rural centre in the Far-North Province, Cameroon. Trop Med Int Health. 2009;14(1):36–43.
Gupta A, et al. Early mortality in adults initiating antiretroviral therapy (ART) in low-and middle-income countries (LMIC): a systematic review and meta-analysis. PLoS One. 2011;6(12):e28691.
Alibhai A, et al. Gender-related mortality for HIV-infected patients on highly active antiretroviral therapy (HAART) in rural Uganda. Int J Womens Health. 2010;2:45–52.
Woldegeorgis BZ, et al. Incidence and predictors of opportunistic infections in adolescents and adults after the initiation of antiretroviral therapy: a 10-year retrospective cohort study in Ethiopia. Front Public Health. 2022;10:1064859.
Nicastri E, et al. Sex issues in HIV-1-infected persons during highly active antiretroviral therapy: a systematic review. J Antimicrob Chemother. 2007;60(4):724–32.
Araujo Chaveron L. et al. Injecting drug use increases the risk of death in HIV patients on antiretroviral therapy in Vietnam. AIDS Care. 2023:1–10. https://doi.org/10.1080/09540121.2023.2224549.
Petoumenos K, Law MG. Smoking, alcohol and illicit drug use effects on survival in HIV-positive persons. Curr Opin HIV AIDS. 2016;11(5):514–20.
Alema HB, et al. HIV positive status disclosure and associated factors among HIV positive adults in Axum health facilities, Tigray, Northern Ethiopia. Sci J Public Health. 2015;3(1):61.
Mi T, et al. HIV disclosure to family members and medication adherence: role of social support and self-efficacy. AIDS Behav. 2020;24(1):45–54.
Dessie G, et al. The effect of disclosure on adherence to antiretroviral therapy among adults living with HIV in Ethiopia: a systematic review and meta-analysis. BMC Infect Dis. 2019;19(1):528.
Yehualashet F, et al. Human immunodeficiency virus positive status disclosure to a sexual partner and its determinant factors in Ethiopia: a systematic review and meta-analysis. BMC Infect Dis. 2020;20(1):1–14.
da Silva Escada RO, et al. Mortality in patients with HIV-1 and tuberculosis co-infection in Rio de Janeiro, Brazil-associated factors and causes of death. BMC Infect Dis. 2017;17(1):1–10.
Ismail I, Bulgiba A. Predictors of death during tuberculosis treatment in TB/HIV co-infected patients in Malaysia. PLoS One. 2013;8(8):e73250.
Johannessen A, et al. Predictors of mortality in HIV-infected patients starting antiretroviral therapy in a rural hospital in Tanzania. BMC Infect Dis. 2008;8:1–10.
Johansson KA, Robberstad B, Norheim OF. Further benefits by early start of HIV treatment in low income countries: survival estimates of early versus deferred antiretroviral therapy. AIDS Res Ther. 2010;7:1–9.
Mocroft A, et al. Anaemia is an independent predictive marker for clinical prognosis in HIV-infected patients from across Europe. AIDS. 1999;13(8):943–50.
Negesse A, et al. Prevalence of anemia and its associated factors in human immuno deficiency virus infected adult individuals in Ethiopia. A systematic review and meta-analysis. BMC Hematol. 2018;18:1–10.
Amuron B, et al. Mortality in an antiretroviral therapy programme in **ja, south-east Uganda: a prospective cohort study. AIDS Res Ther. 2011;8:1–8.
Brinkhof MW, et al. Mortality of HIV-infected patients starting antiretroviral therapy in sub-Saharan Africa: comparison with HIV-unrelated mortality. PLoS Med. 2009;6(4):e1000066.
Losina E, et al. HIV morbidity and mortality in Jamaica: analysis of national surveillance data, 1993–2005. Int J Infect Dis. 2008;12(2):132–8.
Seid A, et al. Prevalence of undernutrition and associated factors among adults taking antiretroviral therapy in sub-Saharan Africa: a systematic review and meta-analysis. PLoS One. 2023;18(3):e0283502.
Severe P, et al. Antiretroviral therapy in a thousand patients with AIDS in Haiti. N Engl J Med. 2005;353(22):2325–34.
Weldehaweria NB, et al. Psychosocial correlates of nutritional status among people living with HIV on antiretroviral therapy: a matched case-control study in Central zone of Tigray, Northern Ethiopia. PLoS One. 2017;12(3):e0174082.
Oumer A, Kubsa ME, Mekonnen BA. Malnutrition as predictor of survival from anti-retroviral treatment among children living with HIV/AIDS in Southwest Ethiopia: survival analysis. BMC Pediatr. 2019;19(1):1–10.
Muenchhoff M, et al. Malnutrition in HIV-infected children is an indicator of severe disease with an impaired response to antiretroviral therapy. AIDS Res Hum Retroviruses. 2018;34(1):46–55.
Getaneh MB, Aklilu E, Amare DA. Late presentation of HIV positive adults and its predictors to HIV/AIDS care in Ethiopia: a systematic review and meta-analysis. BMC Infect Dis. 2019;19:534.
Woldegeorgis BZ, et al. Prevalence and determinants of opportunistic infections among HIV-infected adults receiving antiretroviral therapy in Ethiopia: a systematic review and meta-analysis. Front Med (Lausanne). 2023;10:1087086.
Muhula SO. et al. Effects of highly active antiretroviral therapy on the survival of HIV-infected adult patients in urban slums of Kenya. Pan Afr Med J. 2015;20(1).
Bhatta L, et al. Survival on antiretroviral treatment among adult HIV-infected patients in Nepal: a retrospective cohort study in far-western Region, 2006–2011. BMC Infect Dis. 2013;13(1):1–9.
Rai S, et al. Adherence to antiretroviral therapy and its effect on survival of HIV-infected individuals in Jharkhand, India. PLoS One. 2013;8(6):e66860.
Wood E, et al. Effect of medication adherence on survival of HIV-infected adults who start highly active antiretroviral therapy when the CD4+ cell count is 0.200 to 0.350× 109 cells/L. Ann Intern Med. 2003;139(10):810–6.
Cohen MS, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505.
Mouton JP, et al. Serious adverse drug reactions in sub-Saharan Africa in the era of antiretroviral treatment: a systematic review. Pharmacol Res Perspect. 2021;9(6):e00875.
Gudina EK, et al. Magnitude of antiretroviral drug toxicity in adult HIV patients in Ethiopia: a cohort study at seven teaching hospitals. Ethiop J Health Sci. 2017;27(1):39–52.
Acknowledgements
We would like to express our gratitude to the authors of the original papers that were included in this systematic review and meta-analysis.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
BZW conceptualized the study and wrote the original manuscript. BZW,YSA,AH,GAK and ASB participated in the formal analysis, investigation, methodology, project administration, software management, supervision, validation, visualization, review and editing, and approval of the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Woldegeorgis, B.Z., Asgedom, Y.S., Habte, A. et al. Highly active antiretroviral therapy is necessary but not sufficient. A systematic review and meta-analysis of mortality incidence rates and predictors among HIV-infected adults receiving treatment in Ethiopia, a surrogate study for resource-poor settings. BMC Public Health 24, 1735 (2024). https://doi.org/10.1186/s12889-024-19268-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-024-19268-1