Abstract
Background
Regular prenatal physical activity provides numerous health benefits to both mother and fetus. However, little is known about the physical activity status of pregnant women in China and whether they meet the current guidelines for prenatal physical activity. The aims of the study were to assess physical inactivity status and associated factors among pregnant women in Shanghai, China.
Methods
A cross-sectional study of 1636 pregnant women were recruited at a tertiary obstetrics and gynecology hospital in Shanghai. Maternal sociodemographic characteristics and health information were obtained using structured questionnaires or from the electronic medical records. Physical inactivity status was assessed using the International Physical Activity Questionnaire-Short Form. Factors pertinent to physical inactivity were identified by binary logistic regression and were reported with adjusted odds ratios (ORs) and 95% confidence intervals (CIs). All statistical analyses were performed using the SPSS software package.
Results
In total, the prevalence of physical inactivity was 47.5%. Walking was the main form of physical activity and only 2.8% of the pregnant women achieved the goal of at least 150 min of moderate-intensity physical activity weekly. Multivariate logistic regression identified a significant negative association of physical inactivity with personal monthly income (adjusted OR 0.648, 95% CI 0.505–0.831), engagement in regular exercise before pregnancy (adjusted OR 0.575, 95% CI 0.464–0.711) and in the second (adjusted OR 0.534, 95% CI 0.411–0.693) or third (adjusted OR 0.615, 95% CI 0.470–0.806) trimester of pregnancy. Women with nausea or vomiting during pregnancy were more likely to be physically inactive during pregnancy (adjusted OR 1.307, 95% CI 1.002–1.705).
Conclusion
Physical inactivity is highly prevalent among pregnant women in China. Further efforts should be taken to overcome the barriers to prenatal physical activity and to promote moderate- to vigorous-intensity activities among Chinese pregnant women.
Similar content being viewed by others
Background
Physical activity (PA) is defined as any bodily movement produced by skeletal muscles that requires energy expenditure, and is an essential element of a healthy lifestyle [1]. A growing body of evidence suggests that regular prenatal exercise has health benefits for both mother and fetus [2]. Maternal benefits of being active during pregnancy include control of gestational weight gain [3], less risk of gestational diabetes mellitus (GDM) [4], improved cardiovascular function [5], relief of lumbar and pelvic pain [1], a shorter delivery time [6], less likelihood of cesarean section [7], and, to some extent, improved psychological well-being [8]. A randomized controlled trial that included 300 overweight or obese Chinese women with uncomplicated singleton pregnancies found that initiation of cycling exercise for at least 30 min three times per week during the first trimester of pregnancy significantly decreased the amount of gestational weight gain and the risk of GDM with no increase in the risk of preterm birth [4]. Prenatal exercise also has some benefits for the fetus; for example, facilitating neurological development [9] and reducing the risk of obesity at birth and in early childhood [10].
Considering the physiological and psychological benefits of engaging in regular prenatal exercise, the guidelines published by the World Health Organization (WHO) [2], the American College of Obstetricians and Gynecologists (ACOG) [1], the Society of Obstetricians and Gynecologists of Canada (SOGC), the Canadian Society for Exercise Physiology (CSEP) [11], the Department of Health and Social Care, UK [12], and the Australian Government Department of Health [13] all recommend that at least 150 min of moderate-intensity PA per week during pregnancy should be maintained when there are no pregnancy-related contraindications or complications. However, pregnant women are often physically inactive, with many who were previously active choosing to become inactive during pregnancy [14]. Compared with the PA level before conception, the level of PA during pregnancy is expected to be much lower and take a relatively single form. Recent studies showed that the rate of physical inactivity among pregnant women ranged from 27.2 to 88.9% [15,16,17,18,19]. In Brazil, Galliano et al. evaluated the PA level in 2,706 pregnant women with GDM in the second or third trimester using the International Physical Activity Questionnaire (IPAQ) and found that only 34.3% reached the recommended levels [18]. Studies in other countries have yielded similar results [17, 20]. Several studies have investigated PA in pregnant women in China and found that the proportion of women meeting the recommendations ranged from 11% in Tian** [16] to 57.1% in Chengdu [Covariates and other measurements Sociodemographic, behavioral, obstetric, and social support data were collected using a structured questionnaire that was developed in consultation with experts in the field and pilot tested in 10 respondents before the survey. The sociodemographic characteristics consisted of age, ethnicity, level of education, current employment status, personal monthly income, and pre-pregnancy body mass index (BMI), which was calculated as body weight divided by the square of height. Using the Chinese classification, women with a BMI < 18.5 kg/m2 are considered underweight, those with a BMI of 18.5–23.9 kg/m2 as normal weight, those with a BMI of 24–27.9 kg/m2 as overweight, and those with a BMI ≥ 28 kg/m2 as obese [34]. The behavioral characteristics before pregnancy are briefly described below. Regular exercise before pregnancy was defined as having consciously engaged in walking, cycling, yoga, running, or other PA for at least 30 min/week in the preconception period [35]. Pre-pregnancy smoking was defined as daily or intermittent smoking and passive smoking was defined as breathing second-hand tobacco smoke for more than 15 min a day in the 3 months before conception. Alcohol consumption before pregnancy was defined as drinking at least half bottle of beer, 40 mL of white wine, or 125 mL of red wine in a month. The obstetric parameters included history of spontaneous abortion, parity, stage of pregnancy, singleton pregnancy, nausea and vomiting during pregnancy, measures taken to prevent miscarriage, and GDM. The last 3 questions in the structured questionnaire were about social support. We collected information about social support by asking whether the participants had support from family members, friends and medical workers, which were sourced from the Social Support Rating Scale [38]. Prenatal anxiety status was assessed using the Chinese version of the Self-rating Anxiety Scale, which contains 20 items and is scored by calculating the frequency of symptoms corresponding to anxiety in the past week. Each item is scored on a 4-point scale ranging from 1 to 4; the scores for the 20 items are then added to obtain a rough score, which is then multiplied by 1.25 and the integer component taken to derive the standard score. The cut-off score for this scale is 50 points; a score of 50–59 is classified as mild anxiety, 60–69 as moderate anxiety, and > 70 as severe anxiety [39]. Prenatal symptoms of depression were measured by the Edinburgh Postnatal Depression Scale, which is considered to have acceptable reliability, validity and applicability as a screening tool for depression during pregnancy [40]. This scale contains 10 items, each of which is scored from 0 to 3, with higher scores indicating a higher likelihood of perinatal depression. In general, a cut-off score of 10 or higher is recommended for detection of potential depression in Asian pregnant women [41]. The characteristics of the participants were presented as descriptive statistics. Continuous variables were summarized as the mean ± standard deviation if normally distributed and as the median (interquartile range) if not. Categorical variables were shown as the frequency (percentage). The dependent variable in this study was physical inactivity, which was defined as total energy expenditure < 600 MET min/week. A binary logistic regression model was used to identify factors associated with physical inactivity in pregnant women. Adjusted odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. The steps used to identify these factors were as follows. First, we used backward elimination to remove variables with a p-value > 0.2. Second, considering maternal age, level of education, pre-pregnancy BMI, parity, and history of spontaneous abortions to be clinically relevant or related to the level of PA in previous studies [20, 25, 26], we also added these five variables into the model. Finally, maternal age, level of education, current employment status, personal monthly income, pre-pregnancy BMI, regular exercise before pregnancy, alcohol consumption before pregnancy, history of spontaneous abortions, parity, stage of pregnancy, nausea and vomiting during pregnancy, and prenatal sleep quality were entered as independent variables into the logistic regression model. All statistical analyses were performed using the SPSS software package (version 25.0; IBM Corp., Armonk, NY, USA). A p-value of < 0.05 was considered statistically significant.Statistical analysis
Results
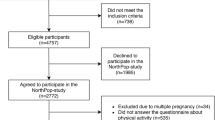
In total, 1636 women were enrolled in the study. Twenty-two of the women did not meet the inclusion criteria, 64 dropped out during the study period, and 35 returned invalid questionnaires (incomplete or with inconsistent responses), leaving data for 1515 participants for analysis.
Characteristics of the participants
Table 1 presented the sociodemographic, behavioral, obstetric and social support characteristics of the study participants. The average age was 30.6 ± 3.7 years, and 18.1% of the women were overweight or obese according to the Chinese BMI classification. The socioeconomic status of the majority of the participants was good, with 75.7% having a personal monthly income of more than 10,000 yuan; 71.7% of the women were still working. More than half of the women (55.5%) undertook regular exercise before pregnancy. In total, 13.1% of the women reported tobacco exposure before pregnancy. Most of the women were nulliparous and 79.3% experienced severe nausea and vomiting during pregnancy. The questionnaires were completed by 668 women in the first trimester, 389 in the second trimester, and 458 in the third trimester. Poor prenatal sleep quality was a common complaint. Overall, the participants were well-educated with 92.8% having completed a college degree.
Physical activity levels of the participants
Table 2 showed the PA level overall during pregnancy and according to intensity. The median energy expenditure on total PA was 693 MET min/week. Overall, 129 women did not report any type of PA. Walking as the main form of PA accounted for 92.7% of total MET. The prevalence of physical inactivity was 47.5%, and only 2.8% of women achieved the goal of at least 150 min of moderate-intensity PA per week. Furthermore, 94.6% of the women who reported sufficient PA did not meet the guideline of more than 150 min of moderate-intensity PA per week (Table 3).
Factors related to physical inactivity during pregnancy
The results of the multivariable binary logistic regression analysis were presented in Table 4. Pregnant women with an individual income of more than 10,000 yuan per month (adjusted OR 0.648, 95% CI 0.505–0.831) were less likely to be physically inactive than those with a lower income. Moreover, regular exercise before conception contributed to maintaining adequate PA during pregnancy (adjusted OR 0.575, 95% CI 0.464–0.711). However, the physical inactivity rate was significantly higher in the first trimester than in the second trimester (adjusted OR 0.534, 95% CI 0.411–0.693) or third trimester (adjusted OR 0.615, 95% CI 0.470–0.806). Women who had nausea and vomiting during pregnancy were 1.307 times more likely to be physically inactive than those who did not have these symptoms (adjusted OR 1.307, 95% CI 1.002–1.705).
Discussion
This study evaluated physical inactivity and related factors in pregnant women in Shanghai, China. We found that 47.5% of our study participants do not engage in sufficient PA during pregnancy, with an average of only 287.78 MET min/week, which is reasonably consistent with the figure of 44.75% reported for the 15 provinces in China [22]. Use of the same criteria with a total energy expenditure of < 600 MET min/week to define physical inactivity may account for these findings. However, the proportion of pregnant women in a study from Serbia [15] who do not have adequate PA was found to be slightly lower despite using the same classification standard, and may reflect a difference in the trimesters studied or a difference in the study populations. In a study that used the Prenatal Physical Activity Questionnaire, 57.1% of pregnant women reached the recommended level (≥ 150 min of moderate-intensity activity per week) in Chengdu [24, 44], and underdeveloped countries in South Africa [20] consistently reported lower PA levels in pregnant women, despite using different measurement tools and evaluation methods.
In our study, only 2.8% of the pregnant women achieved the level of prenatal PA recommended by the international guidelines. In fact, moderate-intensity activity was assigned a MET value of 4 in this investigation, to some extent, the international guidelines of at least 150 min of moderate-intensity activity per week is equivalent to 600 MET min/week. However, more than ninety percent of our study participants who reported sufficient PA of ≥ 600 MET min/week did not meet the international guidelines. The main reason for such a huge difference between the two results may be that walking, a light-intensity activity, was the major form of PA and accounted for 92.7% of total MET values in our population. It has been suggested that some health care workers have conservative views about PA during pregnancy and question the safety of moderate-to-vigorous intensity PA, which may result in recommendations for lighter PA during pregnancy [45]. Our finding that PA during pregnancy consisted of walking which accounted for a relatively large proportion of PA in our study is similar to that of studies in Ethiopia and Poland [17, 27]. In our study, walking was assigned a MET value of 3.3 and included in calculating the result of PA level to define sufficient PA. However, walking is excluded from the international guidelines, which focus only on moderate or vigorous intensity PA. And without any doubt, moderate or vigorous intensity activities can provide substantial health benefits; for example, pregnant women engaged in at least 600 MET min/week of moderate PA (e.g., 140 min of brisk walking and stationary cycling) can reduce their risk of GDM or gestational hypertension by at least 25% [46]. Light-intensity activities, such as walking, can have health benefits too, but the greatest benefit often occurs when sedentary behavior is replaced with moderate or vigorous intensity PA [47]. Therefore, we should suggest a gradual increase in the amount and intensity of activity; for example, increasing their walking pace and choosing exercise that is more acceptable, such as brisk walking, to promote the moderate or vigorous intensity PA recommended by the guidelines.
We used a multivariable logistic regression model to identify factors associated with physical inactivity during pregnancy in Shanghai and found that women who had engaged in regular exercise before pregnancy were more likely to have a higher PA level during pregnancy than women who had not, which is consistent with studies from Arba Minch Town in Ethiopia [25], Campinas in Brazil [24], data from the US Centers for Disease Control and Prevention [19] and a study performed in Chengdu in China [21]. This finding indicates that starting exercise before conception may help women to achieve adequate PA during pregnancy and underscores the importance of lifestyle changes before pregnancy. Of note, a prospective study from Spain found that nearly half of 1,175 pregnant women engaged in sufficient PA before pregnancy but did not maintain their PA level during pregnancy [14]. This may be attributed to physical discomfort during pregnancy, especially nausea and vomiting [48], which is consistent with our findings, in addition to low back pain, fatigue, fear of injury, and uncertainty about the safety of exercising during pregnancy [49].
In our study, women with higher incomes were less likely to have a low PA level during pregnancy, which is in line with the reports by Galliano et al. and Yu et al. [18, 23]. The study in Serbian women found that inadequate PA during pregnancy was more marked in low-income families, which could reflect a lack of leisure time for exercise [15]. However, unlike other studies [26, 50], we did not find any relationship between physical inactivity status during pregnancy and other social factors, such as social support or educational level. We also found a decrease in the likelihood of physical inactivity as pregnancy advanced from the first trimester to the second and third trimesters. A cross-sectional study of 1077 Chinese pregnant women found that pregnant women in the third trimester were more likely to meet the international guidelines than those in the early stages of pregnancy [21]. Likely explanations for this finding include the traditional Chinese practice of protecting the fetus from miscarriage by resting and physical discomfort, particularly morning sickness. However, as mentioned above, the available evidence indicates that interventions to improve the PA level during late pregnancy should be implemented during early pregnancy or even before pregnancy. The cohort study showed that women with adequate PA levels in early pregnancy were more likely to engage in adequate PA in mid-to-late pregnancy [22]. Similarly, Okafor and Goon showed that women who started PA in the first trimester were more active and more likely to meet the guideline than those who started PA in the second trimester [20].
Although various factors including socioeconomic status, PA levels before pregnancy, as well as physiological changes during pregnancy, may affect the ability to engage in PA, there is limited qualitative information in the literature on how these factors affect women’s PA during pregnancy. More qualitative or mix-method studies are needed in the future to improve our understanding about women’s willingness or otherwise to engage in PA during pregnancy and inform interventions in clinical practice. What’s more, walking was the predominant form of PA in our population. It is worth thinking about the effects of different intensities of PA, such as walking or moderate-intensity activities, on pregnancy outcomes or long-term health effects of mothers and offspring. Therefore, further comparative studies are needed.
The results of our study provided some basic information of PA status among Chinese pregnant women, which can also help health professionals as well as policymakers to take tailored measures to promote adequate PA among this population. To be specific, first, early intervention is needed, especially for women who do not exercise regularly before pregnancy. Second, as to the low-income pregnant women, we advocate the government and relevant departments to implement corresponding policies, such as providing free consultations, sports grounds and brochures, to alleviate the impacts of poor economic conditions. In addition, it is necessary to provide systematic training of PA knowledge for medical workers, which is crucial for them to provide evidence-based scientific recommendations of PA for pregnant women.
Limitations
This study had several limitations. First, the PA data were self-reported using the IPAQ, which introduces the possibility of reporting bias. More accurate data on the amount and frequency of PA during pregnancy would be obtained by use of electronic activity monitors, such as wearable bracelets, accelerometers, or pedometers. Second, the study had a cross-sectional design, which meant that we could not obtain information on changes in the PA level as the women moved through the trimesters. Therefore, prospective studies are needed in the future. Third, our study participants were from only one hospital in Shanghai and may not be representative of the entire city. However, despite these limitations, this study provides some useful information concerning physical inactivity during pregnancy in Shanghai that can be used for comparative purposes in future studies.
Conclusion
The prevalence of physical inactivity among pregnant women in China remains high, particularly among women with a lower monthly income, those who are not engaged in regular exercise before pregnancy, and those who experience nausea and vomiting during pregnancy. More efforts should be taken to help these women to overcome the barriers to prenatal PA. Furthermore, we need a better understanding of the dynamic changes in PA that occur during pregnancy and whether these may be affected by the coronavirus pandemic, which is likely to continue into the future. Further researches including a combination of quantitative analysis and qualitative method are needed to obtain more information on how to promote PA during pregnancy.
Availability of data and materials
The data that support the findings of this study can be obtained by contacting the corresponding author Na Wang, upon reasonable request.
Abbreviations
- PA:
-
Physical activity
- GDM:
-
Gestational diabetes mellitus
- WHO:
-
World Health Organization
- ACOG:
-
American College of Obstetricians and Gynecologists
- SOGC:
-
Society of Obstetricians and Gynecologists of Canada
- CSEP:
-
Canadian Society for Exercise Physiology
- IPAQ:
-
International Physical Activity Questionnaire
- MET:
-
Metabolic equivalent
- IPAQ-SF:
-
International Physical Activity Questionnaire Short Form
- TEE:
-
Total energy expenditure
- BMI:
-
Body mass index
- PSQI:
-
Pittsburgh Sleep Quality Index
- ORs:
-
Odds ratios
- CIs:
-
Confidence intervals
- IQR:
-
Interquartile range
References
Birsner ML, Gyamfi-Bannerman C. Practice CoO: Physical activity and exercise during pregnancy and the postpartum period: ACOG committee opinion, number 804. Obstet Gynecol. 2020;135(4):e178–88. https://doi.org/10.1097/aog.0000000000003772.
Bull FC, Al-Ansari SS, Biddle S, Borodulin K, Buman MP, Cardon G, Carty C, Chaput JP, Chastin S, Chou R, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020;54(24):1451–62. https://doi.org/10.1136/bjsports-2020-102955.
Barakat R, Refoyo I, Coteron J, Franco E. Exercise during pregnancy has a preventative effect on excessive maternal weight gain and gestational diabetes. A randomized controlled trial. Brazilian J Phys Ther. 2019;23(2):148–55. https://doi.org/10.1016/j.bjpt.2018.11.005.
Wang C, Wei Y, Zhang X, Zhang Y, Xu Q, Sun Y, Su S, Zhang L, Liu C, Feng Y, et al. A randomized clinical trial of exercise during pregnancy to prevent gestational diabetes mellitus and improve pregnancy outcome in overweight and obese pregnant women. Am J Obstet Gynecol. 2017;216(4):340–51. https://doi.org/10.1016/j.ajog.2017.01.037.
Morales-Suárez-Varela M, Clemente-Bosch E, Peraita-Costa I, Llopis-Morales A, Martínez I, Llopis-González A. Maternal physical activity during pregnancy and the effect on the mother and newborn: a systematic review. J Phys Act Health. 2021;18(1):130–47. https://doi.org/10.1123/jpah.2019-0348.
Rodríguez-Blanque R, Sánchez-García JC, Sánchez-López AM, Aguilar-Cordero MJ. Physical activity during pregnancy and its influence on delivery time: a randomized clinical trial. PeerJ. 2019;7:e6370. https://doi.org/10.7717/peerj.6370.
Russo LM, Harvey MW, Pekow P, Chasan-Taber L. Physical activity and risk of cesarean delivery in Hispanic women. J Phys Act Health. 2019;16(2):116–24. https://doi.org/10.1123/jpah.2018-0072.
Coll CVN, Domingues MR, Stein A, da Silva BGC, Bassani DG, Hartwig FP, da Silva ICM, da Silveira MF, da Silva SG, Bertoldi AD. Efficacy of regular exercise during pregnancy on the prevention of postpartum depression: the PAMELA randomized clinical trial. JAMA Netw Open. 2019;2(1):e186861. https://doi.org/10.1001/jamanetworkopen.2018.6861.
Nakahara K, Michikawa T, Morokuma S, Ogawa M, Kato K, Sanefuji M, Shibata E, Tsuji M, Shimono M, Kawamoto T, et al. Influence of physical activity before and during pregnancy on infant’s sleep and neurodevelopment at 1-year-old. Sci Rep. 2021;11(1):8099. https://doi.org/10.1038/s41598-021-87612-1.
Taylor RW, Gray AR, Heath AM, Galland BC, Lawrence J, Sayers R, Healey D, Tannock GW, Meredith-Jones KA, Hanna M, et al. Sleep, nutrition, and physical activity interventions to prevent obesity in infancy: follow-up of the Prevention of Overweight in Infancy (POI) randomized controlled trial at ages 3.5 and 5 y. Am J Clin Nutr. 2018;108(2):228–36. https://doi.org/10.1093/ajcn/nqy090.
Mottola MF, Davenport MH, Ruchat SM, Davies GA, Poitras VJ, Gray CE, Jaramillo Garcia A, Barrowman N, Adamo KB, Duggan M, et al. 2019 Canadian guideline for physical activity throughout pregnancy. Br J Sports Med. 2018;52(21):1339–46. https://doi.org/10.1136/bjsports-2018-100056.
Smith R, Reid H, Matthews A, Calderwood C, Knight M, Foster C. Infographic: physical activity for pregnant women. Br J Sports Med. 2018;52(8):532–3. https://doi.org/10.1136/bjsports-2017-098037.
Homer CS, Oats J, Middleton P, Ramson J, Diplock S. Updated clinical practice guidelines on pregnancy care. Med J Aust. 2018;209(9):409–12. https://doi.org/10.5694/mja18.00286.
Amezcua-Prieto C, Olmedo-Requena R, Jiménez-Mejías E, Mozas-Moreno J, Lardelli-Claret P, Jiménez-Moleón JJ. Factors associated with changes in leisure time physical activity during early pregnancy. Int J Gynaecol Obstet. 2013;121(2):127–31. https://doi.org/10.1016/j.ijgo.2012.11.021.
Todorovic J, Terzic-Supic Z, Bjegovic-Mikanovic V, Piperac P, Dugalic S, Gojnic-Dugalic M: Factors Associated with the Leisure-Time Physical Activity (LTPA) during the First Trimester of the Pregnancy: The Cross-Sectional Study among Pregnant Women in Serbia. Int J Environ Res Public Health. 2020, 17(4). https://doi.org/10.3390/ijerph17041366
Zhang Y, Dong S, Zuo J, Hu X, Zhang H, Zhao Y. Physical activity level of urban pregnant women in Tian**, China: a cross-sectional study. PLoS One. 2014;9(10):e109624. https://doi.org/10.1371/journal.pone.0109624.
Hailemariam TT, Gebregiorgis YS, Gebremeskel BF, Haile TG, Spitznagle TM. Physical activity and associated factors among pregnant women in Ethiopia: facility-based cross-sectional study. BMC Pregnancy Childbirth. 2020;20(1):92. https://doi.org/10.1186/s12884-020-2777-6.
Galliano LM, Del Vecchio AHM, Silvani J, Façanha C, Del Vecchio FB. Physical activity level in women with gestational diabetes mellitus: Lifestyle INtervention for Diabetes prevention After pregnancy (LINDA-Brasil) study. J Diabetes. 2019;11(6):457–65. https://doi.org/10.1111/1753-0407.12872.
Santo EC, Forbes PW, Oken E, Belfort MB. Determinants of physical activity frequency and provider advice during pregnancy. BMC Pregnancy Childbirth. 2017;17(1):286. https://doi.org/10.1186/s12884-017-1460-z.
Okafor UB, Goon DT: Physical Activity Level during Pregnancy in South Africa: A Facility-Based Cross-Sectional Study. Int J Environ Res Public Health. 2020, 17(21). https://doi.org/10.3390/ijerph17217928
**ang M, Zhang J, Liang H, Zhang Z, Konishi M, Hu H, Nishimaki M, Kim HK, Tabata H, Shimizu H, et al. Physical activity and dietary intake among Chinese pregnant women: an observational study. BMC Pregnancy Childbirth. 2019;19(1):295. https://doi.org/10.1186/s12884-019-2452-y.
Lü Y, Feng Y, Ma S, Jiang Y, Ma L. Changes in physical activity across pregnancy among Chinese women: a longitudinal cohort study. BMC Womens Health. 2021;21(1):236. https://doi.org/10.1186/s12905-021-01377-3.
Yu H, He J, Szumilewicz A: Pregnancy Activity Levels and Impediments in the Era of COVID-19 Based on the Health Belief Model: A Cross-Sectional Study. Intl J Environ Res Public Health. 2022, 19(6). http://doi.org/https://doi.org/10.3390/ijerph19063283
Nascimento SL, Surita FG, Godoy AC, Kasawara KT, Morais SS. Physical activity patterns and factors related to exercise during pregnancy: a cross sectional study. PLoS One. 2015;10(6):e0128953. https://doi.org/10.1371/journal.pone.0128953.
Beyene MM, Shimbre MS, Ukke GG, Gebremichael MA, Gurara MK. Factors associated with antenatal exercise in Arba Minch town, Southern Ethiopia: a community-based cross-sectional study. PLoS One. 2022;17(2):e0260840. https://doi.org/10.1371/journal.pone.0260840.
Syed Nor SF, Idris IB, Md Isa Z. Physical inactivity in early pregnancy and the determinants in an urban city setting of Kuala Lumpur, Malaysia. BMC Public Health. 2022;22(1):93. https://doi.org/10.1186/s12889-022-12513-5.
Walasik I, Kwiatkowska K, Kosińska Kaczyńska K, Szymusik I: Physical Activity Patterns among 9000 Pregnant Women in Poland: A Cross-Sectional Study. Int J Environ Res Public Health. 2020, 17(5). https://doi.org/10.3390/ijerph17051771
Lee DT, Ngai IS, Ng MM, Lok IH, Yip AS, Chung TK. Antenatal taboos among Chinese women in Hong Kong. Midwifery. 2009;25(2):104–13. https://doi.org/10.1016/j.midw.2007.01.008.
Eng J. Sample size estimation: how many individuals should be studied? Radiology. 2003;227(2):309–13. https://doi.org/10.1148/radiol.2272012051.
Martínez-Mesa J, González-Chica DA, Duquia RP, Bonamigo RR, Bastos JL. Sampling: how to select participants in my research study? An Bras Dermatol. 2016;91(3):326–30. https://doi.org/10.1590/abd1806-4841.20165254.
Meh K, Jurak G, Sorić M, Rocha P, Sember V: Validity and Reliability of IPAQ-SF and GPAQ for Assessing Sedentary Behaviour in Adults in the European Union: A Systematic Review and Meta-Analysis. Int J Environ Res Public Health. 2021;18(9). https://doi.org/10.3390/ijerph18094602
Fan M, Lyu J, He P. Chinese guidelines for data processing and analysis concerning the International Physical Activity Questionnaire. Zhonghua Liu **ng Bing Xue Za Zhi. 2014;35(8):961–4.
Guercio BJ, Zhang S, Ou FS, Venook AP, Niedzwiecki D, Lenz HJ, Innocenti F, O’Neil BH, Shaw JE, Polite BN, et al. Associations of physical activity with survival and progression in metastatic colorectal cancer: results from cancer and Leukemia Group B (Alliance)/SWOG 80405. J Clin Oncol. 2019;37(29):2620–31. https://doi.org/10.1200/jco.19.01019.
Wang N, Deng Z, Wen L, Ding Y, He G: Relationships between Maternal Dietary Patterns and Blood Lipid Levels during Pregnancy: A Prospective Cohort Study in Shanghai, China. Int J Environ Res Public Health. 2021;18(7).https://doi.org/10.3390/ijerph18073701
Kim S, Kang W, Cho S, Lim DY, Yoo Y, Park RJ, Lee BC, Moon JD, Park WJ. Associations between blood lead levels and coronary artery stenosis measured using coronary computed tomography angiography. Environ Health Perspect. 2021;129(2):27006. https://doi.org/10.1289/ehp7351.
**ao S. Theoretical foundation and research application about the social support rating scale. J Clin Psychiatry. 1994;02:98–100.
Mollayeva T, Thurairajah P, Burton K, Mollayeva S, Shapiro CM, Colantonio A. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: a systematic review and meta-analysis. Sleep Med Rev. 2016;25:52–73. https://doi.org/10.1016/j.smrv.2015.01.009.
Sedov ID, Cameron EE, Madigan S, Tomfohr-Madsen LM. Sleep quality during pregnancy: A meta-analysis. Sleep Med Rev. 2018;38:168–76. https://doi.org/10.1016/j.smrv.2017.06.005.
Liu X, Chen M, Wang Y, Sun L, Zhang J, Shi Y, Wang J, Zhang H, Sun G, Baker PN, et al. Prenatal anxiety and obstetric decisions among pregnant women in Wuhan and Chongqing during the COVID-19 outbreak: a cross-sectional study. BJOG. 2020;127(10):1229–40. https://doi.org/10.1111/1471-0528.16381.
Lee DT, Yip SK, Chiu HF, Leung TY, Chan KP, Chau IO, Leung HC, Chung TK. Detecting postnatal depression in Chinese women. Validation of the Chinese version of the Edinburgh Postnatal Depression Scale. Br J Psychiatry J Mental Sci. 1998;172:433–7. https://doi.org/10.1192/bjp.172.5.433.
Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. https://doi.org/10.1016/0165-1781(89)90047-4.
Gjestland K, Bø K, Owe KM, Eberhard-Gran M. Do pregnant women follow exercise guidelines? Prevalence data among 3482 women, and prediction of low-back pain, pelvic girdle pain and depression. Br J Sports Med. 2013;47(8):515–20. https://doi.org/10.1136/bjsports-2012-091344.
Walsh JM, McGowan C, Byrne J, McAuliffe FM. Prevalence of physical activity among healthy pregnant women in Ireland. Int J Gynaecol Obstet. 2011;114(2):154–5. https://doi.org/10.1016/j.ijgo.2011.02.016.
Nguyen CL, Pham NM, Lee AH, Nguyen PTH, Chu TK, Ha AVV, Duong DV, Duong TH, Binns CW. Physical activity during pregnancy is associated with a lower prevalence of gestational diabetes mellitus in Vietnam. Acta Diabetol. 2018;55(9):955–62. https://doi.org/10.1007/s00592-018-1174-3.
Koleilat M, Vargas N, vanTwist V, Kodjebacheva GD. Perceived barriers to and suggested interventions for physical activity during pregnancy among participants of the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) in Southern California. BMC Pregnancy Childbirth. 2021;21(1):69. https://doi.org/10.1186/s12884-021-03553-7.
Davenport MH, Ruchat SM, Poitras VJ, Jaramillo Garcia A, Gray CE, Barrowman N, Skow RJ, Meah VL, Riske L, Sobierajski F, et al. Prenatal exercise for the prevention of gestational diabetes mellitus and hypertensive disorders of pregnancy: a systematic review and meta-analysis. Br J Sports Med. 2018;52(21):1367–75. https://doi.org/10.1136/bjsports-2018-099355.
Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, George SM, Olson RD. The Physical Activity Guidelines for Americans. JAMA. 2018;320(19):2020–8. https://doi.org/10.1001/jama.2018.14854.
Padmapriya N, Shen L, Soh SE, Shen Z, Kwek K, Godfrey KM, Gluckman PD, Chong YS, Saw SM, Müller-Riemenschneider F. Physical Activity and Sedentary Behavior Patterns Before and During Pregnancy in a Multi-ethnic Sample of Asian Women in Singapore. Matern Child Health J. 2015;19(11):2523–35. https://doi.org/10.1007/s10995-015-1773-3.
Okafor UB, Goon DT. Uncovering barriers to prenatal physical activity and exercise among South African pregnant women: a cross-sectional. Mixed-Method Analysis Front Public Health. 2022;10:697386. https://doi.org/10.3389/fpubh.2022.697386.
Shum KW, Ang MQ, Shorey S. Perceptions of physical activity during pregnancy among women: a descriptive qualitative study. Midwifery. 2022;107:103264. https://doi.org/10.1016/j.midw.2022.103264.
Acknowledgements
We want to thank all the pregnant women who participated in our study and the collaborating hospital for supporting this project. We acknowledge the contributions of the students, doctor Weihong Hu, nursing administrators Beibei Shen and ** Lin, Feng Xu, ** Lin, Feng Xu & **aoxia Ma
Contributions
Na Wang and Yan Ding conceived and designed the study. Yu** Lin, **aoxia Ma and Feng Xu analyzed and interpreted the data. Tianchun Zhou drafted the manuscript. Na Wang reviewed and edited the paper. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study protocol approved by the Institutional Review Board of the Research Committee of the Obstetrics and Gynecology of Hospital Fudan University (protocol code 202123). All methods were performed in accordance with the relevant guidelines and regulations of the Declaration of Helsinki and all participants provided written informed consent to participate in the study prior to data collection.
Consent for publication
Not applicable.
Competing interests
There is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhou, T., Lin, Y., Xu, F. et al. Factors influencing physical inactivity status among chinese pregnant women: a cross-sectional study. BMC Public Health 22, 2310 (2022). https://doi.org/10.1186/s12889-022-14757-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-022-14757-7