Abstract
Background
People who inject drugs (PWID) experience many health problems which result in a heavy economic and public health burden. To tackle this issue, France opened two drug consumption rooms (DCRs) in Paris and Strasbourg in 2016. This study assessed their long-term health benefits, costs and cost-effectiveness.
Methods
We developed a model to simulate two fictive cohorts for each city (n=2,997 in Paris and n=2,971 in Strasbourg) i) PWID attending a DCR over the period 2016-2026, ii) PWID attending no DCR. The model accounted for HIV and HCV infections, skin abscesses and related infective endocarditis, drug overdoses and emergency department visits. We estimated the number of health events and associated costs over 2016-2026, the lifetime number of quality-adjusted life-years (QALYs) and costs, and the incremental cost-effectiveness ratio (ICER).
Results
The numbers of abscesses and associated infective endocarditis, drug overdoses, and emergency department visits decreased significantly in PWID attending DCRs (-77%, -69%, and -65%, respectively) but the impact on HIV and HCV infections was modest (-11% and -6%, respectively). This resulted in savings of €6.6 (Paris) and €5.8 (Strasbourg) millions of medical costs. The ICER of DRCs was €30,600/QALY (Paris) and €9,200/QALY (Strasbourg). In scenario analysis where drug consumption spaces are implemented inside existing harm reduction structures, these ICERs decreased to €21,400/QALY and €2,500/QALY, respectively.
Conclusions
Our findings show that DCRs are highly effective and efficient to prevent harms in PWID in France, and advocate extending this intervention to other cities by adding drug consumption spaces inside existing harm reduction centers.
Similar content being viewed by others
Background
People who inject drugs (PWID) experience many health problems. The burden of chronic viral infections is high in this population, with an estimated global prevalence of 17.8 and 52.3% for human immunodeficiency virus (HIV) and hepatitis C virus (HCV), respectively [1]. Bacterial infections also represent a major issue in PWID, with 6 to 32% reporting skin and soft tissue infections in the previous month [2]. Finally, 41.5% of PWID report overdosing during their lifetime [3]. All these health events have a huge impact on PWID life expectancy [4, 5] and quality of life [6].
Harm reduction measures have traditionally relied on access to sterile injection equipment through needle/syringe exchange programs, and opiate agonist therapy [7]. Some countries, such as Australia, Switzerland and Canada, have also implemented drug consumption rooms (DCRs). These are places where PWID can consume drugs in good hygienic and sanitary conditions, and access care adapted to their needs. Studies from Australia and Canada show that DCR bring many benefits, including a decrease in the risk of overdose and syringe sharing, improved access to care, and a positive impact on local drug-related violence and trafficking [8, 9].
In France, psychosocial and health services for PWID are usually delivered in harm reduction centers for people who use drugs, namely CAARUD. These services are financed by the national health insurance system (NHI) and are managed either by non-profit organizations or by public hospitals. Two DCRs were opened on an experimental basis in Paris and Strasbourg in 2016. The COSINUS cohort [10] was established the same year to assess the two DCRs’ effectiveness in PWID, especially in terms of fewer infection risk practices and adverse health events. Cohort participants were recruited between June 2016 and October 2018 in both DCRs and in CAARUDs in Paris, Strasbourg, Bordeaux and Marseilles and followed up to 12 months. Results showed that attending DCRs significantly reduced the probability of reporting injecting equipment sharing, abscesses, overdoses, and emergency department (ED) visits [11]. A sociological survey embedded in the evaluation also showed an improvement in regards to public safety [12] and social acceptance, especially among harm reduction and addiction professionals [13]. Furthermore, existing economic evaluation studies suggested that the intervention is cost-effective and in some settings even cost-saving (i.e., avoided medical costs because of avoided health events exceed the cost of DCR). However, all these studies have been conducted in North America settings (Canada and the United States) where the organization of the health system and the epidemiological situation are very specific [1, 14] and all used DCR effectiveness data from one single experience in Vancouver to simulate the impact of this intervention on long-term PWID health.
In the perspective of the potential extension of the DCR experiment to other cities in France, further information on their costs and efficiency is needed for decision-making by national health authorities.
Using a modelling approach combining data from the COSINUS cohort and the literature, this study aims to assess the long-term health benefits, costs avoided and cost-effectiveness of DCR compared to standard services offered in CAARUD in the France setting.
Methods
Analytic overview
We designed a stochastic, individual-based, continuous-time model to simulate health outcomes and related costs in hypothetical populations of PWID attending either the Paris or Strasbourg DCR over a 10-year period. The comparator harm reduction strategy was the situation where only CAARUD are present (i.e., the standard situation without DCR).
The model simulated the occurrence of the following health events: new HIV and HCV infections, abscesses and associated infective endocarditis (IE), overdoses, and ED visits. We stratified analyses by city (Paris and Strasbourg) to account for local specificity (e.g. characteristics of PWID and cost of DCR). We first estimated health outcomes and associated medical costs for both strategies over a 10-year horizon and then conducted the complete cost-effectiveness analysis over a lifetime horizon. In line with recommendations from the French National Authority for Health (HAS) [15], the cost-effectiveness analysis was conducted using the point of view of the national health system (irrespective of the financing body i.e. the Ministry of health or the NHI) and included both medical costs incurred by the NHI and the costs of establishing and running the two DCR, funded by public resources.
Simulated populations
For both Paris and Strasbourg, we simulated two hypothetical populations: i) PWID attending the DCR (plus possibly CARRUD) over a 10-year period starting from the DCR opening date and ii) PWID attending no DCR (but possibly CARRUD) over the same period (See Additional file S1 for further details).
For both strategies, the number of new entrants in the model per unit of time, and their age and gender distribution were defined using DCR activity registers over the period 2016-2019 (Table 1).
Model description
A schematic representation of the model is given Fig. 1, and the full model structure is described in Additional file S2. The model accounted for HCV and HIV infections and simulated the cascade of care associated with both these infections (i.e., access to screening, linkage to care, treatment) and their natural history. HIV and HCV infection rates in susceptible PWID were determined by data from the COSINUS cohort on the sharing of injecting equipment depending on whether or not PWID attended a DCR. Infected PWID then progressed through the HCV and HIV cascade of care which will determine their access or not to treatment and subsequent treatment success (i.e., sustained virological response (SVR) for HCV and viral load suppression for HIV).
Model for a. HCV infection and chronic hepatitis C care cascade, b. HIV infection and cascade of care, c. natural history of chronic hepatitis C and d. natural history of HIV infection. \({\uplambda }_{\text{HCV}}(\text{i})\) = rate of infection according to whether or not injection equipment was shared within one month. \({\text{T}}_{\text{a}}\) = duration of acute hepatitis C. \({\text{p}}_{\text{Rem}}=\) probability of spontaneous remission. \({\updelta }_{\text{HCV}}\) = rate of HCV testing; \({\upphi }_{\text{HCV}}\) = rate of linkage to care. \({\uptau }_{\text{HCV}}\) = rate of loss to follow-up. aVHC = rate of initiation of treatment. \({\text{T}}_{\text{t}}\) = duration of antiviral therapy. \({\text{p}}_{\text{RVS}}\) = probability of sustained virological response. \({\upgamma }_{\text{F}2/3}\) rate of progression to F2/3 fibrosis. \({\upgamma }_{\text{F}4}\) = rate of occurrence of cirrhosis in F2/3. \({\upgamma }_{\text{HCC}}\) = rate of occurrence of hepatocellular carcinoma in cirrhosis. \({\upgamma }_{\text{DC }-\text{HCC}}\) = rate of occurrence of hepatocellular carcinoma in decompensated cirrhosis. \({\upgamma }_{\text{DC}}\) = rate of occurrence of decompensated cirrhosis in cirrhosis. \({\upgamma }_{\text{HCC}-\text{TP}}\) = rate of liver transplants with hepatocellular carcinoma. \({\upgamma }_{\text{DC}-\text{TP}}\) = rate of hepatic transplants with decompensated cirrhosis. \({\upgamma }_{\text{HCC}-\text{Death}}\) = death rate from hepatocellular carcinoma. \({\upgamma }_{\text{DC}-\text{Death}}\) = death rate in decompensated cirrhosis. \({\upgamma }_{\text{TP}-\text{Death}}\) = death rate after liver transplantation. \({\uplambda }_{\text{HIV}}(\text{i})\) = rate of infection by sharing or not sharing injecting equipment in the month. \({\updelta }_{\text{HIV}}\) = HIV testing rate. \({\upphi }_{\text{HIV}}\) = rate of linkage to care. \({\uptau }_{\text{HIV}}\) = rate of loss to follow-up. aHIV = rate of initiation of treatment. \({\upmu }_{\text{x}}\) = mortality rate at CD4 level x. \({\upgamma }_{\text{x}}\) = rate of decline of CD4 level to \(\text{x}\). \({\uptheta }_{\text{x}}\) = rate of improvement in CD4 level towards \(\text{x}\). Abbreviations: ARV=antiretrovirals; HCV=Hepatitis C virus; HIV=Human immunodeficiency virus; SVR=Sustained Virologic Response
Furthermore, the model accounted for the occurrence of skin abscesses (which may be associated with IE) and of overdoses. Each of these events could lead to hospitalization, while IE and overdoses could lead to death. The model also included ED visits, some of which require sending a French mobile emergency and resuscitation service (MERS) ambulance.
Outcomes
First, the following outcomes were compared between the two strategies over a 10-year period: i) morbidity, assessed using the number of HIV and HCV infections, abscesses and associated IE, overdoses and ED visits; ii) mortality, assessed using the number of deaths and life years (LY); iii) medical costs associated with each health event. We calculated health events avoided, life years saved (LYS), and associated total medical cost avoided as the difference between the number of health events/LY/medical costs for the DCR and no DCR strategies.
Second, the following three outcomes were estimated in a lifetime cost-effectiveness analysis: i) incremental costs of the DCR strategy (versus the comparator strategy) which included medical costs, initial DCR implementation costs, and DCR running costs; ii) the number of QALYs gained (preferred outcome [26]); iii) incremental cost-effectiveness ratios (ICERs) in euros per QALY. As recommended by the HAS, outcomes of the cost-effectiveness analysis were discounted at a rate of 4% per year up to 30 years, then using a linear decrease to reach 2% at 40 years [15].
Input parameters for the model
A summary of the key parameter values and sources is provided in Table 1, and additional information is available in Additional file S3. The relative risks of abscesses, ED visits and overdoses with the DCR strategy (compared to the no DCR strategy) were estimated using data from the COSINUS cohort [11]. Using data from the literature, the proportion of abscesses associated with IE, and the proportion of ED visits associated with a MERS ambulance intervention were set at 2.2% [21] and 6.3% [23], respectively. The proportion of abscesses requiring hospital management was estimated at 31.5% [20].
The relative risk of HIV and HCV infection with the DCR strategy was estimated using information on the sharing of injecting equipment from the COSINUS cohort data, combined with values for the relative risk of HIV and HCV infection when sharing injection equipment obtained from the literature (i.e., 2.36 for HIV [17] and 1.94 for HCV [19]). HIV and HCV infection rates in the no DCR strategy were calibrated to reproduce the incidence rates observed in PWID in France prior to the opening of the DCRs in Paris and Strasbourg, i.e., 173/100,000 person-years for HIV [16], and 11.2/100 person-years for HCV [18]. All other estimates for parameters were taken from the literature.
Health-related quality of life
Our model accounted for the deterioration in quality of life associated with the HIV and HCV infections using utility score data from the literature based on CD4 level for HIV infection, and liver disease stage for HCV (Table 2).
Costs
The costs are described in Table 3 and Supplemental Material S4.
The DCRs’ initial implementation costs and their annual running costs were estimated from the financial and accounting documents of both structures. The medical costs associated with the management of chronic HCV (according to liver disease stage) and of HIV (according to the CD4 level) were provided by the scientific and gray literature. The average costs of in-hospital management of abscesses, associated IE, overdoses and ED visits, as well as the average cost of a MERS ambulance intervention (assuming an average intervention time of one and a half hours [36]) were obtained from the French NHI hospitalization database [33,34,35].
All costs were inflated in 2023 euros [37].
Economic and sensitivity analysis
Base-case analysis
The methods employed in the economic analysis were in line with international guidelines [38, 39]. We estimated the lifetime incremental costs and lifetime incremental health benefits of DCRs as the cost and QALY differences between the DCR and no DCR strategies. The ICER was then computed as the ratio of the incremental cost to the number of QALY gained. As the HAS does not provide recommendations on cost-effectiveness thresholds (CET) to use in France [15], we assumed the following CET suggested by the World Health Organization (WHO) in order to provide an indication on the cost-effectiveness of DCR [40]: i) very cost-effective if the ICER is less than one times the 2023 French per-capita gross domestic product (GDP) (€33,300 [41]) and ii) cost-effective if the ICER is less than three times the 2023 French per-capita GDP (€99,900). We also considered a more realistic approach to define the CET in France based on ICERs of interventions that national health authorities considered worthy of NHI funding, i.e. €50,000/QALY gained [42, 43].
In the base-case analysis, we accounted for the uncertainty related to stochasticity (i.e., the relatively small size of the simulated populations) by performing, for each strategy and for each city, 1,000 simulations for each scenario. Using the simulations, we estimated the means and associated 95% confidence intervals (CI 95%) associated with each outcome using bootstrap** (See Additional file S5 for further details).
Scenario and sensitivity analysis
We conducted two alternative scenario analyses. In the first, we assumed a 20% decrease in the DCRs’ entry rates after 2019 compared to the base-case analysis. In the second, we assumed that DCRs were not created as separate structures from existing harm reduction services (i.e., CAARUD) but within them.
Finally, we addressed uncertainty in the model parameters using a probabilistic sensitivity analysis (PSA) with Monte Carlo simulations including 1,000 iterations [44]. This method enables to derive the cost-effectiveness acceptability curve (See Supplemental Material S5).
Role of the funding source
The study’s financial sponsors had no role in the design of the study. Neither were they involved in data collection, analysis or interpretation. Furthermore, they were not involved in the preparation, reviewing or approval of this manuscript.
Results
Base-case analysis
Health events, deaths and medical costs avoided over 10 years (end of 2016 to end of 2026)
The sizes of the simulated populations expected to attend the two DCRs over the 10-year period were estimated at 2,997 and 2,971 PWID in Paris and Strasbourg, respectively.
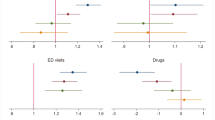
Table 4 presents the mean number [95% confidence interval – CI] of expected health events (HIV and HCV infections, abscesses and related IE, ED visits, overdoses and deaths) and mean [95% CI] expected medical costs (undiscounted) in both strategies (with/without DCR), as well as the mean number [95% CI] of health events and medical costs avoided in the DCR strategy. In addition, Fig. 2 shows the variations in the number of health events and associated costs (i.e., percentage decrease or increase) observed between both strategies.
The mean number of HIV infections decreased by 11.4% (in Paris) and 11.5% (in Strasbourg) in the DCR strategy (compared to the strategy without DCR) and the mean number of HCV infections by 5.6% and 5.9%, respectively. For the other health events (abscesses and related IE, overdoses, and ED visits), large reductions (i.e., between 65.1% and 76.6%) were observed in the DCR strategy compared to strategy without DCR. These avoided health events resulted in a 6.6% and 8.6% reduction in the number of deaths in Paris and Strasbourg, respectively, compared to strategy without DCR, corresponding to an increase in life expectancy of 5 and 6 months, respectively, in the DCR strategy.
The largest expected medical costs avoided concerned ED visits (Paris: k€3,455 [3,437; 3,473] / Strasbourg: k€3,040 [3,022; 3,059]), followed by IE (Paris: k€2,099 [2,063; 2,139] / Strasbourg: k€1,916 [1,873; 1,959]), and abscesses (Paris: k€1,309 [1,302; 1,315] / Strasbourg: k€1,155 [1,148; 1,161]. For HIV, the DCR strategy led to additional costs compared to the strategy without DCR (Paris: k€1,124 [38; 2,162] / Strasbourg: k1,284 [153; 2,348]). However, the total expected medical costs remained lower in the DCR strategy because of the total expected medical costs avoided, which were estimated at k€6,568 [5,512; 7,690] / k€5,793 [4,674; 6,953] in Paris and Strasbourg, respectively.
Cost-effectiveness of DCR over lifetime
When considering the costs of the DCRs and CAARUDs in the cost-effectiveness analysis, the total expected lifetime cost (after discounting) in the DCR strategy was higher than in the comparator strategy, corresponding to an incremental expected lifetime cost of k€16,178 [15,663; 16,700] in Paris and k€5,827 [5,291; 6,346] in Strasbourg.
In addition, in both cities, expected lifetime QALYs were also significantly higher in the DCR strategy than in the comparator, yielding 529 [492; 563] and 635 [599; 671]) QALYs gained with the DRC in Paris and Strasbourg, respectively. The ICER [95% CI] of the DCR strategy (versus the comparator strategy) was estimated at €30,600 [28,500; 33,100] and €9,200 [8,300; 10,100] per QALY in Paris and Strasbourg, respectively (See Table 5).
Alternative scenarios
In the first alternative scenario (i.e., decrease in the DCRs’ new entry rates after 2019), the ICER increased to €33,900 [31,600; 36,500] per QALY in Paris and €12,000 [10,900; 13,200] per QALY in Strasbourg (after discounting), as we assumed that the implementation and running costs of DCRs would remain constant even when the population size decreased as a consequence of lower attendance rates (estimated at -20% compared to the base-case value).
In the second scenario, where we assumed that the DCRs were set up inside CAARUD, health outcomes remained unchanged but the expected incremental cost fell (after discounting) to k€11,318 [10,804; 11,839] in Paris and k€1,592 in Strasbourg [1,055; 2,111], resulting in lower discounted ICERs (i.e €21,400 [19800; 23,200] and €2,500 [1,700; 3,300] per QALY in Paris and Strasbourg, respectively).
Sensitivity analysis
The results of the PSA are presented in Fig. 3.
Results obtained from 1,000 Monte-Carlo simulations for the probabilistic sensitivity analysis. Each simulation is represented according to the incremental effectiveness and the incremental cost of the DCR on the cost-effectiveness plane (A). The acceptability curves represent the proportion of simulations below the willingness-to-pay threshold as a function of the latter (B). The grey short-dashed line represents one-times the French GDP per capita (€33,300); the grey long-dashed line represents three-times the French GDP per capita (€99,900). The grey dot-dashed line represents the incremental cost-effectiveness ratio of interventions adopted in France based on their cost-effectiveness (€50,900). Results are presented for Paris and Strasbourg. Abbreviations: ICER=Incremental cost-effectiveness ratio; GDP=Gross domestic product; QALY=Quality-adjusted life-year
In Paris, the DCR had a 48 % probability of being very cost-effective at the WHO-recommended CET of €33,300/QALY (i.e., one times the 2023 French GDP per capita) and a 67% probability at WHO-recommended CET of €99,900/QALY (i.e. three times the 2023 French GDP per capita).
In Strasbourg, the DCR probability of being very cost-effective was 76% and the probability of cost-effective was 84% at the WHO CET of €33,300/QALY and €99,900/QALY CETs, respectively.
Using as a CET, the ICER of interventions that the national health authorities considered worthy of NHI funding (€50,000/QALY), the probability that DCR was cost-effective was 58% in Paris and 80% in Strasbourg. In addition, in Strasbourg, the DCR was cost-saving in 21% of the simulations.
Discussion
This modelling study provides information on the projected long-term health benefits, costs and cost-effectiveness of two recently established experimental DCRs in Paris and in Strasbourg in France. Our findings highlighted that over a ten-year period, attending a DCR would significantly reduce the occurrence of health events and therefore lead to significant medical costs avoided. Interestingly, the main potential health benefits of the two DCRs in our study were fewer abscesses and associated IEs (three quarters of these events being avoided) and a reduction in ED visits and overdoses (two thirds being avoided). However, only a relatively modest number of HIV (-6%) and HCV (-11%) infections would be avoided over the ten-year period. Besides, a total of 40 deaths would be prevented over 10 years corresponding to an increase in life expectancy of 5 and 6 months in PWID attending the Paris and Strasbourg DCRs, respectively. Overall, the two DCRs would avoid €6.6 million of medical costs in Paris and €5.8 million in Strasbourg over the 10 years. These significant savings are achieved mainly through avoided ED visits and abscesses (the most frequent events), as well as avoided IE (rare but costly events) which exceed the additional costs for HIV care due to concurrent mortality avoided.
In the lifetime cost-effectiveness analysis, ICERs [95% CI] were estimated at €30,600 [28,500; 33,100] per QALY in Paris and €9,200 [8,300; 10,100] per QALY in Strasbourg in the base-case analysis, taking into account stochastic uncertainty. These findings suggest that the two DCRs would be cost-effective in both cities when considering a CET of one times the French per capita GDP (€33,300 in 2023) and when considering CETs defined based on the ICERs of interventions that the national health authorities deem to be worthy of NHI funding (i.e., €50,000/QALY).
Furthermore, the scenario analysis highlighted that the cost-effectiveness of DCRs would be significantly improved if they are established inside existing harm reduction services (specifically CAARUD) as it would considerably reduce the costs. With ICERs [95% CI] decreasing to €21,400 [19,800; 23,200] per QALY in Paris and to €2,500 [1,700; 3,300] per QALY in Strasbourg, the DCRs would be a very cost-effective intervention, particularly in Strasbourg.
Our findings are consistent with those of other cost-effectiveness studies which also demonstrated that DCRs bring important health benefits to PWID and constitute a cost-effective intervention [24, 45,46,47,48,49,50,51,52,53,54,55,56,57]. However, all such studies were conducted in North American settings which differed substantially from the French context [58]. First, unlike in a part North America, harm reduction services and healthcare in case of health events - including hospital care - are provided free of charge in France through the NHI. Furthermore, overdose incidence and associated mortality are much lower in France than in North America (e.g., 463 deaths from opioid overdoses reported in France in 2017 [59] versus 75,673 between May 2020 and April 2021 in the United States [60], a country whose population is six times greater than that of France).
Our study is the first to demonstrate the economic value of DCRs in the setting of a European country characterized by a universal health system. It also provides more comprehensive information than previous studies on the health benefits of DCR by taking into account the effects of this harm reduction intervention on the most frequent health events which PWID face, and by including all-cause ED visits, something which has not been considered to date in the literature.
Nevertheless, this study has several limitations. First, observational data on DCR attendance were only available for the period 2016-2019, and we therefore assumed that the new entry rate would remain stable after 2019. However, the sensitivity analysis highlighted that when considering a lower entry rate, the ICERs would remain acceptable at the two CET defined above. Second, as with any simulation-based analysis, there was a large degree of uncertainty over the values used for the parameters, irrespective of the sources used to define their values (i.e., scientific literature, gray literature, and the COSINUS cohort). As we used a stochastic individual-based model to assess the uncertainty related to the population size, running the model was particularly time-consuming and it was not possible to perform an extensive deterministic, univariate sensitivity analysis to identify the most sensitive parameters. However, we performed a probabilistic sensitivity analysis taking all sources of uncertainties (uncertainty over the key parameters, uncertainty caused by the small study population size, and uncertainty due to the low incidence of certain events). In that analysis, the probability of the DCR being very cost effective was close to 50 % in Paris at the CET of one time the French per capita GDP and below 80% at the CET of three times the French per capita GDP (i.e. 48% and 67%, respectively) but close to 80% in Strasbourg for both CET (76% and 84%, respectively). Furthermore, the probability of DCR being cost-effective was 58% in Paris and 80% in Strasbourg using a CET of €50,000/QALY, corresponding to the ICERs of interventions previously adopted in France by the national health authorities. These results suggest a relatively good confidence in the cost-effectiveness of the DCR in Strasbourg but some uncertainty for Paris. The uncertainty on our results could be decreased by collecting additional data to refine parameters estimates. However, this process can be costly. A value of information study could inform on the interest of such studies. Finally, our study was strongly conservative as the model did not take into account all the potential benefits of DCRs, due to a lack of available data. Attending a DCR could improve PWID quality of life, especially mental health and reduce the occurrence of other bacterial infections common in this population, such as osteomyelitis or sepsis, which may be associated with high mortality and management costs. The omission of these potential benefits may therefore have led to an underestimation of the cost-effectiveness of the intervention [61]. Furthermore, we did not take into account non-health related potential positive social effects of DCRs, for example improved access to rights and reduced delinquency, as suggested by a recent sociological study [12]. Although taking these effects into account is outside the methodological framework of medico-economic analyses, it is important to stress that these potential additional benefits increase the economic value of DCRs.
Conclusion
Our long-term findings for experimental DRCs in France show that they are effective in terms of reductions in infections and overdoses, and are efficient at the standard cost-effectiveness threshold. The creation of a drug consumption space within pre-existing harm reduction structures would make this intervention even more cost-effective and represent a pragmatic approach to its scaling up in the future.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CI:
-
Confidence Intervals
- CAARUD:
-
Centre d'Accueil et d'Accompagnement à la Réduction des risques pour Usagers de Drogues - risk reduction center for drug users in France
- DCR:
-
Drug consumption room
- ED:
-
Emergency department
- GDP:
-
Gross domestic product
- IE:
-
Infective endocarditis
- ICER:
-
Incremental cost-effectiveness ratio
- HAS:
-
Haute Autorité de Santé – French High Health Authority
- HCV:
-
Hepatitis C virus
- HIV:
-
Human immunodeficiency virus
- LY:
-
Life-year
- LYS:
-
Life-year saved
- MERS:
-
Mobile emergency and resuscitation service
- NIH:
-
National health insurance
- OFDT:
-
Observatoire Français des Drogues et des Tendances addictives - French Observatory of Drugs and Drug Addiction
- PWID:
-
People who inject drugs
- QALYs:
-
Quality-adjusted life years
- SVR:
-
Sustained virological response
References
Degenhardt L, Peacock A, Colledge S, Leung J, Grebely J, Vickerman P, et al. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Glob Health. 2017;5(12):e1192-207.
Larney S, Peacock A, Mathers BM, Hickman M, Degenhardt L. A systematic review of injecting-related injury and disease among people who inject drugs. Drug Alcohol Depend. 2017;1(171):39–49.
Colledge S, Peacock A, Leung J, Larney S, Grebely J, Hickman M, et al. The prevalence of non-fatal overdose among people who inject drugs: A multi-stage systematic review and meta-analysis. Int J Drug Policy. 2019;1(73):172–84.
Smyth B, Hoffman V, Fan J, Hser YI. Years of Potential Life Lost among Heroin Addicts 33 Years after Treatment. Prev Med. 2007;44(4):369–74.
Glei DA, Preston SH. Estimating the impact of drug use on US mortality, 1999–2016. PLOS ONE. 2020;15(1): e0226732.
Fischer JA, Conrad S, Clavarino AM, Kemp R, Najman JM. Quality of life of people who inject drugs: characteristics and comparisons with other population samples. Qual Life Res Int J Qual Life Asp Treat Care Rehabil. 2013;22(8):2113–21.
Fatseas M, Denis C, Serre F, Dubernet J, Daulouède JP, Auriacombe M. Change in HIV-HCV risk-taking behavior and seroprevalence among opiate users seeking treatment over an 11-year period and harm reduction policy. AIDS Behav. 2012;16(7):2082–90.
Potier C, Laprévote V, Dubois-Arber F, Cottencin O, Rolland B. Supervised injection services: What has been demonstrated? A systematic literature review. Drug Alcohol Depend. 2014;1(145):48–68.
Tran V, Reid SE, Roxburgh A, Day CA. Assessing Drug Consumption Rooms and Longer Term (5 Year) Impacts on Community and Clients. Risk Manag Healthc Policy. 2021;14:4639–47.
Auriacombe M, Roux P, Briand Madrid L, Kirchherr S, Kervran C, Chauvin C, et al. Impact of drug consumption rooms on risk practices and access to care in people who inject drugs in France: the COSINUS prospective cohort study protocol. BMJ Open. 2019;9(2): e023683.
Roux P, Jauffret-Roustide M, Donadille C, Briand Madrid L, Denis C, Célérier I, et al. Impact of drug consumption rooms on non-fatal overdoses, abscesses and emergency department visits in people who inject drugs in France: results from the COSINUS cohort. Int J Epidemiol. 2022;dyac120.
Houborg E, Jauffret-Roustide M. Drug Consumption Rooms: Welfare State and Diversity in Social Acceptance in Denmark and in France. Am J Public Health. 2022;112(S2):S159-65.
Jauffret-Roustide M, Cailbault I. Drug consumption rooms: Comparing times, spaces and actors in issues of social acceptability in French public debate. Int J Drug Policy. 2018;56:208–17.
Durand-Zaleski I. International Health Care System Profiles, France. Commonw Fund. 2020;
Haute Autorité de Santé. Choix méthodologiques pour l’évaluation économique à la HAS. HAS Paris, France; 2020.
Ndawinz JDA, Costagliola D, Supervie V. New method for estimating HIV incidence and time from infection to diagnosis using HIV surveillance data: results for France. AIDS. 2011;25(15):1905–13.
Roy E, Richer I, Morissette C, Leclerc P, Parent R, Claessens C, et al. Temporal changes in risk factors associated with HIV seroconversion among injection drug users in eastern central Canada. AIDS Lond Engl. 2011;25(15):1897–903.
Leon L, Kasereka S, Barin F, Larsen C, Weill-Barillet L, Pascal X, et al. Age- and time-dependent prevalence and incidence of hepatitis C virus infection in drug users in France, 2004–2011: model-based estimation from two national cross-sectional serosurveys. Epidemiol Infect. 2017;145(5):895–907.
Pouget ER, Hagan H, Des Jarlais DC. Meta-analysis of hepatitis C seroconversion in relation to shared syringes and drug preparation equipment. Addict Abingdon Engl. 2012;107(6):1057–65.
Hope VD, McVeigh J, Marongiu A, Evans-Brown M, Smith J, Kimergård A, et al. Injection site infections and injuries in men who inject image- and performance-enhancing drugs: prevalence, risks factors, and healthcare seeking. Epidemiol Infect. 2015;143(1):132–40.
Spijkerman IJ, van Ameijden EJ, Mientjes GH, Coutinho RA, van den Hoek A. Human immunodeficiency virus infection and other risk factors for skin abscesses and endocarditis among injection drug users. J Clin Epidemiol. 1996;49(10):1149–54.
Foisel C. Endocardite infectieuse du sujet toxicomane [PhD Thesis]. UHP-Université Henri Poincaré; 2009.
Denis B, Dedobbeleer M, Benabderrazik A, Bizimungu DG, Sciera V. Les usagers de drogues suivis en médecine générale: recours fréquent aux services d’urgence mais pas toujours les bienvenus. Santé. 2009;(47).
Hood JE, Behrends CN, Irwin A, Schackman BR, Chan D, Hartfield K, et al. The projected costs and benefits of a supervised injection facility in Seattle, WA, USA. Int J Drug Policy. 2019;67:9–18.
Neale J. A response to Darke et al., ‘The ratio of non-fatal to fatal heroin overdose’. Addict Abingdon Engl. 2003 Aug;98(8):1171.
Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the economic evaluation of health care programmes. Oxford university press; 2015.
Cossais S, Schwarzinger M, Pol S, Fontaine H, Larrey D, Pageaux GP, et al. Quality of life in patients with chronic hepatitis C infection: Severe comorbidities and disease perception matter more than liver-disease stage. PLoS ONE [Internet]. 2019 May 3 [cited 2021 Mar 15];14(5). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6499434/
Pol S, Chevalier J, Branchoux S, Perry R, Milligan G, Gaudin AF. P0747: Health related quality of life and utility values in chronic hepatitis C patients: A cross-sectional study in France, the Uk and Germany. J Hepatol. 2015;62:S606.
Miners A, Phillips A, Kreif N, Rodger A, Speakman A, Fisher M, et al. Health-related quality-of-life of people with HIV in the era of combination antiretroviral treatment: a cross-sectional comparison with the general population. Lancet HIV. 2014;1(1):e32-40.
Sloan CE, Champenois K, Choisy P, Losina E, Walensky RP, Schackmanj BR, et al. Newer drugs and earlier treatment: Impact on lifetime cost of care for HIV-infected adults. AIDS Lond Engl. 2012;26(1):45–56.
Papot E, Landman R, Louni F, Charpentier C, Peytavin G, Certain A, et al. Budget impact of antiretroviral therapy in a French clinic cohort. AIDS Lond Engl. 2017;31(9):1271–9.
Assurance Maladie. Base de données des médicaments et informations tarifaires [Internet]. [cited 2024 Mar 6]. Available from: http://www.codage.ext.cnamts.fr/codif/bdm_it/
Etudes nationales de coûts sanitaires [Internet]. [cited 2020 Aug 17]. Available from: https://www.scansante.fr/applications/enc-mco
MCO par diagnostic ou acte | Stats ATIH [Internet]. [cited 2021 Feb 22]. Available from: https://www.scansante.fr/applications/statistiques-activite-MCO-par-diagnostique-et-actes
Référentiel de coût des unités d’oeuvres (RTC) | Stats ATIH [Internet]. [cited 2024 Mar 5]. Available from: https://www.scansante.fr/applications/cout-dunites-doeuvre/
Borthomieu L. Evaluation de l’activité des Médecins Correspondants SAMU et retour d’expérience des acteurs intervenant dans ce dispositif en Vienne entre 2015 et 2018. [PhD Thesis]. Université de Poitiers; 2018.
Institut national de la statistique et des études économiques. Convertisseur franc-euro [Internet]. [cited 2024 Mar 6]. Available from: https://www.insee.fr/fr/information/2417794
Briggs A, Sculpher M, Claxton K. Decision modelling for health economic evaluation. Oup Oxford; 2006.
Eddy DM, Hollingworth W, Caro JJ, Tsevat J, McDonald KM, Wong JB, et al. Model transparency and validation: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-7. Med Decis Mak Int J Soc Med Decis Mak. 2012;32(5):733–43.
WHO. Making choices in health : WHO guide to cost-effectiveness analysis [Internet]. World Health Organization; 2003 [cited 2019 Jul 23]. Available from: https://apps.who.int/iris/handle/10665/42699
Statistics | Eurostat [Internet]. [cited 2024 Mar 1]. Available from: https://ec.europa.eu/eurostat/databrowser/view/sdg_08_10/default/table?lang=en
Haute Autorité de Santé. KEYTRUDA (pembrolizumab) - Cancer colorectal métastatique [Internet]. Avis sur les médicaments; 2021. Available from: https://www.has-sante.fr/jcms/p_3272286/fr/keytruda-pembrolizumab-cancer-colorectal-metastatique
Haute Autorité de Santé. Elargissement de La Vaccination Contre Les Papillomavirus Aux Garçons [Internet]. Recommandation vaccinale; 2019. Available from: https://www.has-sante.fr/upload/docs/application/pdf/2019-12/recommandation_vaccinale_elargissement_de_la_vaccination_contre_les_papillomavirus_aux_garcons.pdf
Briggs AH, Weinstein MC, Fenwick EAL, Karnon J, Sculpher MJ, Paltiel AD, et al. Model parameter estimation and uncertainty analysis: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force Working Group-6. Med Decis Mak Int J Soc Med Decis Mak. 2012;32(5):722–32.
Andresen MA, Boyd N. A cost-benefit and cost-effectiveness analysis of Vancouver’s supervised injection facility. Int J Drug Policy. 2010;21(1):70–6.
Andresen MA, Jozaghi E. The point of diminishing returns: an examination of expanding Vancouver’s Insite. Urban Stud. 2012;49(16):3531–44.
Bayoumi AM, Zaric GS. The cost-effectiveness of Vancouver’s supervised injection facility. CMAJ Can Med Assoc J J Assoc Medicale Can. 2008;179(11):1143–51.
Enns EA, Zaric GS, Strike CJ, Jairam JA, Kolla G, Bayoumi AM. Potential cost-effectiveness of supervised injection facilities in Toronto and Ottawa. Canada Addict Abingdon Engl. 2016;111(3):475–89.
Irwin A, Jozaghi E, Weir BW, Allen ST, Lindsay A, Sherman SG. Mitigating the heroin crisis in Baltimore, MD, USA: a cost-benefit analysis of a hypothetical supervised injection facility. Harm Reduct J. 2017 12;14(1):29.
Irwin A, Jozaghi E, Bluthenthal RN, Kral AH. A cost-benefit analysis of a potential supervised injection facility in San Francisco, California, USA. J Drug Issues. 2017;47(2):164–84.
Jozaghi E. A cost-benefit/cost-effectiveness analysis of an unsanctioned supervised smoking facility in the Downtown Eastside of Vancouver, Canada. Harm Reduct J. 2014;13(11):30.
Jozaghi E, Jackson A. Examining the potential role of a supervised injection facility in Saskatoon, Saskatchewan, to avert HIV among people who inject drugs. Int J Health Policy Manag. 2015;4(6):373–9.
Jozaghi E, Reid AA. The potential role for supervised injection facilities in Canada’s largest city. Toronto Int Crim Justice Rev. 2015;25(3):233–46.
Jozaghi E, Reid AA, Andresen MA, Juneau A. A cost-benefit/cost-effectiveness analysis of proposed supervised injection facilities in Ottawa, Canada. Subst Abuse Treat Prev Policy. 2014;4(9):31.
Jozaghi E, Reid AA, Andresen MA. A cost-benefit/cost-effectiveness analysis of proposed supervised injection facilities in Montreal, Canada. Subst Abuse Treat Prev Policy. 2013;9(8):25.
Pinkerton SD. Is Vancouver Canada’s supervised injection facility cost-saving? Addict Abingdon Engl. 2010;105(8):1429–36.
Pinkerton SD. How many HIV infections are prevented by Vancouver Canada’s supervised injection facility? Int J Drug Policy. 2011;22(3):179–83.
Nguemeni Tiako MJ, Mori M, Bin Mahmood SU, Shioda K, Mangi A, Yun J, et al. Recidivism Is the Leading Cause of Death Among Intravenous Drug Users Who Underwent Cardiac Surgery for Infective Endocarditis. Semin Thorac Cardiovasc Surg. 2019;31(1):40–5.
Brisacier AC, Díaz-Gómez C, Martinez M. Harms and harm reduction workbook.
Drug Overdose Deaths in the U.S. Top 100,000 Annually [Internet]. 2021 [cited 2022 May 17]. Available from: https://www.cdc.gov/nchs/pressroom/nchs_press_releases/2021/20211117.htm
Gordon RJ, Lowy FD. Bacterial infections in drug users. N Engl J Med. 2005;353(18):1945–54.
Acknowledgments
We would like to thank the French Institute for Public Health Research (IRESP) and the IRESP scientific committee (Marc Bardou, Christian Ben-Lakhdar, Eric Breton, Olivier Cottencin, Helene Donnadieu-Rigole, Xavier Laqueille, Jennifer O’Loughlin, Christophe Tzourio et Frank Zobel), the Gaïa (Paris) and Ithaque (Strasbourg) associations for their financial and active DCR membership file data, the Mésocentre of the University of Lille for the computing resources, Cristina Diaz-Gomez of the French Observatory of Drugs and Addictive Tendencies (OFDT) for sharing data on CAARUD budgets, and Jude Sweeney for English editing of this article.
Funding
This work was supported by the French Government Addiction Agency MILDECA (the inter-ministerial mission to fight against drugs and addictive practices). The study’s financial sponsors had no role in the design of the study. Neither were they involved in data collection, analysis or interpretation. Furthermore, they were not involved in the preparation, reviewing or approval of this manuscript.
Author information
Authors and Affiliations
Contributions
AC, PR, SB: study concept and design; methodology; AC: model implementation, numerical simulations, directly accessed and verified the underlying data reported in the manuscript; AC and SB co-writing first draft; PR and SB: funding acquisition; PR, SB: study supervision; LBM: Data acquisition, literature review; CD: data analysis. MJR, LL, MA, PR: main investigators of the COSINUS cohort. AC, CD, LBM, GM, MJR, LL, MA, PR, SB: interpretation of results; critical revision of the manuscript for important intellectual content. AC, CD, LBM, GM, MJR, LL, MA, PR, SB had full access to all the data in the study and accept responsibility for the decision to submit for publication.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Cousien, A., Donadille, C., Madrid, L.B. et al. Cost-effectiveness of drug consumption rooms in France: a modelling study. BMC Public Health 24, 1426 (2024). https://doi.org/10.1186/s12889-024-18909-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-024-18909-9