Abstract
Breast cancer (BC) is the second-leading factor of mortality for women globally and is brought on by a variety of genetic and environmental causes. The conventional treatments for this disease have limitations, making it difficult to improve the lifespan of breast cancer patients. As a result, extensive research has been conducted over the past decade to find innovative solutions to these challenges. Targeting of the antitumor immune response through the immunomodulatory checkpoint protein B7 family has revolutionized cancer treatment and led to intermittent patient responses. B7-H3 has recently received attention because of its significant demodulation and its immunomodulatory effects in many cancers. Uncontrolled B7-H3 expression and a bad outlook are strongly associated, according to a substantial body of cancer research. Numerous studies have shown that BC has significant B7-H3 expression, and B7-H3 induces an immune evasion phenotype, consequently enhancing the survival, proliferation, metastasis, and drug resistance of BC cells. Thus, an innovative target for immunotherapy against BC may be the B7-H3 checkpoint.
In this review, we discuss the structure and regulation of B7-H3 and its double costimulatory/coinhibitory function within the framework of cancer and normal physiology. Then we expound the malignant behavior of B7-H3 in BC and its role in the tumor microenvironment (TME) and finally focus on targeted drugs against B7-H3 that have opened new therapeutic opportunities in BC.
Similar content being viewed by others
Introduction
BC is the leading cause of disability and death among women globally [1]. The World Health Organization reports that approximately 2.26 million women are given a BC diagnosis every year [1]. Mosaic populations of tumor cells, immune cells, and stromal cells that have different genetic, epigenetic, and phenotypic traits make up breast malignancies. Four molecular subtypes of BC were categorized by gene expression sequence analysis; these include Luminal A, if estrogen receptor alpha-positive (ER) + and/or progesterone-receptor (PR) + , human epidermal growth factor receptor 2 (HER2) − , Ki67 < 14%), Luminal B (if ER + and/or PR + , HER2 overexpressed or Ki67 ≥ 14%), triple-negative breast cancer (TNBC) (if ER − , PR − , HER2 −), and HER2-enriched (if ER − , PR − and HER2 +) [2]. The specific receptors that cancer cells express (or do not express) act as biomarkers for therapy. Anti-estrogens and aromatase inhibitors, both of which disrupt ER activity, are effective against ER-α positive cancers [3]. Therapeutic agents directed at HER2, such as trastuzumab—an anti-HER2 antibody—demonstrate anticancer efficacy specifically in HER2-positive malignancies [4]. Hormone-responsive BC has been successfully treated with endocrine treatment. Regretfully, disease recurrence and relapse are caused by the emergence of drug resistance [5], TNBC has the poorest prognosis because of the high intra-tumor heterogeneity and absence of specific receptors [6]. Therefore, the outlook for women with BC remains grim. The immune and stromal cell subsets that compose the breast tumor ecosystem are extremely complicated, and their makeup, spatial arrangement, and functional orientation all significantly impact how the illness develops and how patients fare. Consequently, it is crucial to establish effective BC treatment techniques and identify new therapeutic targets. Cancer treatment has undergone a paradigm shift as a result of recent developments in immune checkpoint inhibitor (ICI) medicines [7].
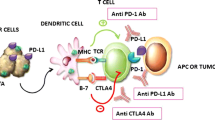
Particular focus has been placed on the B7 family proteins due to its potential use as an ICI to cure cancer. Members of the B7 family closely regulate immunological responses [8] and tumor progression [9]. The 10 members of the B7 family that are now recognized include B7-1/CD80, B7-2/CD86, B7-H1/PD-L1, B7-DC/PD-L2, B7-H2/CD275, B7-H3/CD276, B7-H4/VTCN1, B7-H5/Vista, B7-H6/NCR3LG1, and B7-H7/HHLA2 [10]. It has been demonstrated that B7-H1/PD-L1 and B7-DC/PD-L2 interact with PD-1 (programmed death 1) and stimulate the growth of T cells via secreting IL-10 and interferon-γ [11]. In contrast, the T-cell response is inhibited and immune evasion is facilitated when PD-L1 is expressed on cancer-associated cells [12]. PD-1/PD-L1 pathway proteins have been targeted by antibodies to treat a variety of malignancies [13]. However, certain tumors that exhibit high PD-L1 proteins were found to respond to PD-L1 treatment with a low objective response rate (ORR), likely because the TME significantly affects how well the immune system responds to these inhibitors [14,15,16]. Just 40% of patients have clinically reacted to PD-1/PD-L1 blocking [17]. Thus, it is crucial for therapeutic purposes to find new biomarkers in patients who respond to ICIs.
Among B7 family members, B7-H3 has recently received attention because it is significantly expressed in several malignancies and predict a dismal prognosis [18,19,20,21,22]. The expression of B7-H3 on the surfaces of tumor cells stimulates the growth of tumors by allowing these cells to evade immunosurveillance [23]; Compared to normal tissues, tumor tissues have an excessive expression of B7-H3 [24, 25]. The American Joint Committee on Cancer evaluated B7-H3 expression in stage I to III primary breast cancer and normal breast specimens, results showed that 39% of initial breast cancers had B7-H3 mRNA expression, whereas normal breast tissues did not [26]. Moreover, B7-H3 was substantially linked with tumor formation and lymph node metastasis in primary breast cancers [26]. Elevated expression of B7-H3 was tied to a worse prognosis in a five-year examination of BC patients’ survival rates [27] and bad clinicopathological BC parameters [28]. According to another research, individuals with BC who have high levels of B7-H3 expression in their circulating epithelial tumor cells are more likely to develop metastases [29]. Hence, we propose that the B7-H3 immune checkpoint may be a promising target in BC immunotherapy.
B7-H3’s structure and physiological implications
B7-H3 is a dual-acting immunological checkpoint protein that is expressed on cancer cells and antigen-presenting cells (APCs) including dendritic cells and macrophages. It is effective in both soluble and membrane-associated forms [30]. The soluble form can be produced by selective splicing [31] or, more commonly, by cleavage of B7-H3 present on the surfaces of monocytes, DCs, and T cells by membrane metalloproteinases [32]. The membrane-associated form has an extracellular Ig-like structural domain, a transmembrane part, and a shorter intracellular region [33]. The number of extracellular Ig-like domains that each of the two membrane-bound B7-H3 isoforms, 2IgB7-H3, and 4IgB7-H3, contains serves to distinguish them from one another; the former contains a single IgV (variable) domain and a single IgC (constant) domain, due to exon duplication, the latter has tandemly duplicated IgV and IgC domains [34]. B7-H3 has both stimulatory and inhibitory properties to increase or decrease the activity of T cells, possibly due to its interaction with various receptors that have different functions in specific contexts. However, the B7-H3 receptor’s identification is up for debate. Certain putative receptors, including phospholipase A2 receptor 1, interleukin-20 receptor subunit α, and the trigger receptor expressed on myeloid cells-like transcript 2, have not been conclusively verified [153, 154]. FOXP3 is crucial for Treg function [155,156,157]. Treg cells that express FOXP3 are thus effective peripheral immunological tolerance mediators. B7-H3 expression and the quantity of FOXP3 + Treg cells have a strong positive connection [158], indicating that the recruitment of Treg cells may be a partial mediator of the immunosuppressive action of B7-H3.
CAFs
Many stromal variables either repress or encourage genetic epithelial alterations to impact the complex ecosystems that makeup tumors. While normal fibroblasts suppress tumor formation [159], Cancer-associated fibroblasts (CAFs) promote tumor characteristics such as ECM remodeling, inflammation, and cancer cell proliferation and invasiveness [160,161,162]. It has been reported that different CAF populations produce various cytokine patterns in malignancies [163, 164]. CAFs produce alpha-smooth muscle actin (α-SMA) [165]. The development of several malignant tumors is strongly correlated with α-SMA expression [166, 167]. Increased stromal myofibroblasts in human BC are linked to aggressive adenocarcinomas and foretell disease recurrence [168]. Some tumor subtypes have also been linked to CAF subtypes, and CAFs that are positive for these CAF-associated markers have been predominantly found in HER2 and TNBC [169]. As mentioned earlier, BC often metastasizes to bone. It has been shown that CAFs play a crucial role in develo** characteristics that enable cells in the original TME to metastasize to bone [170]. One study showed that primary tumor stroma enriched in CAFs could imitate the CXCL12-rich bone metastatic niche and promote the preselection of cancer cells that possess the potential to metastasize to bone [171].
Using an orthotopic xenograft tumor model they established in nude mice, Zhang et al. confirmed that B7-H3 + CAFs play a significant role in tumor growth and metastatic progression [172]. Another research revealed that the lack of B7-H3 reduced the release of cytokines, including interleukin (IL)-6 and vascular endothelial growth factor (VEGF), as well as the capacity of CAFs to migrate and invade [173]. In a subgroup of breast cancers, high B7-H3 expression on CAFs was shown to alter T-cell activity toward more regulatory activities [174]. Hence, more research is required into the role of B7-H3 expression in immune cell-connected fibroblasts.
The above observations, considered together, reiterate how crucial the immunological environment is for influencing clinical outcomes. Develo** more effective treatment plans for BC will undoubtedly need combination therapy that targets both tumor cells and TME.
B7-H3 as an attractive immunotherapy target
The ability to target B7-H3 via diverse effector pathways has recently been made available by developments in molecular biology and antibody design. Most of these tactics have been examined in mice and in vitro, and the testing has yielded safety and/or antitumor data, laying the foundation for clinical trials targeting B7-H3. It is regrettable that, as of now, no targeted drug has received FDA approval. Table 1 lists the current therapeutic studies being conducted to treat B7-H3.
Targeting B7-H3 with monoclonal antibodies
Strong justification exists for using B7-H3-specific inhibitory monoclonal antibodies (mAbs) in the management of solid tumors due to the substantial alterations in cancer cells brought about by silencing of B7-H3 and the remarkable therapeutic outcomes of mAbs that block checkpoint molecules. It has been shown that using mAbs to block B7-H3 activity increases CD8 + T and NK cell tumor infiltration, prevents tumor growth, and/or lengthens life [176]. A mouse IgG1 mAb targeting B7-H3, 8H9, was shown to effectively against primary brain cancers [177]. 8H9 is currently being tested in phase 1 clinical studies to treat advanced CNS malignancies and desmoplastic small round cell tumors [178]. When the Fc part of an antibody interacts with immune cells to assault targets, the process is known as antibody-dependent cellular cytotoxicity (ADCC) [179]. Enoblituzumab (MGA271), a monoclonal antibody targeting the Fc region of B7-H3 with the potential to activate killer T cells through FcR binding, has demonstrated potent Antibody-Dependent Cellular Cytotoxicity (ADCC) against various xenograft tumors. It is currently undergoing clinical trials for the treatment of resistant malignancies (NCT02982941, NCT02923180, NCT02381314, NCT04630769, NCT02475213 and NCT01391143) [180].
Targeting B7-H3 with bispecific antibodies
Nisonoff and his colleagues originally introduced the idea of a bispecific antibody (bsAb), a synthetic antibody-based molecule with two distinct antigen-binding sites, more than 60 years ago [181]. The ensuing conceptual and technical developments in the production of bsAbs evolved in tandem with groundbreaking developments in antibody design and physiology disciplines [182]. BsAbs’ ability to allow dual-targeting ideas holds significant therapeutic potential. For example, the anti-CD3 mAb scFv was combined with the anti-B7-H3 mAb scFv to create obrindatamab [183]. Obrindatamab instructs T lymphocytes to attack B7-H3 + tumor cells by attaching simultaneously to CD3 and B7-H3. Obrindatamab demonstrated an enhancement in T-cell cytotoxicity by stimulating the production of IL-2, TNF-α, and IFN-γ. This resulted in a substantial reduction in tumor development, leading to increased survival in immunodeficient animals [183]. The B7-H3-targeting bispecific antibody now undergoing clinical review, is being investigated for its potential synergy with anti-PD-1 treatment, although no results have been made public as of yet. Recently, Huang et al. created a BiTE-based mRNA therapy by encasing the mRNA that codes for B7-H3CD3 BiTE inside brand-new ionizable lipid nanoparticles (LNPs). These findings imply that treatment approaches based on B7-H3 × CD3 BiTE mRNA expression may be beneficial and have good clinical application possibilities [184].
Targeting B7-H3 through ADC therapies
Antibody–drug conjugates (ADCs), hybrid molecules designed for targeted therapy, have demonstrated considerable promise in facilitating a paradigm change in cancer therapy through antibody-antigen interactions [185]. ADCs comprise a potent cytotoxic payload, a humanized antibody that targets tumors, and a linker that connects them [186]. Antibody–drug conjugation systems are sophisticated, cutting-edge strategies that can deliver the best outcomes in BC therapy. MGC018 is a DNA-alkylating anti-B7-H3 ADC that has been studied in phase 1 dose-expansion trials and has been shown to have robust anticancer efficacy in various cancer models (NCT03729596) [187]. In a more recent clinical study, DS-7300a, an ADC that combines a humanized anti-B7-H3 antibody that contains an inhibitor of DNA topoisomerase I, has shown to be secure and reliable in the treatment [188]; the published interim results show good tolerability in patients with advanced tumors. Scientists have been immensely enthused by the DS-7300a’s early achievements, and a fresh trial testing DS-7300a’s efficiency has started (NCT05280470).
Targeting B7-H3 with CAR T cells and CAR NK cells
Two types of immune cells, CD8 + cytotoxic T and NK cells, destroy their target cells through similar cytotoxic processes. While HLA class I antigen expression is not required to detect tumor cells by Chimeric Antigen Receptor (CAR) T cells, the CAR T cells detect tumor cells quickly and with solid cytotoxicity [189]. B7-H3 CAR T cells with different B7-H3-specific scFvs exhibit potent in vitro antitumor efficacy against several tumor types [190,191,192,193]. In the case of reports, B7-H3-targeted CAR-T cells exhibited excellent tolerance in patients with relapsed basal cell carcinoma, glioblastoma, and recurrent anaplastic meningioma [194]. Combinatorial approaches that increase CAR-T cell antitumor efficacy and the vulnerability of tumor cells to the effector mechanism are being studied. Regarding cost-effectiveness, while CAR-T therapy has shown remarkable clinical outcomes, its economic implications, including manufacturing costs, accessibility, and long-term sustainability, need careful consideration.
As a crucial component of the innate immune response against malignancy, NK cells are capable of directly destroying tumors [195]. Nonetheless, it has been demonstrated that the cytotoxicity of NK cells is functionally compromised by the immunosuppressive characteristics of B7-H3 in several cancers [196]. It is possible to obtain CAR with distinct specificity for cancer immunotherapy and use it to enhance NK cell function in malignancy. Several clinical scenarios have demonstrated the superior safety of CAR-NK cell immunotherapy and shown that it has a lower risk of causing neurotoxicity and cytokine release syndrome [197, 198]. Findings from the first large-scale study using CAR-NK cells in individuals with CD19 + chronic lymphocytic leukemia and B-cell lymphoma demonstrated safety and showed encouraging clinical efficacy [199]. Tumor heterogeneity, the disappearance of the targeted antigen, and antagonistic TME are the insurmountable difficulties that CAR-NK cell therapy now confronts. Several strategies should be taken into consideration in the future to optimize the efficacy of CAR-based NK cell treatment.
Radiotherapy
Radioimmunotherapy slows tumor growth by attaching radionucleotides to tumor-targeting antibodies, producing radiation-induced cytotoxicity [199]. The carrier most often utilized in radioimmunoconjugates is omburtamab. In phase I trials, intrathecal omburtamab was well tolerated by patients treated for metastatic central nervous system neuroblastoma and intraperitoneal 131I-mAb 8H9 in desmoplastic small round cell tumors (NCT04022213) [200]. Delivering 124I-mAb 8H9 to diffuse pontine glioma through convection-enhanced brainstem caused low systemic exposure and no harm (NCT01502917) [175]. Control of radiotoxicity remains a significant obstacle that must be overcome when attempting to treat other solid tumors using radioimmunotherapy against B7-H3.
B7-H3 small-molecule inhibitors
By combining computational modeling with an in silico technique, synthetic chemical libraries can be screened to identify compounds with apparent inhibitory effects on B7-H3. These compounds provide various observable advantages; their small size and solubility allow them to readily cross membrane barriers such as the blood–brain barrier, allowing precise penetration into different tissues, including TMEs. This makes them particularly helpful for the treatment of central nervous system cancers. Compared to antibody-based or CAR therapy, the cost of producing small-molecule inhibitors is minimal, and the conditions required for their storage are less rigorous [201]. Thus, targeting B7-H3 with small-molecule inhibitors might be an appealing alternative or supplementary treatment approach.
Application of B7-H3 in tumor imaging
B7-H3 has shown promise for therapeutic use in tumor imaging in addition to being a prognostic marker and an immunotherapy target. The first line of defense in BC screening programs is mammography. The median size of lesions identified with mammography screening is 1.5 cm; however, the median size identified through clinical detection is 2.6 cm [202], and digital mammogram analysis greatly boosts screening sensitivity [203]. Unfortunately, mammograms frequently lead to overdiagnosis and pointless biopsies, and half of the women who receive multiple screenings report experiencing false-positive results [204].
It has been established that B7-H3 is a target for BC molecular ultrasound imaging. As molecular targeting contrast agents, microbubbles functionalized with B7-H3-targeted affibodies [205] or antibodies [206] have shown excellent promise. While nontargeted microbubbles produced lower imaging signals in normal mammary tissues and malignancies that block B7-H3, Strong imaging signals were obtained in tumors expressing hB7-H3 by microbubbles conjugated to the B7-H3-targeted affibody (MBABY-B7-H3) [205], proving the B7-H3’s diagnostic utility in BC imaging. Spectroscopic photoacoustic imaging is a new focused approach [207]. Using an affibody or antibody that is specific for B7-H3 and conjugated to indocyanine green, researchers can detect BC [208], assess the tumor’s grade [209], and direct the resection during surgery.
Conclusion
BC is the primary cancer-related killer of women worldwide and is regarded as a lethal malignant tumor in most countries. The threat of BC lies not only in its widespread incidence but also in its cunning ability to relapse and metastasize. The BC patient’s treatment journey is often accompanied by multiple treatment modes such as surgery, radiotherapy, chemotherapy. Given the strain on the patient’s body and the fact that conventional procedures may not always appear sufficient, new effective and gentle therapeutic approaches are especially required.
Within this context, the stable high expression of B7-H3 in a variety of cancers is of great interest to researchers, especially in BC. The close correlation between elevated expression levels of B7-H3 and an unfavorable prognosis provides compelling evidence for its potential as a promising therapeutic target. Furthermore, preclinical studies and early trials have also shown the value of B7-H3 as a serum marker for use in BC diagnosis and prognosis. Its integration into breast ultrasound imaging further underscores its potential as a non-invasive tool for early disease detection and monitoring.
Overall, while B7-H3 shows promise in BC treatment and may serve as a therapeutic target, continued research is needed to fully understand its complex receptor interactions and overcome barriers to develo** potent B7-H3 inhibitors. By overcoming these challenges, new therapeutic approaches may be developed, instilling renewed hope in BC patients worldwide.
Availability of data and materials
No datasets were generated or analysed during the current study.
Abbreviations
- BC:
-
Breast cancer
- HR + :
-
Hormone receptor-positive
- ER + :
-
Estrogen receptor alpha-positive
- PR + :
-
Progesterone receptor
- HER2 + :
-
Human epidermal growth factor receptor 2 positive
- TNBC:
-
Triple negative breast cancer
- ICI:
-
Immune checkpoint inhibitor
- PD-1:
-
Programmed death 1
- TME:
-
Tumor microenvironment
- APCs:
-
Antigen-presenting cells
- IgV:
-
Variable
- IgC:
-
Constant
- sB7-H3:
-
Soluble B7-H3
- BBD:
-
Benign breast disease
- CSCs:
-
Cancer stem cells
- BCSCs:
-
Breast cancer stem cells
- EMT:
-
Epithelial-mesenchymal transition
- ROS:
-
Reactive oxygen species
- MDSCs:
-
Myeloid-derived suppressor cells
- NK:
-
Natural killer
- TAMs:
-
Tumor-associated macrophages
- ECM:
-
Extracellular matrix
- Th1:
-
T-helper 1
- FOXP3:
-
Forkhead Box P3
- Th2:
-
T-helper cells
- CTL:
-
Cytotoxic T-cells
- TILs:
-
Tumor-infiltrating lymphocytes
- TN:
-
Triple negative
- OS:
-
Overall survival
- Treg:
-
T-regulatory
- CAFs:
-
Cancer-associated fibroblasts
- α-SMA:
-
Alpha-smooth muscle actin
- VEGF:
-
Vascular endothelial growth factor
- mAbs:
-
Monoclonal Antibodies
- ADCC:
-
Antibody-dependent cellular cytotoxicity
- bsAb:
-
Bispecific antibody
- LNPs:
-
Lipid nanoparticles
- ADCs:
-
Antibody–drug conjugates
- CAR:
-
Chimeric Antigen Receptor
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ. Strategies for subtypes–dealing with the diversity of breast cancer: highlights of the St. Gallen international expert consensus on the primary therapy of early breast cancer 2011. Ann Oncol. 2011;22(8):1736–47.
Davies C, Godwin J, Gray R, Clarke M, Cutter D, Darby S, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378(9793):771–84.
Hudis CA. Trastuzumab–mechanism of action and use in clinical practice. N Engl J Med. 2007;357(1):39–51.
Garcia-Martinez L, Zhang Y, Nakata Y, Chan HL, Morey L. Epigenetic mechanisms in breast cancer therapy and resistance. Nat Commun. 2021;12(1):1786.
So JY, Ohm J, Lipkowitz S, Yang L. Triple negative breast cancer (TNBC): non-genetic tumor heterogeneity and immune microenvironment: emerging treatment options. Pharmacol Ther. 2022;237:108253.
Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. 2020;20(11):651–68.
Bolandi N, Derakhshani A, Hemmat N, Baghbanzadeh A, Asadzadeh Z, Afrashteh Nour M, et al. The positive and negative immunoregulatory role of B7 family: promising novel targets in gastric cancer treatment. Int J Mol Sci. 2021;22(19):10719.
Sadreddini S, Baradaran B, Aghebati-Maleki A, Sadreddini S, Shanehbandi D, Fotouhi A, et al. Immune checkpoint blockade opens a new way to cancer immunotherapy. J Cell Physiol. 2019;234(6):8541–9.
Wang C, Feng H, Cheng X, Liu K, Cai D, Zhao R. Potential therapeutic targets of B7 family in colorectal cancer. Front Immunol. 2020;11:681.
Wang S, Bajorath J, Flies DB, Dong H, Honjo T, Chen L. Molecular modeling and functional map** of B7–H1 and B7-DC uncouple costimulatory function from PD-1 interaction. J Exp Med. 2003;197(9):1083–91.
Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7–H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800.
Hossain MA, Liu G, Dai B, Si Y, Yang Q, Wazir J, et al. Reinvigorating exhausted CD8(+) cytotoxic T lymphocytes in the tumor microenvironment and current strategies in cancer immunotherapy. Med Res Rev. 2021;41(1):156–201.
Yi M, Jiao D, Xu H, Liu Q, Zhao W, Han X, et al. Biomarkers for predicting efficacy of PD-1/PD-L1 inhibitors. Mol Cancer. 2018;17(1):129.
Cai D, Li J, Liu D, Hong S, Qiao Q, Sun Q, et al. Tumor-expressed B7–H3 mediates the inhibition of antitumor T-cell functions in ovarian cancer insensitive to PD-1 blockade therapy. Cell Mol Immunol. 2020;17(3):227–36.
Callahan MK, Postow MA, Wolchok JD. Targeting T Cell co-receptors for cancer therapy. Immunity. 2016;44(5):1069–78.
Pitt JM, Vétizou M, Daillère R, Roberti MP, Yamazaki T, Routy B, et al. Resistance mechanisms to immune-checkpoint blockade in cancer: tumor-intrinsic and -extrinsic factors. Immunity. 2016;44(6):1255–69.
Kanchan RK, Perumal N, Atri P, Chirravuri Venkata R, Thapa I, Klinkebiel DL, et al. MiR-1253 exerts tumor-suppressive effects in medulloblastoma via inhibition of CDK6 and CD276 (B7–H3). Brain Pathol. 2020;30(4):732–45.
Kanchan RK, Doss D, Khan P, Nasser MW, Mahapatra S. To kill a cancer: Targeting the immune inhibitory checkpoint molecule, B7–H3. Biochim Biophys Acta Rev Cancer. 2022;1877(5):188783.
Crispen PL, Sheinin Y, Roth TJ, Lohse CM, Kuntz SM, Frigola X, et al. Tumor cell and tumor vasculature expression of B7–H3 predict survival in clear cell renal cell carcinoma. Clin Cancer Res. 2008;14(16):5150–7.
Getu AA, Tigabu A, Zhou M, Lu J, Fodstad Ø, Tan M. New frontiers in immune checkpoint B7–H3 (CD276) research and drug development. Mol Cancer. 2023;22(1):43.
Chen H, Duan X, Deng X, Huang Y, Zhou X, Zhang S, et al. EBV-upregulated B7–H3 inhibits NK cell-mediated antitumor function and contributes to nasopharyngeal carcinoma progression. Cancer Immunol Res. 2023;11:830–46.
Wang C, Li Y, Jia L, Kim JK, Li J, Deng P, et al. CD276 expression enables squamous cell carcinoma stem cells to evade immune surveillance. Cell Stem Cell. 2021;28(9):1597-613.e7.
Ganesan B, Parameswaran S, Sharma A, Krishnakumar S. Clinical relevance of B7H3 expression in retinoblastoma. Sci Rep. 2020;10(1):10185.
Zang X, Thompson RH, Al-Ahmadie HA, Serio AM, Reuter VE, Eastham JA, et al. B7–H3 and B7x are highly expressed in human prostate cancer and associated with disease spread and poor outcome. Proc Natl Acad Sci U S A. 2007;104(49):19458–63.
Arigami T, Narita N, Mizuno R, Nguyen L, Ye X, Chung A, et al. B7–h3 ligand expression by primary breast cancer and associated with regional nodal metastasis. Ann Surg. 2010;252(6):1044–51.
Cong F, Yu H, Gao X. Expression of CD24 and B7–H3 in breast cancer and the clinical significance. Oncol Lett. 2017;14(6):7185–90.
Liu C, Liu J, Wang J, Liu Y, Zhang F, Lin W, et al. B7–H3 expression in ductal and lobular breast cancer and its association with IL-10. Mol Med Rep. 2013;7(1):134–8.
Pizon M, Schott DS, Pachmann U, Pachmann K. B7–H3 on circulating epithelial tumor cells correlates with the proliferation marker, Ki-67, and may be associated with the aggressiveness of tumors in breast cancer patients. Int J Oncol. 2018;53(5):2289–99.
Zhou WT, ** WL. B7–H3/CD276: an emerging cancer immunotherapy. Front Immunol. 2021;12:701006.
Chen W, Liu P, Wang Y, Nie W, Li Z, Xu W, et al. Characterization of a soluble B7–H3 (sB7-H3) spliced from the intron and analysis of sB7-H3 in the sera of patients with hepatocellular carcinoma. PLoS One. 2013;8(10):e76965.
Zhang G, Hou J, Shi J, Yu G, Lu B, Zhang X. Soluble CD276 (B7–H3) is released from monocytes, dendritic cells and activated T cells and is detectable in normal human serum. Immunology. 2008;123(4):538–46.
Hwang JY, Jeong JM, Kwon MG, Seo JS, Hwang SD, Son MH, et al. Olive flounder CD276 (B7–H3) a coinhibitory molecule for T cells: responses during viral hemorrhagic septicemia virus (VHSV) stimulation. Fish Shellfish Immunol. 2018;73:228–33.
Hofmeyer KA, Ray A, Zang X. The contrasting role of B7–H3. Proc Natl Acad Sci U S A. 2008;105(30):10277–8.
Zhao B, Li H, **a Y, Wang Y, Wang Y, Shi Y, et al. Immune checkpoint of B7–H3 in cancer: from immunology to clinical immunotherapy. J Hematol Oncol. 2022;15(1):153.
Oh Y, Park R, Kim SY, Park SH, Jo S, Kim TH, et al. B7–H3 regulates osteoclast differentiation via type I interferon-dependent IDO induction. Cell Death Dis. 2021;12(11):971.
Suh WK, Wang SX, Jheon AH, Moreno L, Yoshinaga SK, Ganss B, et al. The immune regulatory protein B7–H3 promotes osteoblast differentiation and bone mineralization. Proc Natl Acad Sci U S A. 2004;101(35):12969–73.
Picarda E, Galbo PM Jr, Zong H, Rajan MR, Wallenius V, Zheng D, et al. The immune checkpoint B7–H3 (CD276) regulates adipocyte progenitor metabolism and obesity development. Sci Adv. 2022;8(17):eabm7012.
Flem-Karlsen K, Fodstad Ø, Nunes-Xavier CE. B7–H3 immune checkpoint protein in human cancer. Curr Med Chem. 2020;27(24):4062–86.
Flem-Karlsen K, Fodstad Ø, Tan M, Nunes-Xavier CE. B7–H3 in cancer - beyond immune regulation. Trends Cancer. 2018;4(6):401–4.
Dong P, **ong Y, Yue J, Hanley SJB, Watari H. B7H3 As a promoter of metastasis and promising therapeutic target. Front Oncol. 2018;8:264.
Picarda E, Ohaegbulam KC, Zang X. Molecular pathways: targeting B7–H3 (CD276) for human cancer immunotherapy. Clin Cancer Res. 2016;22(14):3425–31.
Nygren MK, Tekle C, Ingebrigtsen VA, Mäkelä R, Krohn M, Aure MR, et al. Identifying microRNAs regulating B7–H3 in breast cancer: the clinical impact of microRNA-29c. Br J Cancer. 2014;110(8):2072–80.
Wang ET, Sandberg R, Luo S, Khrebtukova I, Zhang L, Mayr C, et al. Alternative isoform regulation in human tissue transcriptomes. Nature. 2008;456(7221):470–6.
Avci O, Çavdar E, İriağaç Y, Karaboyun K, Çelikkol A, Özçağlayan TİK, et al. Soluble B7H3 level in breast cancer and its relationship with clinicopathological variables and T cell infiltration. Contemp Oncol (Pozn). 2022;26(1):27–31.
Dube DH, Bertozzi CR. Glycans in cancer and inflammation–potential for therapeutics and diagnostics. Nat Rev Drug Discov. 2005;4(6):477–88.
Huang Y, Zhang HL, Li ZL, Du T, Chen YH, Wang Y, et al. FUT8-mediated aberrant N-glycosylation of B7H3 suppresses the immune response in triple-negative breast cancer. Nat Commun. 2021;12(1):2672.
Dittmer J. Breast cancer stem cells: features, key drivers and treatment options. Semin Cancer Biol. 2018;53:59–74.
Gupta PB, Fillmore CM, Jiang G, Shapira SD, Tao K, Kuperwasser C, et al. Stochastic state transitions give rise to phenotypic equilibrium in populations of cancer cells. Cell. 2011;146(4):633–44.
Atashzar MR, Baharlou R, Karami J, Abdollahi H, Rezaei R, Pourramezan F, et al. Cancer stem cells: a review from origin to therapeutic implications. J Cell Physiol. 2020;235(2):790–803.
Kreso A, Dick JE. Evolution of the cancer stem cell model. Cell Stem Cell. 2014;14(3):275–91.
Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100(7):3983–8.
Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1(5):555–67.
Phillips TM, McBride WH, Pajonk F. The response of CD24(-/low)/CD44+ breast cancer-initiating cells to radiation. J Natl Cancer Inst. 2006;98(24):1777–85.
Beck B, Blanpain C. Unravelling cancer stem cell potential. Nat Rev Cancer. 2013;13(10):727–38.
Zhang L, Chen W, Liu S, Chen C. Targeting breast cancer stem cells. Int J Biol Sci. 2023;19(2):552–70.
Liu Z, Zhang W, Phillips JB, Arora R, McClellan S, Li J, et al. Immunoregulatory protein B7–H3 regulates cancer stem cell enrichment and drug resistance through MVP-mediated MEK activation. Oncogene. 2019;38(1):88–102.
Lito P, Saborowski A, Yue J, Solomon M, Joseph E, Gadal S, et al. Disruption of CRAF-mediated MEK activation is required for effective MEK inhibition in KRAS mutant tumors. Cancer Cell. 2014;25(5):697–710.
Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023–75.
Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147(2):275–92.
Waks AG, Winer EP. Breast cancer treatment: a review. JAMA. 2019;321(3):288–300.
Tulotta C, Ottewell P. The role of IL-1B in breast cancer bone metastasis. Endocr Relat Cancer. 2018;25(7):R421–34.
**ong Z, Deng G, Huang X, Li X, **e X, Wang J, et al. Bone metastasis pattern in initial metastatic breast cancer: a population-based study. Cancer Manag Res. 2018;10:287–95.
Smid M, Wang Y, Zhang Y, Sieuwerts AM, Yu J, Klijn JG, et al. Subtypes of breast cancer show preferential site of relapse. Cancer Res. 2008;68(9):3108–14.
Pentheroudakis G, Fountzilas G, Bafaloukos D, Koutsoukou V, Pectasides D, Skarlos D, et al. Metastatic breast cancer with liver metastases: a registry analysis of clinicopathologic, management and outcome characteristics of 500 women. Breast Cancer Res Treat. 2006;97(3):237–44.
Lin NU, Bellon JR, Winer EP. CNS metastases in breast cancer. J Clin Oncol. 2004;22(17):3608–17.
Wang Y, Ye F, Liang Y, Yang Q. Breast cancer brain metastasis: insight into molecular mechanisms and therapeutic strategies. Br J Cancer. 2021;125(8):1056–67.
Yu TT, Zhang T, Lu X, Wang RZ. B7–H3 promotes metastasis, proliferation, and epithelial-mesenchymal transition in lung adenocarcinoma. Onco Targets Ther. 2018;11:4693–700.
Castellanos JR, Purvis IJ, Labak CM, Guda MR, Tsung AJ, Velpula KK, et al. B7–H3 role in the immune landscape of cancer. Am J Clin Exp Immunol. 2017;6(4):66–75.
Tekle C, Nygren MK, Chen YW, Dybsjord I, Nesland JM, Maelandsmo GM, et al. B7–H3 contributes to the metastatic capacity of melanoma cells by modulation of known metastasis-associated genes. Int J Cancer. 2012;130(10):2282–90.
Wang L, Zhang Q, Chen W, Shan B, Ding Y, Zhang G, et al. B7–H3 is overexpressed in patients suffering osteosarcoma and associated with tumor aggressiveness and metastasis. PLoS One. 2013;8(8):e70689.
Liu F, Zhang T, Zou S, Jiang B, Hua D. B7–H3 promotes cell migration and invasion through the Jak2/Stat3/MMP9 signaling pathway in colorectal cancer. Mol Med Rep. 2015;12(4):5455–60.
Liao H, Ding M, Zhou N, Yang Y, Chen L. B7–H3 promotes the epithelial-mesenchymal transition of NSCLC by targeting SIRT1 through the PI3K/AKT pathway. Mol Med Rep. 2022;25(3):79.
**e J, Sun M, Zhang D, Chen C, Lin S, Zhang G. Fibronectin enhances tumor metastasis through B7–H3 in clear cell renal cell carcinoma. FEBS Open Bio. 2021;11(11):2977–87.
**e C, Liu D, Chen Q, Yang C, Wang B, Wu H. Soluble B7–H3 promotes the invasion and metastasis of pancreatic carcinoma cells through the TLR4/NF-κB pathway. Sci Rep. 2016;6:27528.
Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J Gen Physiol. 1927;8(6):519–30.
Li L, Liang Y, Kang L, Liu Y, Gao S, Chen S, et al. Transcriptional regulation of the Warburg effect in cancer by SIX1. Cancer Cell. 2018;33(3):368-85.e7.
Wang Y, Tahiri H, Yang C, Gu M, Ruan X, Hardy P. Overexpression of miR-181a regulates the Warburg effect in triple-negative breast cancer. Climacteric. 2023;26(1):64–71.
Jiang H, Wei H, Wang H, Wang Z, Li J, Ou Y, et al. Zeb1-induced metabolic reprogramming of glycolysis is essential for macrophage polarization in breast cancer. Cell Death Dis. 2022;13(3):206.
Li J, Qu P, Zhou XZ, Ji YX, Yuan S, Liu SP, et al. Pimozide inhibits the growth of breast cancer cells by alleviating the Warburg effect through the P53 signaling pathway. Biomed Pharmacother. 2022;150:113063.
Wu H, Jiao Y, Zhou C, Guo X, Wu Z, Lv Q. miR-140-3p/usp36 axis mediates ubiquitination to regulate PKM2 and suppressed the malignant biological behavior of breast cancer through Warburg effect. Cell Cycle. 2023;22(6):680–92.
Zhang X, Luo F, Luo S, Li L, Ren X, Lin J, et al. Transcriptional repression of aerobic glycolysis by OVOL2 in breast cancer. Adv Sci (Weinh). 2022;9(27):e2200705.
Lim S, Liu H, Madeira da Silva L, Arora R, Liu Z, Phillips JB, et al. Immunoregulatory protein B7–H3 reprograms glucose metabolism in cancer cells by ROS-mediated stabilization of HIF1α. Cancer Res. 2016;76(8):2231–42.
Nunes-Xavier CE, Karlsen KF, Tekle C, Pedersen C, Øyjord T, Hongisto V, et al. Decreased expression of B7–H3 reduces the glycolytic capacity and sensitizes breast cancer cells to AKT/mTOR inhibitors. Oncotarget. 2016;7(6):6891–901.
Liu J, Yang S, Cao B, Zhou G, Zhang F, Wang Y, et al. Targeting B7–H3 via chimeric antigen receptor T cells and bispecific killer cell engagers augments antitumor response of cytotoxic lymphocytes. J Hematol Oncol. 2021;14(1):21.
Abad E, Graifer D, Lyakhovich A. DNA damage response and resistance of cancer stem cells. Cancer Lett. 2020;474:106–17.
Raman D, Cimpean AM, De Miglio MR. Editorial: drug resistance in breast cancer - mechanisms and approaches to overcome chemoresistance. Front Oncol. 2022;12:1080684.
Brown KA, Andreopoulou E, Andreopoulou P. Endocrine therapy-related endocrinopathies-biology, prevalence and implications for the management of breast cancer. Oncol Hematol Rev. 2020;16(1):17–22.
Beyaz H, Uludag H, Kavaz D, Rizaner N. Mechanisms of drug resistance and use of nanoparticle delivery to overcome resistance in breast cancers. Adv Exp Med Biol. 2021;1347:163–81.
Zhang P, Chen Z, Ning K, ** J, Han X. Inhibition of B7–H3 reverses oxaliplatin resistance in human colorectal cancer cells. Biochem Biophys Res Commun. 2017;490(3):1132–8.
Pulido R, Nunes-Xavier CE. Hopes on immunotherapy targeting B7–H3 in neuroblastoma. Transl Oncol. 2023;27:101580.
Fan R, Chen C, Hu J, Mu M, Chuan D, Chen Z, et al. Multifunctional gold nanorods in low-temperature photothermal interactions for combined tumor starvation and RNA interference therapy. Acta Biomater. 2023;159:324–37.
Nunes-Xavier CE, Emaldi M, Guldvik IJ, Ramberg H, Taskén KA, Mælandsmo GM, et al. Correlation of expression of Major Vault Protein with androgen receptor and immune checkpoint protein B7–H3, and with poor prognosis in prostate cancer. Pathol Res Pract. 2023;241:154243.
Guo C, Figueiredo I, Gurel B, Neeb A, Seed G, Crespo M, et al. B7–H3 as a therapeutic target in advanced prostate cancer. Eur Urol. 2023;83(3):224–38.
Mahmoud AM, Frank I, Orme JJ, Lavoie RR, Thapa P, Costello BA, et al. Evaluation of PD-L1 and B7–H3 expression as a predictor of response to adjuvant chemotherapy in bladder cancer. BMC Urol. 2022;22(1):90.
Liu H, Tekle C, Chen YW, Kristian A, Zhao Y, Zhou M, et al. B7–H3 silencing increases paclitaxel sensitivity by abrogating Jak2/Stat3 phosphorylation. Mol Cancer Ther. 2011;10(6):960–71.
Qi M, Elion EA. MAP kinase pathways. J Cell Sci. 2005;118(Pt 16):3569–72.
Lee S, Rauch J, Kolch W. Targeting MAPK signaling in cancer: mechanisms of drug resistance and sensitivity. Int J Mol Sci. 2020;21(3):1102.
Jiménez-Martínez M, Stamatakis K, Fresno M. The Dual-Specificity Phosphatase 10 (DUSP10): its role in cancer, inflammation, and immunity. Int J Mol Sci. 2019;20(7):1626.
Flem-Karlsen K, Tekle C, Øyjord T, Flørenes VA, Mælandsmo GM, Fodstad Ø, et al. p38 MAPK activation through B7-H3-mediated DUSP10 repression promotes chemoresistance. Sci Rep. 2019;9(1):5839.
Cánovas B, Igea A, Sartori AA, Gomis RR, Paull TT, Isoda M, et al. Targeting p38α increases DNA damage, chromosome instability, and the anti-tumoral response to taxanes in breast cancer cells. Cancer Cell. 2018;33(6):1094-110.e8.
Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19(11):1423–37.
Zhu S, Zhang T, Zheng L, Liu H, Song W, Liu D, et al. Combination strategies to maximize the benefits of cancer immunotherapy. J Hematol Oncol. 2021;14(1):156.
Motz GT, Coukos G. Deciphering and reversing tumor immune suppression. Immunity. 2013;39(1):61–73.
Talmadge JE, Gabrilovich DI. History of myeloid-derived suppressor cells. Nat Rev Cancer. 2013;13(10):739–52.
Groth C, Hu X, Weber R, Fleming V, Altevogt P, Utikal J, et al. Immunosuppression mediated by myeloid-derived suppressor cells (MDSCs) during tumour progression. Br J Cancer. 2019;120(1):16–25.
Sui H, Dongye S, Liu X, Xu X, Wang L, ** CQ, et al. Immunotherapy of targeting MDSCs in tumor microenvironment. Front Immunol. 2022;13:990463.
Stewart TJ, Abrams SI. How tumours escape mass destruction. Oncogene. 2008;27(45):5894–903.
Tannenbaum CS, Rayman PA, Pavicic PG, Kim JS, Wei W, Polefko A, et al. Mediators of inflammation-driven expansion, trafficking, and function of tumor-infiltrating MDSCs. Cancer Immunol Res. 2019;7(10):1687–99.
Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12(4):253–68.
Casbon AJ, Reynaud D, Park C, Khuc E, Gan DD, Schepers K, et al. Invasive breast cancer reprograms early myeloid differentiation in the bone marrow to generate immunosuppressive neutrophils. Proc Natl Acad Sci U S A. 2015;112(6):E566–75.
Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58(1):49–59.
Yu J, Wang Y, Yan F, Li H, Ren X. Response to comment on “Myeloid-derived suppressor cells suppress antitumor immune responses through IDO expression and correlate with lymph node metastasis in patients with breast cancer.” J Immunol. 2013;190(11):5341–2.
Li W, Tanikawa T, Kryczek I, **a H, Li G, Wu K, et al. Aerobic glycolysis controls myeloid-derived suppressor cells and tumor immunity via a specific CEBPB isoform in triple-negative breast cancer. Cell Metab. 2018;28(1):87-103.e6.
Zhang G, Huang H, Zhu Y, Yu G, Gao X, Xu Y, et al. A novel subset of B7–H3(+)CD14(+)HLA-DR(-/low) myeloid-derived suppressor cells are associated with progression of human NSCLC. Oncoimmunology. 2015;4(2):e977164.
Liu T, Gonzalez De Los Santos F, Rinke AE, Fang C, Flaherty KR, Phan SH. B7H3-dependent myeloid-derived suppressor cell recruitment and activation in pulmonary fibrosis. Front Immunol. 2022;13:901349.
Fang C, Rinke AE, Wang J, Flaherty KR, Phan SH, Liu T. B7H3 expression and significance in idiopathic pulmonary fibrosis. J Pathol. 2022;256(3):310–20.
Lee YH, Martin-Orozco N, Zheng P, Li J, Zhang P, Tan H, et al. Inhibition of the B7–H3 immune checkpoint limits tumor growth by enhancing cytotoxic lymphocyte function. Cell Res. 2017;27(8):1034–45.
Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51.
Cassetta L, Fragkogianni S, Sims AH, Swierczak A, Forrester LM, Zhang H, et al. Human tumor-associated macrophage and monocyte transcriptional landscapes reveal cancer-specific reprogramming, biomarkers, and therapeutic targets. Cancer Cell. 2019;35(4):588-602.e10.
Li H, Yang P, Wang J, Zhang J, Ma Q, Jiang Y, et al. HLF regulates ferroptosis, development and chemoresistance of triple-negative breast cancer by activating tumor cell-macrophage crosstalk. J Hematol Oncol. 2022;15(1):2.
Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124(2):263–6.
Goswami S, Sahai E, Wyckoff JB, Cammer M, Cox D, Pixley FJ, et al. Macrophages promote the invasion of breast carcinoma cells via a colony-stimulating factor-1/epidermal growth factor paracrine loop. Cancer Res. 2005;65(12):5278–83.
Coniglio SJ, Eugenin E, Dobrenis K, Stanley ER, West BL, Symons MH, et al. Microglial stimulation of glioblastoma invasion involves epidermal growth factor receptor (EGFR) and colony stimulating factor 1 receptor (CSF-1R) signaling. Mol Med. 2012;18(1):519–27.
Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958–69.
Mao Y, Chen L, Wang F, Zhu D, Ge X, Hua D, et al. Cancer cell-expressed B7–H3 regulates the differentiation of tumor-associated macrophages in human colorectal carcinoma. Oncol Lett. 2017;14(5):6177–83.
Miyamoto T, Murakami R, Hamanishi J, Tanigaki K, Hosoe Y, Mise N, et al. B7–H3 suppresses antitumor immunity via the CCL2-CCR2-M2 macrophage axis and contributes to ovarian cancer progression. Cancer Immunol Res. 2022;10(1):56–69.
Cheng N, Bei Y, Song Y, Zhang W, Xu L, Zhang W, et al. B7–H3 augments the pro-angiogenic function of tumor-associated macrophages and acts as a novel adjuvant target for triple-negative breast cancer therapy. Biochem Pharmacol. 2021;183:114298.
Gao Y, Fang P, Li WJ, Zhang J, Wang GP, Jiang DF, et al. LncRNA NEAT1 sponges miR-214 to regulate M2 macrophage polarization by regulation of B7–H3 in multiple myeloma. Mol Immunol. 2020;117:20–8.
Zitvogel L, Galluzzi L, Kepp O, Smyth MJ, Kroemer G. Type I interferons in anticancer immunity. Nat Rev Immunol. 2015;15(7):405–14.
Tan AH, Goh SY, Wong SC, Lam KP. T helper cell-specific regulation of inducible costimulator expression via distinct mechanisms mediated by T-bet and GATA-3. J Biol Chem. 2008;283(1):128–36.
Pagès F, Kirilovsky A, Mlecnik B, Asslaber M, Tosolini M, Bindea G, et al. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol. 2009;27(35):5944–51.
Dieu-Nosjean MC, Antoine M, Danel C, Heudes D, Wislez M, Poulot V, et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol. 2008;26(27):4410–7.
Hwang WT, Adams SF, Tahirovic E, Hagemann IS, Coukos G. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: a meta-analysis. Gynecol Oncol. 2012;124(2):192–8.
Denkert C, Loibl S, Noske A, Roller M, Müller BM, Komor M, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28(1):105–13.
Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02–98. J Clin Oncol. 2013;31(7):860–7.
Adams S, Gatti-Mays ME, Kalinsky K, Korde LA, Sharon E, Amiri-Kordestani L, et al. Current landscape of immunotherapy in breast cancer: a review. JAMA Oncol. 2019;5(8):1205–14.
Monnot GC, Romero P. Rationale for immunological approaches to breast cancer therapy. Breast. 2018;37:187–95.
Dieci MV, Mathieu MC, Guarneri V, Conte P, Delaloge S, Andre F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in two phase III randomized adjuvant breast cancer trials. Ann Oncol. 2015;26(8):1698–704.
Zhou XY, Yashiro-Ohtani Y, Nakahira M, Park WR, Abe R, Hamaoka T, et al. Molecular mechanisms underlying differential contribution of CD28 versus non-CD28 costimulatory molecules to IL-2 promoter activation. J Immunol. 2002;168(8):3847–54.
Jain J, Loh C, Rao A. Transcriptional regulation of the IL-2 gene. Curr Opin Immunol. 1995;7(3):333–42.
Quintana Á, Peg V, Prat A, Moliné T, Villacampa G, Paré L, et al. Immune analysis of lymph nodes in relation to the presence or absence of tumor infiltrating lymphocytes in triple-negative breast cancer. Eur J Cancer. 2021;148:134–45.
Ignatiadis M, Van den Eynden G, Roberto S, Fornili M, Bareche Y, Desmedt C, et al. Tumor-infiltrating lymphocytes in patients receiving trastuzumab/pertuzumab-based chemotherapy: A TRYPHAENA substudy. J Natl Cancer Inst. 2019;111(1):69–77.
Mei J, Cai Y, Zhu H, Jiang Y, Fu Z, Xu J, et al. High B7-H3 expression with low PD-L1 expression identifies armored-cold tumors in triple-negative breast cancer. NPJ Breast Cancer. 2024;10(1). https://doi.org/10.1038/s41523-024-00618-6.
Göschl L, Scheinecker C, Bonelli M. Treg cells in autoimmunity: from identification to Treg-based therapies. Semin Immunopathol. 2019;41(3):301–14.
Gasteiger G, Hemmers S, Bos PD, Sun JC, Rudensky AY. IL-2-dependent adaptive control of NK cell homeostasis. J Exp Med. 2013;210(6):1179–87.
Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6(4):295–307.
de la Cruz-Merino L, Chiesa M, Caballero R, Rojo F, Palazón N, Carrasco FH, et al. Breast cancer immunology and immunotherapy: current status and future perspectives. Int Rev Cell Mol Biol. 2017;331:1–53.
Azizi E, Carr AJ, Plitas G, Cornish AE, Konopacki C, Prabhakaran S, et al. Single-cell map of diverse immune phenotypes in the breast tumor microenvironment. Cell. 2018;174(5):1293-308.e36.
De Simone M, Arrigoni A, Rossetti G, Gruarin P, Ranzani V, Politano C, et al. Transcriptional landscape of human tissue lymphocytes unveils uniqueness of tumor-infiltrating T regulatory cells. Immunity. 2016;45(5):1135–47.
Qiu J, Xu L, Zeng X, Wu H, Liang F, Lv Q, et al. CCL5 mediates breast cancer metastasis and prognosis through CCR5/Treg cells. Front Oncol. 2022;12:972383.
Iida K, Miyake M, Onishi K, Hori S, Morizawa Y, Gotoh D, et al. Prognostic impact of tumor-infiltrating CD276/Foxp3-positive lymphocytes and associated circulating cytokines in patients undergoing radical nephrectomy for localized renal cell carcinoma. Oncol Lett. 2019;17(4):4004–10.
Zheng Y, Josefowicz SZ, Kas A, Chu TT, Gavin MA, Rudensky AY. Genome-wide analysis of Foxp3 target genes in develo** and mature regulatory T cells. Nature. 2007;445(7130):936–40.
Gavin MA, Rasmussen JP, Fontenot JD, Vasta V, Manganiello VC, Beavo JA, et al. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445(7129):771–5.
Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4(4):330–6.
Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057–61.
Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4(4):337–42.
** Y, Zhang P, Li J, Zhao J, Liu C, Yang F, et al. B7–H3 in combination with regulatory T cell is associated with tumor progression in primary human non-small cell lung cancer. Int J Clin Exp Pathol. 2015;8(11):13987–95.
Dotto GP, Weinberg RA, Ariza A. Malignant transformation of mouse primary keratinocytes by Harvey sarcoma virus and its modulation by surrounding normal cells. Proc Natl Acad Sci U S A. 1988;85(17):6389–93.
Costa A, Scholer-Dahirel A, Mechta-Grigoriou F. The role of reactive oxygen species and metabolism on cancer cells and their microenvironment. Semin Cancer Biol. 2014;25:23–32.
Gascard P, Tlsty TD. Carcinoma-associated fibroblasts: orchestrating the composition of malignancy. Genes Dev. 2016;30(9):1002–19.
Gentric G, Mieulet V, Mechta-Grigoriou F. Heterogeneity in cancer metabolism: new concepts in an old field. Antioxid Redox Signal. 2017;26(9):462–85.
Öhlund D, Handly-Santana A, Biffi G, Elyada E, Almeida AS, Ponz-Sarvise M, et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med. 2017;214(3):579–96.
Sugimoto H, Mundel TM, Kieran MW, Kalluri R. Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer Biol Ther. 2006;5(12):1640–6.
Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16(9):582–98.
Lee HW, Park YM, Lee SJ, Cho HJ, Kim DH, Lee JI, et al. Alpha-smooth muscle actin (ACTA2) is required for metastatic potential of human lung adenocarcinoma. Clin Cancer Res. 2013;19(21):5879–89.
Parajuli H, Teh MT, Abrahamsen S, Christoffersen I, Neppelberg E, Lybak S, et al. Integrin α11 is overexpressed by tumour stroma of head and neck squamous cell carcinoma and correlates positively with alpha smooth muscle actin expression. J Oral Pathol Med. 2017;46(4):267–75.
Toullec A, Gerald D, Despouy G, Bourachot B, Cardon M, Lefort S, et al. Oxidative stress promotes myofibroblast differentiation and tumour spreading. EMBO Mol Med. 2010;2(6):211–30.
Piersma B, Hayward MK, Weaver VM. Fibrosis and cancer: a strained relationship. Biochim Biophys Acta Rev Cancer. 2020;1873(2):188356.
Guise TA. Breast cancer bone metastases: it’s all about the neighborhood. Cell. 2013;154(5):957–9.
Zhang XH, ** X, Malladi S, Zou Y, Wen YH, Brogi E, et al. Selection of bone metastasis seeds by mesenchymal signals in the primary tumor stroma. Cell. 2013;154(5):1060–73.
Zhang S, Zhou C, Zhang D, Huang Z, Zhang G. The anti-apoptotic effect on cancer-associated fibroblasts of B7–H3 molecule enhancing the cell invasion and metastasis in renal cancer. Onco Targets Ther. 2019;12:4119–27.
Zhan S, Liu Z, Zhang M, Guo T, Quan Q, Huang L, et al. Overexpression of B7–H3 in α-SMA-positive fibroblasts is associated with cancer progression and survival in gastric adenocarcinomas. Front Oncol. 2019;9:1466.
Costa A, Kieffer Y, Scholer-Dahirel A, Pelon F, Bourachot B, Cardon M, et al. Fibroblast heterogeneity and immunosuppressive environment in human breast cancer. Cancer Cell. 2018;33(3):463-79.e10.
Souweidane MM, Kramer K, Pandit-Taskar N, Zhou Z, Haque S, Zanzonico P, et al. Convection-enhanced delivery for diffuse intrinsic pontine glioma: a single-centre, dose-escalation, phase 1 trial. Lancet Oncol. 2018;19(8):1040–50.
Lu H, Shi T, Wang M, Li X, Gu Y, Zhang X, et al. B7–H3 inhibits the IFN-γ-dependent cytotoxicity of Vγ9Vδ2 T cells against colon cancer cells. Oncoimmunology. 2020;9(1):1748991.
Modak S, Kramer K, Gultekin SH, Guo HF, Cheung NK. Monoclonal antibody 8H9 targets a novel cell surface antigen expressed by a wide spectrum of human solid tumors. Cancer Res. 2001;61(10):4048–54.
Kramer K, Kushner BH, Modak S, Pandit-Taskar N, Smith-Jones P, Zanzonico P, et al. Compartmental intrathecal radioimmunotherapy: results for treatment for metastatic CNS neuroblastoma. J Neurooncol. 2010;97(3):409–18.
Kohrt HE, Houot R, Marabelle A, Cho HJ, Osman K, Goldstein M, et al. Combination strategies to enhance antitumor ADCC. Immunotherapy. 2012;4(5):511–27.
Loo D, Alderson RF, Chen FZ, Huang L, Zhang W, Gorlatov S, et al. Development of an Fc-enhanced anti-B7-H3 monoclonal antibody with potent antitumor activity. Clin Cancer Res. 2012;18(14):3834–45.
Nisonoff A, Wissler FC, Lipman LN. Properties of the major component of a peptic digest of rabbit antibody. Science. 1960;132(3441):1770–1.
Labrijn AF, Janmaat ML, Reichert JM, Parren P. Bispecific antibodies: a mechanistic review of the pipeline. Nat Rev Drug Discov. 2019;18(8):585–608.
Ma J, Ma P, Zhao C, Xue X, Han H, Liu C, et al. B7–H3 as a promising target for cytotoxicity T cell in human cancer therapy. Oncotarget. 2016;7(20):29480–91.
Huang C, Duan X, Wang J, Tian Q, Ren Y, Chen K, et al. Lipid nanoparticle delivery system for mRNA encoding B7H3-redirected bispecific antibody displays potent antitumor effects on malignant tumors. Adv Sci (Weinh). 2023;10(3):e2205532.
Bouchard H, Viskov C, Garcia-Echeverria C. Antibody-drug conjugates—a new wave of cancer drugs. Bioorg Med Chem Lett. 2014;24(23):5357–63.
Hafeez U, Parakh S, Gan HK, Scott AM. Antibody-drug conjugates for cancer therapy. Molecules. 2020;25(20):4764.
Scribner JA, Brown JG, Son T, Chiechi M, Li P, Sharma S, et al. Preclinical development of MGC018, a Duocarmycin-based antibody-drug conjugate targeting B7–H3 for solid cancer. Mol Cancer Ther. 2020;19(11):2235–44.
Patel MR, Johnson ML, Falchook GS, Doi T, Friedman CF, Piha-Paul SA, et al. DS-7300 (B7-H3 DXd-ADC) in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC): a subgroup analysis of a phase 1/2 multicenter study. J Clin Oncol. 2022;40:87. https://doi.org/10.1200/JCO.2022.40.6_suppl.087.
Cai L, Michelakos T, Yamada T, Fan S, Wang X, Schwab JH, et al. Defective HLA class I antigen processing machinery in cancer. Cancer Immunol Immunother. 2018;67(6):999–1009.
Tang X, Zhao S, Zhang Y, Wang Y, Zhang Z, Yang M, et al. B7–H3 as a novel CAR-T therapeutic target for glioblastoma. Mol Ther Oncolytics. 2019;14:279–87.
Nehama D, Di Ianni N, Musio S, Du H, Patané M, Pollo B, et al. B7-H3-redirected chimeric antigen receptor T cells target glioblastoma and neurospheres. EBioMedicine. 2019;47:33–43.
Zhang Z, Jiang C, Liu Z, Yang M, Tang X, Wang Y, et al. B7-H3-targeted CAR-T cells exhibit potent antitumor effects on hematologic and solid tumors. Mol Ther Oncolytics. 2020;17:180–9.
Zheng M, Yu L, Hu J, Zhang Z, Wang H, Lu D, et al. Efficacy of B7-H3-redirected BiTE and CAR-T immunotherapies against extranodal nasal natural killer/T cell lymphoma. Transl Oncol. 2020;13(5):100770.
Tang X, Liu F, Liu Z, Cao Y, Zhang Z, Wang Y, et al. Bioactivity and safety of B7-H3-targeted chimeric antigen receptor T cells against anaplastic meningioma. Clin Transl Immunol. 2020;9(6): e1137.
Pierce S, Geanes ES, Bradley T. Targeting natural killer cells for improved immunity and control of the adaptive immune response. Front Cell Infect Microbiol. 2020;10:231.
Burger MC, Zhang C, Harter PN, Romanski A, Strassheimer F, Senft C, et al. CAR-engineered NK cells for the treatment of glioblastoma: turning innate effectors into precision tools for cancer immunotherapy. Front Immunol. 2019;10:2683.
Chou CK, Turtle CJ. Insight into mechanisms associated with cytokine release syndrome and neurotoxicity after CD19 CAR-T cell immunotherapy. Bone Marrow Transplant. 2019;54(Suppl 2):780–4.
Liu E, Marin D, Banerjee P, Macapinlac HA, Thompson P, Basar R, et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N Engl J Med. 2020;382(6):545–53.
Leaman Alcibar O, Candini D, López-Campos F, Albert Antequera M, Morillo Macías V, Conde AJ, et al. Time for radioimmunotherapy: an overview to bring improvements in clinical practice. Clin Transl Oncol. 2019;21(8):992–1004.
Modak S, Zanzonico P, Grkovski M, Slotkin EK, Carrasquillo JA, Lyashchenko SK, et al. B7H3-directed intraperitoneal radioimmunotherapy with radioiodinated omburtamab for desmoplastic small round cell tumor and other peritoneal tumors: results of a phase I study. J Clin Oncol. 2020;38(36):4283–91.
Adams JL, Smothers J, Srinivasan R, Hoos A. Big opportunities for small molecules in immuno-oncology. Nat Rev Drug Discov. 2015;14(9):603–22.
Berg WA, Bandos AI, Mendelson EB, Lehrer D, Jong RA, Pisano ED. Ultrasound as the primary screening test for breast cancer: analysis from ACRIN 6666. J Natl Cancer Inst. 2016;108(4):djv367.
Plevritis SK, Munoz D, Kurian AW, Stout NK, Alagoz O, Near AM, et al. Association of screening and treatment with breast cancer mortality by molecular subtype in US women, 2000–2012. JAMA. 2018;319(2):154–64.
Elmore JG, Barton MB, Moceri VM, Polk S, Arena PJ, Fletcher SW. Ten-year risk of false positive screening mammograms and clinical breast examinations. N Engl J Med. 1998;338(16):1089–96.
Bam R, Lown PS, Stern LA, Sharma K, Wilson KE, Bean GR, et al. Efficacy of affibody-based ultrasound molecular imaging of vascular B7–H3 for breast cancer detection. Clin Cancer Res. 2020;26(9):2140–50.
Bachawal SV, Jensen KC, Wilson KE, Tian L, Lutz AM, Willmann JK. Breast cancer detection by B7-H3-targeted ultrasound molecular imaging. Cancer Res. 2015;75(12):2501–9.
Wilson KE, Bachawal SV, Abou-Elkacem L, Jensen K, Machtaler S, Tian L, et al. Spectroscopic photoacoustic molecular imaging of breast cancer using a B7-H3-targeted ICG contrast agent. Theranostics. 2017;7(6):1463–76.
Bam R, Laffey M, Nottberg K, Lown PS, Hackel BJ, Wilson KE. Affibody-indocyanine green based contrast agent for photoacoustic and fluorescence molecular imaging of B7–H3 expression in breast cancer. Bioconjug Chem. 2019;30(6):1677–89.
Bachawal S, Bean GR, Krings G, Wilson KE. Evaluation of ductal carcinoma in situ grade via triple-modal molecular imaging of B7–H3 expression. NPJ Breast Cancer. 2020;6:14.
Acknowledgements
Not applicable.
Funding
The study was supported by Major project of Wuxi Science and Technology Bureau (N20201006), Wuxi Double-Hundred Talent Fund Project (BJ2023075), Wuxi Health Commission Precision Medicine Project (J202106), Jiangsu Provincial Maternal and Child Health Research Project (F202034) and Jiangsu Provincial Six Talent Peaks Project (No. YY-124), Jiangnan University Wuxi School of Medicine, Graduate Research and Practice Innovation Project.
Author information
Authors and Affiliations
Contributions
J.Y., L.J.Y. and C.L.Y. conceived the review; J.Y. and Q.Z.W. wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jiang, Y., Liu, J., Chen, L. et al. A promising target for breast cancer: B7-H3. BMC Cancer 24, 182 (2024). https://doi.org/10.1186/s12885-024-11933-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-024-11933-3