Abstract
Background
To evaluate the influence of decentration of plate-haptic toric intraocular lens (IOLs) on visual quality.
Methods
This study enrolled 78 eyes of 78 patients. Patients in group A were implanted with toric IOLs, and patients in group B were implanted with monofocal IOLs. All patients were divided into group A1 and B1 (decentration below 0.3 mm) and group A2 and B2 (decentration above 0.3 mm). The uncorrected distance visual acuity (UDVA), best corrected visual acuity (BCVA), modulation transfer function cutoff (MTF cutoff), objective scatter index (OSI), strehl ratio (SR), optical interference and patients’ satisfaction were measured in different pupils at three months postoperatively. The associations between decentration and visual quality were analyzed by Spearman correlation.
Results
There were no significant differences in UDVA, BCVA, MTF cutoff, OSI, SR, optical interference and patients’ satisfaction among subgroups. The differences in decentration between groups A and B were not statistically significant. In group A2, the total higher order aberrations (tHOAs) at pupil sizes of 3 mm (P = 0.046), 5 mm (P = 0.014), spherical aberrations at pupil sizes of 3 mm (P = 0.011), 4 mm (P = 0.014), 5 mm (P = 0.000), secondary astigmatism at pupil sizes of 3 mm (P = 0.002), 4 mm (P = 0.005) were higher than in group B2. Compared to group A1, group A2 had higher spherical aberrations at pupil sizes of 4 mm (P = 0.042), 5 mm (P = 0.001), 6 mm (P = 0.038), secondary astigmatism at pupil sizes of 3 mm (P = 0.013), 4 mm (P = 0.005), 6 mm (P = 0.013). Group B2 has higher coma and secondary astigmatism than group B1 at 6-mm pupil (P = 0.014, P = 0.045). Significant positive correlations were found between spherical aberrations and the decentration of group A1 and A2 at 6-mm pupils.
Conclusion
The decentration above 0.3 mm negatively affected visual quality due to increased tHOAs, spherical aberrations, coma and secondary astigmatism aberrations, the influence become larger with increasing pupil diameter. And toric IOLs are more affected by decentration than monofocal IOLs.
Similar content being viewed by others
Introduction
Astigmatism correction in cataract surgery is a common surgical challenge. Anderson DF et al. [1] systematically reviewed the prevalence of astigmatism and summarized that the proportion of cataract patients with astigmatism more than one diopter (D) ranges from 23 to 47% globally. Residual astigmatism after cataract surgery can lead to poor vision and patient dissatisfaction [2].
The advantages of toric intraocular lens (IOL) for correcting astigmatism have been demonstrated in several studies [3,4,5]. Multiple previous studies have reported excellent rotational stability of toric IOLs, but few studies have focused on their decentration. Chen X et al. [6] investigated decentration characteristics and related factors of IOLs. Miháltz K et al. [7] and Osawa R et al. [8] compared visual function and decentration of toric IOLs with different haptic designs. Hirnschall N et al. [9] found that decentration contributed to the refractive astigmatic error to some extent. It is critical to ensure a centered position for IOLs in the capsular bag. However, no previous study has divided subgroups according to decentration levels and explored the correlation between varying decentration and visual quality. Therefore, we conducted the current study to assess and compare decentration levels and their impact on visual quality of patients implanted with plate-haptic toric IOLs and monofocal IOLs.
Materials and methods
Research design
This is a prospective, nonrandomized controlled clinical study, which complied with the Declaration of Helsinki and was approved by the Ethics Committee of People’s Hospital of Guangxi Zhuang Autonomous Region, China (Ethical Approval Code: KY-LW-202,028). Patients were given detailed explanations of the study protocol and operative complications. They signed an informed consent form to participate in the study and provided permission for the results to be published anonymously.
Patient selection
Patients who underwent cataract surgery were enrolled at the People’s Hospital of Guangxi Zhuang Autonomous Region between November 2020 and June 2022. Exclusion criteria included a history of vision-limiting ocular diseases or ocular surgeries. In addition, patients with a tilt greater than 7° or the axis rotation of the toric IOL exceeded 10° were excluded.
Patients whose corneal regular astigmatism exceeded 1.0 D were implanted with toric IOLs and included in group A, whereas patients with corneal astigmatism less than 1.0 D were implanted with monofocal IOLs and included in group B. Ale JB et al. [10] reported that a 0.2–0.3 mm decentration are common and clinically unnoticed for any design of IOL. Large magnitude aberrations can be caused by decentrations below 0.5 mm, resulting in significant visual symptoms [11]. In order to investigate whether decentration of 0.3 mm can negatively affect postoperative visual quality, subgroups were divided according to decentration, group A was categorized into group A1 (decentration below 0.3 mm) and group A2 (decentration above 0.3 mm). Group B was categorized as group B1 (decentration below 0.3 mm) and group B2 (decentration above 0.3 mm).
Sample size estimation
The sample size is calculated based on the sample size calculation formula (powerandsamplesize.com/). The following assumptions were made for the sample size calculation: type 1 error (alpha) was set at 5%, and power (1-beta) on 0.90. The proportion of cases between the experimental and control groups was set to 1:1. Mean decentration of group A was set to greater than 0.5 mm and mean decentration of group B was set to less than 0.5 mm. Then the sample calculator showed a minimum sample size of 21 patients.
Intraocular lens
The AT TORBI 709 M IOL (Carl Zeiss Meditec AG, Germany) was used as the toric IOL and the CT ASPHINA 509 M (Carl Zeiss Meditec AG, Germany) was used as the monofocal IOL. All patients’ biometric measurements were taken by the IOL Master 700 (Carl Zeiss Meditec, Jena, Germany) after setting the target refraction to 0 D. The IOL power, alignment axis, and expected residual astigmatism were calculated using the toric IOL calculator program, available at https://zcalc.meditec.zeiss.com/.
Preoperative examinations
A complete ocular examination was performed on each patient, which included measuring uncorrected visual acuity (UDVA) and best corrected visual acuity (BCVA) and fundus examination. Corneal topographic maps were measured with the iTrace wavefront aberrometer (Tracey Technologies, Houston, TX).
Surgical technique
With the patients sitting upright in front of a slit-lamp microscope, the skillful assistant precisely marked the axis positions of the toric IOL at the corneal limbus before mydriasis. All operations were performed by experienced surgeons. During the operation, the toric IOL was inserted into the capsular bag and rotated to the planned axis. There were no stitches to close the wound.
Postoperative examinations
Visual acuity and refractive result
Patients were examined at 1 day, 1 week, 1 month, and 3 months postoperatively. UDVA and BCVA were measured at a distance of 5 m with a Standard Logarithmic Visual Acuity chart, then convert to the logarithm of the minimum angle of resolution (LogMAR) vision chart. Refractive error was measured by optometry.
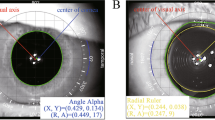
Assessment of IOL position
The orientation of the toric IOL is highly stable at postoperative 1 day [12], therefore the postoperative results at 3 months can infer the long-term stability of the toric IOL. The decentration was measured by drawing a circle that overlapped the IOL with the iTrace wavefront aberrometer after mydriasis at 3 months postoperatively. (Fig. 1A). During each postoperative visit, patients’ eyes were scanned with a slit lamp for visualizing IOL rotation (Fig. 1B). Patient’s eyes were scanned with six meridians (0, 30, 60, 90, 120, and 150 degrees) to determine the tilt level with IOL Master 700. The measurement method is the same as that of previous research[13].
Objective visual quality
The Optical Quality Analysis System (OQAS, Visiometrics, Terrassa, Spain) was used to measure objective visual quality including modulation transfer function cutoff (MTF cutoff), objective scatter index (OSI), strehl ratio (SR), OQAS values at contrasts of 100%, 20%, and 9% (OV, the mean of OV 100%, OV 20%, and OV 9%). Since uncorrected spherical and cylindrical errors could directly affect the optical results, the refractive errors of the subjects were completely corrected.
Aberration measurements
The root mean square (RMS) of whole-eye higher-order aberration (HOA) was measured by using the iTrace wavefront aberrometer. The total higher-order aberrations (tHOAs), coma, trefoil, spherical aberration, and secondary astigmatism were recorded. The entrance pupil scan sizes were 3 millimeters (mm), 4 mm, 5 mm as well as 6 mm respectively.
Patient satisfaction
[14][15]In this study, patients were interviewed by questionnaire about the frequency and degree of interference from glare, halo, and starburst. During the survey, the researchers explained the definitions of the technical terms in the visual quality questionnaire to the patients to ensure that they all understood the contents of the visual quality questionnaire and to avoid any indiscriminate or missing selections.
Statistical analysis
IBM SPSS Statistics 25.0 software was used to analyze the data obtained in the study statistically. The normal distribution of numerical data was evaluated with the Shapiro-Wilk test. The Kruskal–Wallis H test was used to compare the postoperative values and Fisher’s exact tests were used to assess the results of a questionnaire. Correlation analysis was conducted by Spearman correlation analysis. For all statistical analysis, data were expressed as mean ± SD. P < 0.05 was taken as a significant difference.
Results
Patient characteristics and visual acuity
There was no complication occurred in this study. Seven patients were excluded from the analysis due to missing preoperative or postoperative data. Group A included 38 eyes of 38 patients. Group B included 40 eyes of 40 patients. Subject demographics and preoperative and postoperative visual acuity were presented in Table 1.
Postoperative decentration, rotation and tilt
The decentration did not conform to a normal distribution, it was expressed as median and interquartile interval (first quartile; third quartile). Decentration values were 0.26 (0.20, 0.32) mm in group A and 0.25 (0.16, 0.36) mm in group B (Fig. 2). And no statistical significance in decentration was observed between the groups (P = 0.598). The mean postoperative rotation was 3.11 ± 2.02 degrees in group A. No tilt of more than 7 degrees in any direction was observed throughout the follow-up period.
Objective visual quality and aberrations
No statistically significant was observed in objective visual quality characteristics among subgroups (Table 2).
The results of HOAs were shown in Fig. 3. The tHOAs, spherical aberrations and secondary astigmatisms were significantly higher in group A2 than in group B2 with 3 mm pupil (P = 0.046, P = 0.011, P = 0.002). Group A1 showed lower secondary astigmatism than group A2 (P = 0.013; Fig. 3A). The spherical aberrations and secondary astigmatism were higher in group A2 than in group A1 (P = 0.042, P = 0.005) and group B2 (P = 0.014, P = 0.005) with 4.0 mm pupil (Fig. 3B). The tHOAs, spherical aberrations were higher in group A2 than in group B2 (P = 0.014, P = 0.000). Compared to group A1, group A2 had higher spherical aberrations with pupil sizes of 5.0 mm (P = 0.001; Fig. 3C). The tHOAs, spherical and secondary astigmatism were higher in A2 than in group A1 (P = 0.030, P = 0.038, P = 0.013). And the coma and secondary astigmatism were smaller in group B1 than in group B2 with 6.0 mm pupil (P = 0,014, P = 0.045; Fig. 3D).
Patient satisfaction
Questionnaire responses were reported in Fig. 4. There were no significant differences among subgroups in the incidence of optical interference and overall satisfaction (P>0.05). Patients rarely reported experiencing optical interference phenomena including glare, halo, and starburst. The majority of the patients reported being very satisfied or satisfied with the procedure.
Correlation analysis
The relationships between decentration and HOAs were described in Fig. 5. In group A, decentration was normalized against spherical aberrations (r = 0.531, P = 0.001) with 5.0 mm pupil, tHOAs (r = 0.366, P = 0.024), spherical aberrations (r = 0.543, P = 0.000) and secondary astigmatism (r = 0.366, P = 0.024) with 6.0 mm pupil (Fig. 5A). In group B, decentration was positively correlated with tHOAs (r = 0.355, P = 0.025), coma (r = 0.473, P = 0.002) and secondary astigmatism (r = 0.322, P = 0.042) with 6.0 mm pupil (Fig. 5B). In group A1, decentration was positively linked to spherical aberrations (r = 0.517, P = 0.007) with 6.0 mm pupil (Fig. 5C). And we observed a significant positive correlation between decentration and spherical aberrations (r = 0.752, P = 0.005) with 6.0 mm pupil in group A2 (Fig. 5D).
Discussion
Previous research has summarized that toric IOLs demonstrated excellent rotational as well as centration stability [16, 17]. IOL decentration may lead to residual cylindrical errors and poor uncorrected visual acuity[18, 19]. Lawu T et al. [20] have compared the effect of decentration and tilt on the optical performance of different aspheric IOL designs in model eyes, the results showed that IOL decentration and tilt increased residual astigmatism and HOAs, and decreased optical quality. Pérez-Vives C et al. [21] evaluate the impact of different decentration levels on the optical quality of three aspheric toric IOLs and indicated that coma aberration increased significantly with IOL decentration. In summary, it is particularly important to ensure that the toric IOL is centered in the capsular bag. However, there are few reports investigating the effect of decentration of the plate-haptic toric IOL on visual quality. In addition, a few studies divided the study subjects into different subgroups based on decentration to further explore the effect of different decentrations on visual quality for different pupil diameters. Therefore, the aim of this study is to compare in the visual quality of plate-haptic toric IOLs and monofocal IOLs in relation to different degrees of decentration at 3-mm, 4-mm, 5-mm, and 6-mm pupils.
According to previous studies, a larger extent of decentration could negatively affect the postoperative optical performance and patients’ satisfaction. Korynta J et al. [22] indicated that astigmatism may be visually significant when decentration exceeds 1.0 mm based on an ophthalmological model. The more complex the optic design of the IOL, the more vulnerable it is to the decentration and tilt [23]. For toric IOLs, decentrations make the result of astigmatism unpredictable. However, the mean decentrations of patients were in the range of 0.2 to 0.3 mm in this study, which was almost consistent with the decentration in previous study [10]. [22][23]And no statistically significant differences were found among the four groups in UDVA and BCVA. This is consistent with previous study, which demonstrated decentration and tilt values after IOL implantations seem to have an effect on HOAs, but not on vision [19].This result implies that the effect of decentration within this range on visual acuity is minor, and probably clinically irrelevant.
Efficacy after cataract surgery can be assessed by optometry results, MTF cutoff, HOAs, patient satisfaction, and so on. The MTF cutoff can be used to quantify the loss of resolution, and the higher the MTF cutoff is, the better the visual quality is reflected [28] found that decentration led to greater horizontal coma, astigmatism, and defocus aberration at 4.5 mm pupil. Ning Y et al. [29] suggested that coma changes significantly only when the decentration was above 0.2 mm and the tilt was above 2.8º [30]. In case of decentration greater than 0.5 mm, aspheric IOLs lose their advantages [11]. When decentration was equal or above 0.9 mm, VA did not reach 0.2 LogMAR in any eyes [31]. With IOLs decentered by 1.0 mm, the contrast reduction rates of transitional conic toric IOL, bitoric IOL, posterior toric surface IOL, and anterior toric surface IOL were 5.1%, 3.1%, 12.2%, and 15.8%, respectively [32]. In this study, significant differences in HOAs were observed with respect to different subgroups. The HOAs of the subgroup with high decentration were greater than that of the subgroup with low decentration. Group A1 had statistically significantly lower spherical aberrations than group A2 at 3-mm, 4-mm, and 5-mm pupils, which indicated that decentration of toric IOLs mainly affected spherical aberration. In summary of the above results of this study and of previous reports, a decentration exceeding 0.30 mm can result in increased HOAs and reduced visual quality. Toric IOLs with good position and well-centered could decrease HOAs and improve optical performance.
Previous studies have shown that high coma often leads to double vision [33], and spherical aberrations are associated with glare and halo [34]. In this study, noobody complained of photic phenomena frequently, and most patients were very satisfied or satisfied with the postoperative results after 3 months postoperatively. And there was no difference in satisfaction and various types of photic phenomena within the four subgroups. Therefore, we summarized that the effects of decentration on photic phenomena and patients’ satisfaction were so small that it might be clinically insignificant. In addition, we had to consider that these results were directly related to the small number of subjects.
In our research, we detected that spherical aberrations were getting larger with increasing decentration of group A1 and group A2 at 6-mm pupils. When the pupil gets larger at night, so do the spherical aberrations. Thus, a high level of decentration may contribute to the elevated glare and halo risk in the evening. We summarized previous studies and our research find that the HOAs after toric IOL implantation may be increased when the decentration is more than 0.3 mm, which could decrease the visual quality of patients. Therefore, the postoperative decentration should be monitored after the implantation of toric IOLs.
There have been many advances in methods of measuring IOL decentration and tilt recently. In previous studies, tilt and decentration were measured by the Purkinje imaging technique, Scheimpflug imaging, ultrasound biomicroscopy, and anterior segment OCT [11]. In our study, we used the iTrace wavefront aberrometer for decentration and aberration measurements. In order to meet the increasing visual requirements of patients, the iTrace wavefront aberrometer is a good device for further research on decentration and visual quality in hospitals without the above ophthalmological examination devices, which is beneficial to promote the development of refractive cataract surgery.
The current studies demonstrate that the position of the preoperative crystalline lens and AL were the crucial influencing factors of IOL decentration and tilt [35]. Moreover, capsulorhexis and capsular fibrosis have an effect on IOL decentration. Severe eccentric capsulorhexis predisposes patients to greater IOL decentration [36]. Capsular stability plays an important role in the optical performance of IOLs [37]. In conclusion, it is important to select patients rationally, examine patients delicately, mark axial positions accurately and perform surgical operations skillfully to reduce postoperative decentration of toric IOL.
There are some limitations of this study. Firstly, the results were directly affected by the small sample size of patients, especially the small number of patients with high decentration. Secondly, patients were followed for 3 months without assessment of clinical outcomes over a longer period. In future studies, the follow-up period should be extended., In addition, there was an inevitable error in the results because the decentration was measured using iTrace by manually drawing a circle until the circle coincided with the edge of IOLs’ optical area. In order to reduce the errors, the inspector measured decentration multiple times and took the average value in this study.
In conclusions, the toric and monofocal IOLs in this study are capable of showing a low degree of decentration and providing excellent visual quality and patient satisfaction. And eyes are tolerant to the mild decentration below 0.3 mm of toric IOLs. Compared with monofocal IOLs, the decentration above 0.3 mm of toric IOLs have a greater negative effect on the visual quality due to increased tHOAs, spherical aberrations, coma and secondary astigmatism aberrations, the influence become larger with increasing pupil diameter.
Data Availability
The datasets generated and analyzed during the current study are not publicly available due to ethical restrictions but are available from the corresponding author on reasonable request. The data that support the findings of this study are available from the corresponding author, Fan Xu, upon reasonable request.
Abbreviations
- IOL:
-
Intraocular len
- UDVA:
-
Uncorrected distance visual acuity
- BCVA:
-
Best corrected visual acuity
- LogMAR:
-
Logarithm of the minimum angle of resolution
- OQAS:
-
Optical Quality Analysis System
- MTF cutoff:
-
Modulation transfer function cutoff
- OSI:
-
Objective scatter index
- SR:
-
Strehl ratio
- OV100%, OV20%, and OV9%:
-
Mean OQAS values 100%, 20% and 9%
- HOAs:
-
Higher order aberrations
- D:
-
Diopter
- AL:
-
Axial length
- SE:
-
Spherical equivalent
- RMS:
-
The root mean square
References
Anderson DF, Dhariwal M, Bouchet C, Keith MS. Global prevalence and economic and humanistic burden of astigmatism in cataract patients: a systematic literature review. Clin Ophthalmol. 2018;12:439–52.
Behndig A, Montan P, Stenevi U, Kugelberg M, Zetterstrom C, Lundstrom M. Aiming for emmetropia after cataract surgery: Swedish National Cataract Register study. J Cataract Refract Surg. 2012;38(7):1181–6.
Osawa R, Sano M, Yuguchi T, Kaiya T, Oshika T. Effects of Modified Haptics on Surgical Outcomes and Rotational Stability of Toric intraocular Lens Implantation. J Refract Surg. 2022;38(10):648–53.
Takaku R, Nakano S, Iida M, Oshika T. Influence of frosted haptics on rotational stability of toric intraocular lenses. Sci Rep. 2021;11(1):15099.
Zhong H, Qin H, Wang HJ, Wang ZY. Objective visual quality one year after toric IOL implantation for correction of moderate and high corneal astigmatism. Int J Ophthalmol. 2021;14(2):245–9.
Chen X, Gu X, Wang W, **ao W, ** G, Wang L, Dai Y, Zhang E, Ruan X, Liu Z, et al. Characteristics and factors associated with intraocular lens tilt and decentration after cataract surgery. J Cataract Refract Surg. 2020;46(8):1126–31.
Mihaltz K, Lasta M, Burgmuller M, Vecsei-Marlovits PV, Weingessel B. Comparison of two Toric IOLs with different Haptic Design: Optical Quality after 1 year. J Ophthalmol. 2018;2018:4064369.
Osawa R, Oshika T, Sano M, Yuguchi T, Kaiya T. Rotational stability of modified toric intraocular lens. PLoS ONE. 2021;16(3):e0247844.
Hirnschall N, Findl O, Bayer N, Leisser C, Norrby S, Zimper E, Hoffmann P. Sources of Error in Toric intraocular Lens Power calculation. J Refract Surg. 2020;36(10):646–52.
Ale JB. Intraocular lens tilt and decentration: a concern for contemporary IOL designs. Nepal J Ophthalmol. 2011;3(1):68–77.
Ashena Z, Maqsood S, Ahmed SN, Nanavaty MA. Effect of intraocular Lens Tilt and Decentration on Visual Acuity, Dysphotopsia and Wavefront Aberrations. Vis (Basel) 2020, 4(3).
Inoue Y, Takehara H, Oshika T. Axis Misalignment of Toric intraocular Lens: Placement Error and postoperative rotation. Ophthalmology. 2017;124(9):1424–5.
Hirnschall N, Buehren T, Bajramovic F, Trost M, Teuber T, Findl O. Prediction of postoperative intraocular lens tilt using swept-source optical coherence tomography. J Cataract Refract Surg. 2017;43(6):732–6.
Toprak AB, Eser E, Guler C, Baser FE, Mayali H. Cross-validation of the turkish version of the 25-item National Eye Institute Visual Functioning Questionnaire (NEI-VFQ 25). Ophthalmic Epidemiol. 2005;12(4):259–69.
Zhang Y, Bian A, Hang Q, Zhou Q. Optical quality assessed by Optical Quality Analysis System in Chinese Primary Open-Angle Glaucoma patients and its correlations with psychological disturbances and vision-related quality of life. Ophthalmic Res. 2021;64(1):15–21.
Buckhurst PJ, Lau G, Williams JI, Packer M. Efficacy of a one-piece aberration Neutral Hydrophobic Acrylic Toric intraocular Lens. Clin Ophthalmol. 2022;16:3763–74.
Weikert MP, Golla A, Wang L. Astigmatism induced by intraocular lens tilt evaluated via ray tracing. J Cataract Refract Surg. 2018;44(6):745–9.
Singh VM, Ramappa M, Murthy SI, Rostov AT. Toric intraocular lenses: expanding indications and preoperative and surgical considerations to improve outcomes. Indian J Ophthalmol. 2022;70(1):10–23.
Martinez-Plaza E, Lopez-de la Rosa A, Papadatou E, Habib NE, Del Aguila-Carrasco AJ, Lopez-Miguel A, Maldonado MJ, Buckhurst PJ. Influence of decentration and tilt of Tecnis ZCB00 on visual acuity and higher order aberrations. Eye (Lond) 2022.
Lawu T, Mukai K, Matsushima H, Senoo T. Effects of decentration and tilt on the optical performance of 6 aspheric intraocular lens designs in a model eye. J Cataract Refract Surg. 2019;45(5):662–8.
Perez-Vives C, Ferrer-Blasco T, Madrid-Costa D, Garcia-Lazaro S, Montes-Mico R. Optical quality of aspheric toric intraocular lenses at different degrees of decentering. Graefes Arch Clin Exp Ophthalmol. 2014;252(6):969–75.
Korynta J, Bok J, Cendelin J, Michalova K. Computer modeling of visual impairment caused by intraocular lens misalignment. J Cataract Refract Surg. 1999;25(1):100–5.
Tandogan T, Son HS, Choi CY, Knorz MC, Auffarth GU, Khoramnia R. Laboratory evaluation of the influence of decentration and pupil size on the Optical performance of a Monofocal, Bifocal, and Trifocal intraocular Lens. J Refract Surg. 2017;33(12):808–12.
Yin Y, Lu Y, **ang A, Fu Y, Zhao Y, Li Y, Hu T, Du K, Hu S, Fu Q, et al. Comparison of the optical quality after SMILE and FS-LASIK for high myopia by OQAS and iTrace analyzer: a one-year retrospective study. BMC Ophthalmol. 2021;21(1):292.
Yao L, Xu Y, Han T, Qi L, Shi J, Zou Z, Zhou X. Relationships between Haloes and Objective Visual Quality in healthy eyes. Transl Vis Sci Technol. 2020;9(10):13.
Zhang B, Ma JX, Liu DY, Du YH, Guo CR, Cui YX. Optical performance of toric intraocular lenses in the presence of decentration. Int J Ophthalmol. 2015;8(4):730–5.
Perez-Merino P, Marcos S. Effect of intraocular lens decentration on image quality tested in a custom model eye. J Cataract Refract Surg. 2018;44(7):889–96.
Eppig T, Scholz K, Loffler A, Messner A, Langenbucher A. Effect of decentration and tilt on the image quality of aspheric intraocular lens designs in a model eye. J Cataract Refract Surg. 2009;35(6):1091–100.
Ning Y, Shao Y, Zhao J, Zhang J, Wang M, Qin Y. Stability of various types of Aspheric intraocular lenses after implantation: a one-year retrospective study. Int J Gen Med. 2021;14:2183–90.
Oshika T, Sugita G, Miyata K, Tokunaga T, Samejima T, Okamoto C, Ishii Y. Influence of tilt and decentration of scleral-sutured intraocular lens on ocular higher-order wavefront aberration. Br J Ophthalmol. 2007;91(2):185–8.
Hayashi K, Hayashi H, Nakao F, Hayashi F. Correlation between pupillary size and intraocular lens decentration and visual acuity of a zonal-progressive multifocal lens and a monofocal lens. Ophthalmology. 2001;108(11):2011–7.
Kim MJ, Yoo YS, Joo CK, Yoon G. Evaluation of optical performance of 4 aspheric toric intraocular lenses using an optical bench system: influence of pupil size, decentration, and rotation. J Cataract Refract Surg. 2015;41(10):2274–82.
Zhu XJ, Chen MJ, Zhang KK, Yang J, Lu Y. Elevated TGF-beta2 level in aqueous humor of cataract patients with high myopia: potential risk factor for capsule contraction syndrome. J Cataract Refract Surg. 2016;42(2):232–8.
Casprini F, Balestrazzi A, Tosi GM, Miracco F, Martone G, Cevenini G, Caporossi A. Glare disability and spherical aberration with five foldable intraocular lenses: a prospective randomized study. Acta Ophthalmol Scand. 2005;83(1):20–5.
Chen X, Gu X, Wang W, ** G, Wang L, Zhang E, Xu J, Liu Z, Luo L, Liu Y. Distributions of crystalline lens tilt and decentration and associated factors in age-related cataract. J Cataract Refract Surg. 2021;47(10):1296–301.
Findl O, Hirnschall N, Draschl P, Wiesinger J. Effect of manual capsulorhexis size and position on intraocular lens tilt, centration, and axial position. J Cataract Refract Surg. 2017;43(7):902–8.
Liu X, **e L, Huang Y. Effects of decentration and tilt at different orientations on the optical performance of a rotationally asymmetric multifocal intraocular lens. J Cataract Refract Surg. 2019;45(4):507–14.
Acknowledgements
None.
Funding
The study was supported by Guangxi Medical Health Appropriate Technology Development and Application Project (S2020077), Science and Technology Plan Project of Qingxiu District in Nanning City (2020036), Guangxi Clinical Ophthalmic Research Center (No. Guike AD19245193), Natural Science Foundation of Guangxi Province (2021GXNSFBA075051). The sponsor or funding organization had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Contributions
QL designed research; CD and JL conducted research and wrote the paper; CD and QC performed statistical analysis; SZ, GY, ZZ, LL, WH, PL, JL provided essential technical and material support; ML and FX had primary responsibility for final content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of People’s Hospital of Guangxi Zhuang Autonomous Region, China (Ethical Approval Code: KY-LW-202028). All patients have signed an informed consent form to participate in the study and provided permission for the results to be published anonymously.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Diao, C., Lan, Q., Liao, J. et al. Influence of decentration of plate-haptic toric intraocular lens on postoperative visual quality. BMC Ophthalmol 23, 332 (2023). https://doi.org/10.1186/s12886-023-03061-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12886-023-03061-6