Abstract
The incidence of prostate cancer (PCa), the most prevalent malignancy, is currently at the forefront. RNA modification is a subfield of the booming field of epigenetics. To date, more than 170 types of RNA modifications have been described, and N6-methyladenosine (m6A) is the most abundant and well-characterized internal modification of mRNAs involved in various aspects of cancer progression. METTL3, the first identified key methyltransferase, regulates human mRNA and non-coding RNA expression in an m6A-dependent manner. This review elucidates the biological function and role of METTL3 in PCa and discusses the implications of METTL3 as a potential therapeutic target for future research directions and clinical applications.
Similar content being viewed by others
Introduction
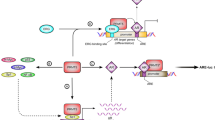
Prostate cancer (PCa) is one of the most prevalent malignant tumors in the world, with the second-highest incidence rate after lung cancer and the eighth-highest mortality rate. It is also a leading cause of cancer-related deaths in men. The incidence rates of PCa have substantial geographical and ethnic differences. Australia/New Zealand, North America, and Europe have a higher incidence rate of 85/100,000, while Asia has the lowest incidence rate of 4.5/100,000–10.5/100,000 [1,2,3,4]. There is no standard treatment protocol for high-risk patients because of the highly aggressive nature of tumors and the complex tumor microenvironment in high-risk patients; androgen deprivation therapy (ADT) is often combined with novel endocrine therapy [5,6,7,8]. Therefore, there is an urgent need to explore the mechanisms of PCa development and therapeutic targets. The rapid development of epigenetics in recent years has provided new avenues to search for the mechanisms of PCa progression, metastasis, and potential therapeutic targets. N6-methyladenosine (m6A), a well-known post-transcriptional modification first identified in 1974, is thought to be the most frequent internal modification in mammalian mRNAs and also occurs in small ncRNAs and lncRNAs in eukaryotic species [9,10,11,12]. This modification is enriched in 3′ UTRs, near-stop codons, long inner exons, intergenic regions, introns, and 5′ UTRs [13, 14]. m6A methyltransferases mainly comprise METTL3, METTL5, METTL14, METTL16, RBM15, WTAP, VIRMA, and ZCCHC “writer” proteins. METTL3 is the only subunit that binds to the methyl donor S-adenosylmethionine (SAM) and catalyzes the methyltransferase domain responsible for converting adenosine to m6A (See Fig. 1) [15,16,17,18]. Furthermore, there are “erasers” proteins with demethylation capabilities, including FTO and ALKBH5 [19, 20], and unique protein binding recognition elements “readers,” including YTHDC1/2 and YTHDF1/2/3 [21, 22].
Studies have demonstrated that METTL3 expression is upregulated in various tumors, including breast, lung, liver, stomach, colorectal, and pancreatic cancers [23,24,25,26,27,28].In PCa, METTL3 upregulation appears to play an important role. METTL3 expression is upregulated in PCa cell lines, and METTL3 knockdown induces apoptosis in cancer cells [29].METTL3 upregulation is also associated with poor prognosis in PCa patients, and METTL3 expression is upregulated in PCa tissues, particularly bone metastases [30, 31].These studies suggest that exploring the specific mechanisms of METTL3 in PCa genesis and metastasis through m6A modification helps us gain a deeper understanding and that identifying and targeting these essential genes involved in PCa metastasis play a key role in the future treatment of metastatic PCa. The influence of current research findings on the clinical translation of PCa and whether they can contribute to the clinical treatment of PCa are discussed in the current study.
m6A modifications
To date, more than 100 types of RNA chemical modifications have been identified for modifying coding and non-coding RNAs [32].As research on these modifications is emerging, they have significantly affected human diseases [33,34,35]. m6A is the most abundant and well-characterized internal modification of mRNAs, which regulates self-renewal in embryonic stem and cancer cells and facilitates cell survival after heat shock or DNA damage [36,37,38]. In addition to their role in mRNAs, m6A modifications are also present in non-coding RNAs, such as miRNAs, lncRNAs, and circRNAs, which regulate their biological functions [39,40,41,42,43,44]. RNA modifications precisely regulate the biological functions of numerous molecules, diversifying genetic information. A protein group has been identified, thereby influencing the outcome of RNA [45].We referred to these proteins that specifically deposit, remove, and recognize RNA as “writer,” “eraser,” and “reader” proteins, respectively (See Table 1 and Fig. 2).
m6A RNA methylation and m6A modification mechanism. METTL3, METTL14, and WTAP form the core component of the methyltransferase complex and catalyze the methylation of N6 adenosine with other regulatory cofactors VIRMA, RBM15, ZC3H13, and METTL16. However, m6A deposition is reversible and depends on the demethylases FTO and ALKBH5. m6A can also be recognized by m6A binding proteins. YTHDC1 can alternative splicing and RNA export; YTHDF1/2/3 regulates RNA translation and degradation. IGFBP1/2/3 promotes RNA stability. hnRNPG/C and hnRNPA2B1 can regulate mRNA splicing
m6A writers
During transcription, m6A is deposited in the nascent pre-mRNAs by the methyltransferase complex in the nucleus. These complexes are composed of METTL3, METTL14, METTL16, RBM15, WTAP, VIRMA, and ZC3H13 [46,47,138,139,140]. Chen et al. [141] reported that METTL3 could directly affect the expression of ARHGDIA, a key migration-related protein that regulates the growth, migration, and polarity of tumor cells [142]. Ectopic expression of ARHGDIA effectively attenuated the effect of METTL3 knockdown on the invasive ability of PCa cells, and that METTL3 promoted PCa metastasis by upregulating ARHGDIA expression through m6A modifications.
Remarkably, these studies focused on METTL3 regulation of downstream genes to promote PCa progression. However, whether METTL3 directly affects molecular studies related to proliferation and migration has received less attention and warrants further investigation. Taken together, these findings suggest that METTL3 plays an essential role as an oncogene in PCa progression and metastasis.
METTL3 promotes PCa bone metastasis
Bone metastases from PCa are the main cause of death in PCa patients, and the overall 5-year survival rate for patients with bone metastases is approximately 30%, with many serious complications, such as bone pain, spinal cord compression, and pathological fractures, affecting the quality of life and survival rate of patients [143, 144]. However, the mechanism underlying PCa bone-specific metastasis remains unclear, and the role of m6A modification in bone metastasis may provide new insights. Lang et al. identified a novel molecular mechanism of bone metastasis in which METTL3-mediated m6A modification promotes PCAT6 upregulation in an IGF2BP2-dependent manner. Furthermore, PCAT6 enhances IGF1R mRNA stability via the PCAT6/IGF2BP2/IGF1R RNA-protein trimer, thereby upregulating IGF1R expression and promoting PCa bone metastasis and tumor growth [93]. METTL3 increased the mRNA level of the adhesion molecule ITGB1 and adhesion to bone marrow stromal type I collagen in PCa cells through m6A modification, thereby increasing the possibility of bone metastasis in PCa [31].
Role of METTL3 in PCa glucose metabolism
As a hallmark of cancer, metabolic reprogramming maximizes energy consumption and production, contributing to tumor growth, invasion, and metastasis [145, 146]. Glycolysis is the preferred pathway for cancer cells to obtain energy, but glycolysis is not a hallmark of primary PCa and only plays a key role in advanced tumors [146,147,148]. METTL3 enhances the stability of SNHG7 and recruits SRSF1 to regulate c-Myc expression by regulating m6A modification of SNHG7, further promoting glycolysis in PCa cells [149]. Current METTL3 research on glucose metabolism in cancer mainly focuses on gastrointestinal tumors, including gastric, liver, and colorectal cancers [103, 150, 151]. Further research is required to determine whether METTL3 acts as a regulator of PCa to target other related molecules that affect glucose metabolism.
Role of METTL3 in PCa drug-resistance
Clinical resistance to PCa is mainly noted in metastatic depot-resistant PCa (mCRPC), and resistance to late treatment with enzalutamide and abiraterone, second-generation androgen receptor (AR) inhibitors, cannot be avoided [152, 153].Low levels of METTL3 are associated with dysregulation of AR signaling and render PCa cells resistant to AR inhibitors in an AR-independent manner via upregulation of NR5A2 [154].This finding suggests that METTL3-mediated m6A modification may regulate the therapeutic sensitivity of AR inhibitors and that patients with varying METTL3 expression levels may respond differently to AR inhibitors, which warrants further investigation.
Targeting of METTL3 for potential clinical application
METTL3 plays a crucial role in cancer progression, and METTL3 inhibition has attracted the attention of pharmaceutical companies. The research and development of m6A-modified inhibitors as therapeutic targets is receiving increasing attention [155].Based on the multiple roles of METTL3, targeting METTL3 may offer new hope for individualized tumor treatment.
Nucleoside METTL3 inhibitors
Most studies on METTL3 inhibitors are still in the early stages, are limited in number, and are mainly divided into two types: nucleoside and non-nucleoside analogs. Bedi et al. [156] identified an N-substituted amide adenosine analog of ribonucleic acid as a potent METTL3 inhibitor after screening 4000 adenosine partial analogs and derivatives of SAM by in silico high-throughput docking. This binding mode was validated by protein crystallography and demonstrated a good ligand efficiency. However, its anticancer effects have not yet been tested.
Non-nucleoside METTL3 inhibitors
As adenosine analogs are less cell-permeable and less binding, non-nucleoside-selective METTL3 inhibitors can compensate for these disadvantages. UZH1a is a high-nanomolar inhibitor discovered through protein structure-based optimization and potency assessment of compounds in HTRF. UZH1a inhibits the activity of METTL3 by occupying its SAM-binding site [157], resulting in a dependent decrease in mRNA m6A methylation levels in leukemic MOLM-13 cells, osteosarcoma U2OS cells, and human embryonic kidney immortalized cells [157].Dolbois et al. optimized the UZH1a analog to obtain a more potent METTL3 inhibitor—UZH2—with a decrease of m6A in UZH2 polyadenylated RNA of 0.7 and 2.5 mM in MOLM-13 and PC-3 cell lines, respectively. For other m6A “writer” proteins, RNA methyltransferases were selectively probed, and no off-target was found [158].
STM2457 is a non-nucleoside METTL3 inhibitor developed for treating hematologic malignancies. Yankova et al. identified STM1760 as a non-SAM-related analog through high-throughput screening of 250,000 drug compounds, including pharmacodynamic optimization and ex vivo studies to obtain STM2457. STM2457 has been revealed to block proliferation and colony formation in MOLM-13 cell lines, promote apoptosis, and not affect normal hematopoietic function. Regarding In vivo studies, STM2457 inhibited the proliferation of acute myeloid leukemia (AML) in patient-derived xenograft and leukemia mouse models [159].
Oral small molecule inhibitors
Rosenfeld et al. invented STC-15, a novel oral small-molecule inhibitor of METTL3. In preclinical cancer models, STC-15 treatment results in the activation of innate immune pathways, inhibition of tumor growth, and enhancement of the anti-tumor properties of anti-PD-1 therapy to produce a durable anti-tumor immune response [160].
Diagnostic and prognostic biomarkers
METTL3 plays a key role in many biological processes, particularly tumorigenesis and development. In most cases, METTL3 functions as an oncogene in cancers. This causes alterations in mRNA translation, leading to tumor progression. METTL3 expression is higher in many tumor tissues than in normal tissues. Therefore, it is a potential clinical diagnostic and prognostic biomarker of cancer. In most cancers, high METTL3 expression predicts a poor prognosis. Examples include osteosarcoma [161], glioblastoma [162, 163], gastric cancer (GC) [26, 103, 162, 164, 165], colorectal cancer (CRC) [166], ovarian cancer [176]. METTL3 is involved in all aspects of tumor progression, including cancer cell proliferation, migration, invasion, apoptosis, metastasis, angiogenesis, drug resistance, glycolipid metabolism, and tumor stem cell maintenance [177].Recent METTL3 studies in PCa have addressed biological functions, including proliferation, migration, invasion, metastasis, drug resistance, and glucose metabolism (See Table 2), whereas tumor angiogenesis, lipid metabolism, and tumor stem cell maintenance have hardly been investigated (See Fig. 6). Other cancer studies in this area have reported that METTL3 mediates the upregulation of the mRNA levels of tumor angiogenesis-related cytokines and angiogenic factors. The stability of ncRNAs leads to tumor angiogenesis in an m6A-dependent manner [103, 178,179,180]. METTL3-mediated m6A methylation renders the mRNA of lipid metabolism-related genes unstable and affects downstream lipid accumulation [181].This instability may lead to dysregulation of lipid metabolism and facilitate tumor cell growth and immune escape. m6A mRNA modification is essential for cancer stem cell self-renewal and tumor metastasis, enhancing the frequency of tumor stem cell self-renewal, and cancer cell genesis and initiation by promoting the expression of SOX2 mRNA, a cancer stem cell marker [182,183,184]. In summary, whether METTL3 is involved in tumor angiogenesis, lipid metabolism, and tumor stem cells in PCa is a worthy target for investigation, which will help us better understand the specific mechanisms of PCa genesis and metastasis.METTL3 plays a crucial role in the progression of PCa, suggesting that it may be a promising molecular biomarker for clinical diagnosis and prognostic relevance. Ji et al. reported that the overexpression of m6A methylation regulators resulted in a worse survival benefit for patients with high levels of mRNA methylation by affecting the subcellular localization of proteins in PCa [185].METTL3 is also associated with higher tumor stage and poorer prognosis in PCa [29],although more studies are required to demonstrate its feasibility.
The role played by METTL3 in prostate cancer cells. METTL3 is involved in prostate cancer development and progression by mediating RNA stabilization and translation and regulating the expression of oncogenes and oncogenes at the post-transcriptional level. METTL3 is involved in cell proliferation, migration, invasion, angiogenesis, tumor radiotherapy, bone metastasis, glycolipid metabolism, and tumor stem cell maintenance. However, further investigation needs to investigate whether METTL3 plays these functional roles in angiogenesis, chemoresistance, lipid metabolism, and tumor stem cell maintenance in prostate cancer cells
The androgen receptor (AR) plays a crucial role in PCa pathogenesis, and METTL3 may play a functional role. Roy et al. [186] found that METTL3 expression was higher in AR-expressing PCa cell lines than in AR-negative PCa cell lines, and similar findings were observed at the protein level. This finding suggests a potential interaction between METTL3, which is elevated at the onset of PCa, and androgen signaling. Notably, the expression of the AR target gene NKX3.1 was increased after METTL3 knockdown, whereas the expression of prostate-specific antigen (PSA) decreased, suggesting a direct role of METTL3 in AR expression [186]. METTL3 knockdown also leads to the elevation of key regulatory factors, such as KDM1A, which is involved in PCa initiation and progression and regulates AR expression and function [187,188,189]. Further studies on the effect of METTL3 deletion on overall androgen signaling are needed. Because of the role of m6A methylation in the splicing process [190, 191], future research must investigate whether METTL3 functions in the progression of PCa to CRPC because of the AR splicing process.
METTL3 has an oncogenic function in most cancers but has also been shown to be a tumor suppressor in some cases [192]. For example, lower METTL3 expression was detected in renal cell carcinoma (RCC) tissues, suggesting that higher METTL3 expression may predict a better prognosis for RCC patients, possibly due to the inhibition of tumor growth by promoting cell cycle arrest in the G1 phase [193]. It has also been shown that the self-renewal of glioblastoma stem cells (GSC) is regulated by m6A mRNA modification, and METTL3 downregulation significantly promotes tumor progression [182]. Similar results were found in melanoma studies, where Jia et al. found that METTL3 downregulation led to reduced m6A levels in melanoma, predicting early recurrence and enhanced aggressiveness, and verified that METTL3-mediated m6A modification promoted the translation of the tumor suppressor gene HINT2 [194]. The opposing roles of METTL3 in different cancers may be related to tumor heterogeneity and METTL3 complex physiological functions, and METTL3 produces inconsistent effects in the same type of cancer. For example, METTL3 elevation promotes the progression of non-small cell lung cancer (NSCLC), but METTL3 also inhibits tumorigenesis in NSCLC [195, 196]. In HCC, METTL3 and METTL14 have opposing effects on the migration of hepatocellular carcinoma cells [24, 42]. These results suggest that some functions of METTL3 are independent of m6A modifications, and the potential mechanisms need further exploration.
Conclusions and prospects
In recent years, RNA m6A modification has emerged as a prominent field in cancer research. Dysregulation of is frequently observed across various types of cancer, exerting significant influence on cancer progression by modulating the expression of oncogenes and tumor suppressor genes. Aberrant m6A modification is closely associated with tumor progression and the prognosis of cancer patients, highlighting the potential for targeting m6A regulators as a promising approach for cancer therapy. However, despite the identification of numerous m6A modification modulators, only a limited number have demonstrated efficacy and actionable targets for cancer treatment. None of the reported inhibitors or activators targeting m6A modification have been approved for clinical use in treating cancer. Thorough investigation through pre trials is necessary before these targeted therapies can be approved for clinical application.
Currently, the m6A content in RNA can be detected by various methods, including two-dimensional thin-layer chromatography [197, 198], m6A dot-blot [19], and high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) [19, 20]. However, these methods are unsuitable for extensively characterizing modification sites [199]. Before the development of methylated RNA immunoprecipitation followed by high-throughput sequencing (MeRIP-seq), m6A distribution throughout the transcriptome was unknown, and this method attracted much attention for its accuracy and reproducibility [13, 200]. However, because MeRIP-seq relies on RNA fragments with a resolution of approximately 100–200 nt, it is not possible to detect methylation sites with single-nucleotide resolution [201]. Other methods such as photo-crosslinking-assisted m6A-sequencing (PA-m6A-Seq), site-specific cleavage, and radioactive-labelling followed by ligation-assisted extraction and thin-layer chromatography (SCARLET) have been used, but they are time-consuming and unsuitable for high-throughput applications [202, 203]. However, a new method called m6A individual nucleotide resolution crosslinking immunoprecipitation (miCLIP), which accurately detects m6A modification sites, is an important step in this field [204]. In addition, CRISPR-based genetically engineered groups can directly detect the effects of altering m6A modification sites in many organisms [205]. As a complementary method, it is valuable to study the function of m6A methylation. Although many methods for detecting m6A methylation have been developed, several challenges and difficulties remain.
As a malignant tumor with the highest incidence in men worldwide, late metastasis is fatal and incurable for patients, and the relationship between RNA modification and PCa may lead to novel strategies for treating PCa. Current research on METTL3 in PCa mainly involves its biological functions and mechanisms, but some functions have not been further investigated. Future research on METTL3 in PCa should mainly focus on tumor angiogenesis, glycolipid metabolism, maintenance of tumor stem cells, and its effect on the tumor microenvironment. In clinical applications, although studies have incorporated METTL3 into tumor biomarkers and inhibitor development with great potential, they remain in the early stages and require continued attention.
Availability of data and materials
Not applicable.
References
Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Dy GW, Gore JL, Forouzanfar MH, Naghavi M, Fitzmaurice C. Global burden of urologic cancers, 1990-2013. Eur Urol. 2017;71(3):437–46.
Wong MC, Goggins WB, Wang HH, et al. Global incidence and mortality for prostate cancer: analysis of temporal patterns and trends in 36 countries. Eur Urol. 2016;70(5):862–74.
Gillessen S, Armstrong A, Attard G, et al. Management of patients with advanced prostate cancer: report from the advanced prostate cancer consensus conference 2021. Eur Urol. 2022;82(1):115–41.
Teo MY, Rathkopf D, Kantoff P. Treatment of advanced prostate cancer. Annu Rev Med. 2019;70:479–99.
Gamat M, DG MN. Androgen deprivation and immunotherapy for the treatment of prostate cancer. Endocr Relat Cancer. 2017;24(12):t297–310.
Masud N. Symphony in the crowd: key genetic alterations in prostate cancer. Cancer Innov. 2023; https://doi.org/10.1002/cai2.52.
Desrosiers R, Friderici K, Rottman F. Identification of methylated nucleosides in messenger rna from novikoff hepatoma cells. Proc Natl Acad Sci U S A. 1974;71(10):3971–5.
Yue Y, Liu J, He C. Rna n6-methyladenosine methylation in post-transcriptional gene expression regulation. Genes Dev. 2015;29(13):1343–55.
Ding Z, Liu Y, Huang Q, Cheng C, Song L, Zhang C, et al. M6a-and immune-related lncrna signature confers robust predictive power for immune efficacy in lung squamous cell carcinoma. View. 2023;4(3):20220083.
Yin X, Lin H, Lin L, Miao L, He J, Zhuo Z. Lncrnas and circrnas in cancer. Medcomm (2020). 2022;3:e141. https://doi.org/10.1002/mco2.141.
Dominissini D, Moshitch-Moshkovitz S, Schwartz S, Salmon-Divon M, Ungar L, Osenberg S, et al. Topology of the human and mouse m6a rna methylomes revealed by m6a-seq. Nature. 2012;485:201–6. https://doi.org/10.1038/nature11112.
Geula S, Moshitch-Moshkovitz S, Dominissini D, et al. Stem cells. M6a mrna methylation facilitates resolution of naïve pluripotency toward differentiation. Science. 2015;347(6225):1002–6.
Horiuchi K, Kawamura T, Iwanari H, et al. Identification of wilms' tumor 1-associating protein complex and its role in alternative splicing and the cell cycle. J Biol Chem. 2013;288(46):33292–302.
Schwartz S, Mumbach M, Jovanovic M, et al. Perturbation of m6a writers reveals two distinct classes of mrna methylation at internal and 5’ sites. Cell Rep. 2014;8(1):284–96.
Chen XY, Zhang J, Zhu JS. The role of m(6)a rna methylation in human cancer. Mol Cancer. 2019;18(1):103.
Huang H, Weng H, Chen J. M(6)a modification in coding and non-coding rnas: roles and therapeutic implications in cancer. Cancer Cell. 2020;37(3):270–88.
Jia G, Fu Y, Zhao X, Dai Q, Zheng G, Yang Y, et al. N6-methyladenosine in nuclear rna is a major substrate of the obesity-associated fto. Nat Chem Biol. 2011;7:885–7. https://doi.org/10.1038/nchembio.687.
Zheng G, Dahl JA, Niu Y, Fedorcsak P, Huang CM, Li CJ, et al. Alkbh5 is a mammalian rna demethylase that impacts rna metabolism and mouse fertility. Mol Cell. 2013;49:18–29. https://doi.org/10.1016/j.molcel.2012.10.015.
Xu C, Wang X, Liu K, et al. Structural basis for selective binding of m6a rna by the ythdc1 yth domain. Nat Chem Biol. 2014;10(11):927–9.
Li F, Zhao D, Wu J, Shi Y. Structure of the yth domain of human ythdf2 in complex with an m(6)a mononucleotide reveals an aromatic cage for m(6)a recognition. Cell Res. 2014;24(12):1490–2.
Cai X, Wang X, Cao C, Gao Y, Zhang S, Yang Z, et al. Hbxip-elevated methyltransferase mettl3 promotes the progression of breast cancer via inhibiting tumor suppressor let-7g. Cancer Lett. 2018;415:11–9. https://doi.org/10.1016/j.canlet.2017.11.018.
Chen M, Wei L, Law CT, Tsang FH, Shen J, Cheng CL, et al. Rna n6-methyladenosine methyltransferase-like 3 promotes liver cancer progression through ythdf2-dependent posttranscriptional silencing of socs2. Hepatology. 2018;67:2254–70. https://doi.org/10.1002/hep.29683.
Li T, Hu PS, Zuo Z, et al. Mettl3 facilitates tumor progression via an m(6)a-igf2bp2-dependent mechanism in colorectal carcinoma. Mol Cancer. 2019;18(1):112.
He H, Wu W, Sun Z, Chai L. Mir-4429 prevented gastric cancer progression through targeting mettl3 to inhibit m(6)a-caused stabilization of sec62. Biochem Biophys Res Commun. 2019;517:581–7. https://doi.org/10.1016/j.bbrc.2019.07.058.
Zhang J, Bai R, Li M, Ye H, Wu C, Wang C, et al. Excessive mir-25-3p maturation via n(6)-methyladenosine stimulated by cigarette smoke promotes pancreatic cancer progression. Nat Commun. 2019;10:1858. https://doi.org/10.1038/s41467-019-09712-x.
Wei W, Huo B, Shi X. Mir-600 inhibits lung cancer via downregulating the expression of mettl3. Cancer Manag Res. 2019;11:1177–87. https://doi.org/10.2147/cmar.s181058.
Cai J, Yang F, Zhan H, Situ J, Li W, Mao Y, et al. Rna m(6)a methyltransferase mettl3 promotes the growth of prostate cancer by regulating hedgehog pathway. Onco Targets Ther. 2019;12:9143–52. https://doi.org/10.2147/ott.s226796.
Yuan Y, Du Y, Wang L, Liu X. The m6a methyltransferase mettl3 promotes the development and progression of prostate carcinoma via mediating myc methylation. J Cancer. 2020;11:3588–95. https://doi.org/10.7150/jca.42338.
Li E, Wei B, Wang X, Kang R. Mettl3 enhances cell adhesion through stabilizing integrin β1 mrna via an m6a-hur-dependent mechanism in prostatic carcinoma. Am J Cancer Res. 2020;10:1012–25.
Boccaletto P, Stefaniak F, Ray A, et al. Modomics: a database of rna modification pathways. 2021 update. Nucleic Acids Res. 2022;50(d1):d231–5.
Barbieri I, Kouzarides T. Role of rna modifications in cancer. Nat Rev Cancer. 2020;20(6):303–22.
Jonkhout N, Tran J, Smith MA, Schonrock N, Mattick JS, Novoa EM. The rna modification landscape in human disease. RNA. 2017;23(12):1754–69.
Fang Z, Mei W, Qu C, et al. Role of m6a writers, erasers and readers in cancer. Exp Hematol Oncol. 2022;11(1):45.
Davalos V, Blanco S, Esteller M. Snapshot: messenger rna modifications. Cell. 2018;174(2):498–498.e1.
Frye M, Harada BT, Behm M, He C. Rna modifications modulate gene expression during development. Science. 2018;361(6409):1346–9.
**ang Y, Laurent B, Hsu CH, et al. Rna m(6)a methylation regulates the ultraviolet-induced dna damage response. Nature. 2017;543(7646):573–6.
Alarcón CR, Goodarzi H, Lee H, Liu X, Tavazoie S, Tavazoie SF. Hnrnpa2b1 is a mediator of m(6)a-dependent nuclear rna processing events. Cell. 2015;162(6):1299–308.
Alarcón CR, Lee H, Goodarzi H, Halberg N, Tavazoie SF. N6-methyladenosine marks primary micrornas for processing. Nature. 2015;519(7544):482–5.
Yang Y, Fan X, Mao M, et al. Extensive translation of circular rnas driven by n(6)-methyladenosine. Cell Res. 2017;27(5):626–41.
Ma JZ, Yang F, Zhou CC, Liu F, Yuan JH, Wang F, et al. Mettl14 suppresses the metastatic potential of hepatocellular carcinoma by modulating n(6) -methyladenosine-dependent primary microrna processing. Hepatology. 2017;65:529–43. https://doi.org/10.1002/hep.28885.
Liu N, Dai Q, Zheng G, He C, Parisien M, Pan T. N(6)-methyladenosine-dependent rna structural switches regulate rna-protein interactions. Nature. 2015;518(7540):560–4.
As W, Kretschmer J, Hackert P, et al. Human mettl16 is a n(6)-methyladenosine (m(6)a) methyltransferase that targets pre-mrnas and various non-coding rnas. EMBO Rep. 2017;18(11):2004–14.
Deng LJ, Deng WQ, Fan SR, et al. M6a modification: recent advances, anticancer targeted drug discovery and beyond. Mol Cancer. 2022;21(1):52.
Niu Y, Zhao X, Wu YS, Li MM, Wang XJ, Yang YG. N6-methyl-adenosine (m6a) in rna: an old modification with a novel epigenetic function. Genom Proteom Bioinform. 2013;11(1):8–17.
Zhu W, Wang JZ, Wei JF, Lu C. Role of m6a methyltransferase component virma in multiple human cancers (review). Cancer Cell Int. 2021;21(1):172.
Chen Z, Zhong X, **a M, Zhong J. The roles and mechanisms of the m6a reader protein ythdf1 in tumor biology and human diseases. Mol Ther Nucleic Acids. 2021;26:1270–9.
Wang P, Doxtader KA, Nam Y. Structural basis for cooperative function of mettl3 and mettl14 methyltransferases. Mol Cell. 2016;63(2):306–17.
** XL, Sun BF, Wang L, et al. Mammalian wtap is a regulatory subunit of the rna n6-methyladenosine methyltransferase. Cell Res. 2014;24(2):177–89.
Patil DP, Chen CK, Pickering BF, et al. M(6)a rna methylation promotes xist-mediated transcriptional repression. Nature. 2016;537(7620):369–73.
Knuckles P, Lence T, Haussmann IU, et al. Zc3h13/flacc is required for adenosine methylation by bridging the mrna-binding factor rbm15/spenito to the m(6)a machinery component wtap/fl(2)d. Genes Dev. 2018;32(5–6):415–29.
Wen J, Lv R, Ma H, et al. Zc3h13 regulates nuclear rna m(6)a methylation and mouse embryonic stem cell self-renewal. Mol Cell. 2018;69(6):1028–1038.e6.
Wei J, Liu F, Lu Z, et al. Differential m(6)a, m(6)a(m), and m(1)a demethylation mediated by fto in the cell nucleus and cytoplasm. Mol Cell. 2018;71(6):973–985.e5.
Liu J, Jia G. Methylation modifications in eukaryotic messenger rna. J Genet Genomics. 2014;41(1):21–33.
Zou S, Toh JD, Wong KH, Gao YG, Hong W, Woon EC. N(6)-methyladenosine: a conformational marker that regulates the substrate specificity of human demethylases fto and alkbh5. Sci Rep. 2016;6:25677.
Ueda Y, Ooshio I, Fusamae Y, et al. Alkb homolog 3-mediated trna demethylation promotes protein synthesis in cancer cells. Sci Rep. 2017;7:42271.
Cd A, Jenuwein T. The molecular hallmarks of epigenetic control. Nat Rev Genet. 2016;17(8):487–500.
Huang H, Weng H, Sun W, et al. Recognition of rna n(6)-methyladenosine by igf2bp proteins enhances mrna stability and translation. Nat Cell Biol. 2018;20(3):285–95.
Liu N, Zhou KI, Parisien M, Dai Q, Diatchenko L, Pan T. N6-methyladenosine alters rna structure to regulate binding of a low-complexity protein. Nucleic Acids Res. 2017;45(10):6051–63.
Petri BJ, Klinge CM. M6a readers, writers, erasers, and the m6a epitranscriptome in breast cancer. J Mol Endocrinol. 2023;70(2)
Sun T, Wu R, Ming L. The role of m6a rna methylation in cancer. Biomed Pharmacother. 2019;112:108613.
Chen M, Wong CM. The emerging roles of n6-methyladenosine (m6a) deregulation in liver carcinogenesis. Mol Cancer. 2020;19(1):44.
Zhao Y, Hu J, Sun X, Yang K, Yang L, Kong L, et al. Loss of m6a demethylase alkbh5 promotes post-ischemic angiogenesis via post-transcriptional stabilization of wnt5a. Clin Transl Med. 2021;11:e402. https://doi.org/10.1002/ctm2.402.
Li Y, Su R, Deng X, Chen Y, Chen J. Fto in cancer: functions, molecular mechanisms, and therapeutic implications. Trends Cancer. 2022;8(7):598–614.
Wang J, Wang J, Gu Q, et al. The biological function of m6a demethylase alkbh5 and its role in human disease. Cancer Cell Int. 2020;20:347.
Zaccara S, Ries Rj J Sr. Reading, writing and erasing mrna methylation. Nat Rev Mol Cell Biol. 2019;20(10):608–24.
Shi H, Wei J, He C. Where, when, and how: context-dependent functions of rna methylation writers, readers, and erasers. Mol Cell. 2019;74(4):640–50.
Wu R, Li A, Sun B, et al. A novel m(6)a reader prrc2a controls oligodendroglial specification and myelination. Cell Res. 2019;29(1):23–41.
Zhang F, Kang Y, Wang M, et al. Fragile x mental retardation protein modulates the stability of its m6a-marked messenger rna targets. Hum Mol Genet. 2018;27(22):3936–50.
Wang J, Chen L, Qiang P. The role of igf2bp2, an m6a reader gene, in human metabolic diseases and cancers. Cancer Cell Int. 2021;21(1):99.
Wang S, Lv W, Li T, et al. Dynamic regulation and functions of mrna m6a modification. Cancer Cell Int. 2022;22(1):48.
Liao S, Sun H, Xu C. Yth domain: a family of n(6)-methyladenosine (m(6)a) readers. Genom Proteom Bioinform. 2018;16(2):99–107.
Patil DP, Pickering BF, Jaffrey SR. Reading m(6)a in the transcriptome: m(6)a-binding proteins. Trends Cell Biol. 2018;28(2):113–27.
Yang Y, Hsu PJ, Ys C, Yang YG. Dynamic transcriptomic m(6)a decoration: writers, erasers, readers and functions in rna metabolism. Cell Res. 2018;28(6):616–24.
Peer E, Moshitch-Moshkovitz S, Rechavi G, Dominissini D. The epitranscriptome in translation regulation. Cold Spring Harb Perspect Biol. 2019;11(8)
Livneh I, Moshitch-Moshkovitz S, Amariglio N, Rechavi G, Dominissini D. The m(6)a epitranscriptome: transcriptome plasticity in brain development and function. Nat Rev Neurosci. 2020;21(1):36–51.
Zhao Y, Shi Y, Shen H, **e W. M(6)a-binding proteins: the emerging crucial performers in epigenetics. J Hematol Oncol. 2020;13(1):35.
Liu Z, Zhong J, Zeng J, Duan X, Lu J, Sun X, et al. Characterization of the m6a-associated tumor immune microenvironment in prostate cancer to aid immunotherapy. Front Immunol. 2021;12:735170. https://doi.org/10.3389/fimmu.2021.735170.
Wang Y, Chen J, Gao WQ, Yang R. Mettl14 promotes prostate tumorigenesis by inhibiting thbs1 via an m6a-ythdf2-dependent mechanism. Cell Death Discov. 2022;8:143. https://doi.org/10.1038/s41420-022-00939-0.
Wu A, Cremaschi P, Wetterskog D, Conteduca V, Franceschini GM, Kleftogiannis D, et al. Genome-wide plasma dna methylation features of metastatic prostate cancer. J Clin Invest. 2020;130:1991–2000. https://doi.org/10.1172/jci130887.
Zhu K, Li Y, Xu Y. The fto m(6)a demethylase inhibits the invasion and migration of prostate cancer cells by regulating total m(6)a levels. Life Sci. 2021;271:119180. https://doi.org/10.1016/j.lfs.2021.119180.
Li S, Cao L. Demethyltransferase fto alpha-ketoglutarate dependent dioxygenase (fto) regulates the proliferation, migration, invasion and tumor growth of prostate cancer by modulating the expression of melanocortin 4 receptor (mc4r). Bioengineered. 2022;13:5598–612. https://doi.org/10.1080/21655979.2021.2001936.
Li X, Liu B, Wang S, Li J, Ge X. Mir-141-3p promotes malignant progression in prostate cancer through alkb homolog 5-mediated m(6)a modification of protein arginine methyltransferase 6. Chin J Physiol. 2023;66:43–51. https://doi.org/10.4103/cjop.cjop-d-22-00071.
Luxton HJ, Simpson BS, Mills IG, Nr B, Ahmed Z, Stavrinides V, et al. The oncogene metadherin interacts with the known splicing proteins ythdc1, sam68 and t-star and plays a novel role in alternative mrna splicing. Cancers (basel). 2019;11 https://doi.org/10.3390/cancers11091233.
Song J, You G, Yin X, Zhu G, Wang W, Yu Y, et al. Overexpression of ythdc2 contributes to the progression of prostate cancer and predicts poor outcomes in patients with prostate cancer. J Biochem Mol Toxicol. 2023:e23308. https://doi.org/10.1002/jbt.23308.
Li P, Shi Y, Gao D, Xu H, Zou Y, Wang Z, et al. Elk1-mediated ythdf1 drives prostate cancer progression by facilitating the translation of polo-like kinase 1 in an m6a dependent manner. Int J Biol Sci. 2022;18:6145–62. https://doi.org/10.7150/ijbs.75063.
Li W, Chen G, Feng Z, Zhu B, Zhou L, Zhang Y, et al. Ythdf1 promotes the proliferation, migration, and invasion of prostate cancer cells by regulating trim44. Genes Genom. 2021;43:1413–21. https://doi.org/10.1007/s13258-021-01175-z.
Li J, **e H, Ying Y, Chen H, Yan H, He L, et al. Ythdf2 mediates the mrna degradation of the tumor suppressors to induce akt phosphorylation in n6-methyladenosine-dependent way in prostate cancer. Mol Cancer. 2020;19:152. https://doi.org/10.1186/s12943-020-01267-6.
Du C, Lv C, Feng Y, Yu S. Activation of the kdm5a/mirna-495/ythdf2/m6a-mob3b axis facilitates prostate cancer progression. J Exp Clin Cancer Res. 2020;39:223. https://doi.org/10.1186/s13046-020-01735-3.
Li J, Meng S, Xu M, Wang S, He L, Xu X, et al. Downregulation of n(6)-methyladenosine binding ythdf2 protein mediated by mir-493-3p suppresses prostate cancer by elevating n(6)-methyladenosine levels. Oncotarget. 2018;9:3752–64. https://doi.org/10.18632/oncotarget.23365.
Cheng Y, Li L, Qin Z, Li X, Qi F. Identification of castration-resistant prostate cancer-related hub genes using weighted gene co-expression network analysis. J Cell Mol Med. 2020;24:8006–17. https://doi.org/10.1111/jcmm.15432.
Lang C, Yin C, Lin K, Li Y, Yang Q, Wu Z, et al. M(6) a modification of lncrna pcat6 promotes bone metastasis in prostate cancer through igf2bp2-mediated igf1r mrna stabilization. Clin Transl Med. 2021;11:e426. https://doi.org/10.1002/ctm2.426.
Chromecki TF, Cha EK, Pummer K, Scherr DS, Tewari AK, Sun M, et al. Prognostic value of insulin-like growth factor ii mrna binding protein 3 in patients treated with radical prostatectomy. BJU Int. 2012;110:63–8. https://doi.org/10.1111/j.1464-410x.2011.10703.x.
Yu YZ, Lv DJ, Wang C, Song XL, **e T, Wang T, et al. Hsa_circ_0003258 promotes prostate cancer metastasis by complexing with igf2bp3 and sponging mir-653-5p. Mol Cancer. 2022;21:12. https://doi.org/10.1186/s12943-021-01480-x.
Zhou KI, Pan T. Structures of the m(6)a methyltransferase complex: two subunits with distinct but coordinated roles. Mol Cell. 2016;63(2):183–5.
Oerum S, Meynier V, Catala M, Tisné C. A comprehensive review of m6a/m6am rna methyltransferase structures. Nucleic Acids Res. 2021;49(13):7239–55.
Śledź P, **ek M. Structural insights into the molecular mechanism of the m(6)a writer complex. Elife. 2016;5
Huang J, Dong X, Gong Z, et al. Solution structure of the rna recognition domain of mettl3-mettl14 n(6)-methyladenosine methyltransferase. Protein Cell. 2019;10(4):272–84.
Jones DT. Protein secondary structure prediction based on position-specific scoring matrices. J Mol Biol. 1999;292(2):195–202.
Ward JJ, Mcguffin LJ, Bryson K, Buxton BF, Jones DT. The disopred server for the prediction of protein disorder. Bioinformatics. 2004;20(13):2138–9.
Schöller E, Weichmann F, Treiber T, et al. Interactions, localization, and phosphorylation of the m(6)a generating mettl3-mettl14-wtap complex. RNA. 2018;24(4):499–512.
Wang Q, Chen C, Ding Q, Zhao Y, Wang Z, Chen J, et al. Mettl3-mediated m(6)a modification of hdgf mrna promotes gastric cancer progression and has prognostic significance. Gut. 2020;69:1193–205. https://doi.org/10.1136/gutjnl-2019-319639.
Chen T, Hao YJ, Zhang Y, Li MM, Wang M, Han W, et al. M(6)a rna methylation is regulated by micrornas and promotes reprogramming to pluripotency. Cell Stem Cell. 2015;16:289–301. https://doi.org/10.1016/j.stem.2015.01.016.
Yan J, Huang X, Zhang X, Chen Z, Ye C, **ang W, et al. Lncrna linc00470 promotes the degradation of pten mrna to facilitate malignant behavior in gastric cancer cells. Biochem Biophys Res Commun. 2020;521:887–93. https://doi.org/10.1016/j.bbrc.2019.11.016.
Zhu L, Zhu Y, Han S, Chen M, Song P, Dai D, et al. Impaired autophagic degradation of lncrna arhgap5-as1 promotes chemoresistance in gastric cancer. Cell Death Dis. 2019;10:383. https://doi.org/10.1038/s41419-019-1585-2.
Du Y, Hou G, Zhang H, Dou J, He J, Guo Y, et al. Sumoylation of the m6a-rna methyltransferase mettl3 modulates its function. Nucleic Acids Res. 2018;46:5195–208. https://doi.org/10.1093/nar/gky156.
Garcia-Campos MA, Edelheit S, Toth U, Safra M, Shachar R, Viukov S, et al. Deciphering the "m(6)a code" via antibody-independent quantitative profiling. Cell. 2019;178:731–47.e16. https://doi.org/10.1016/j.cell.2019.06.013.
Huang H, Weng H, Zhou K, Wu T, Zhao BS, Sun M, et al. Histone h3 trimethylation at lysine 36 guides m(6)a rna modification co-transcriptionally. Nature. 2019;567:414–9. https://doi.org/10.1038/s41586-019-1016-7.
Aguilo F, Zhang F, Sancho A, Fidalgo M, Di Cecilia S, Vashisht A, et al. Coordination of m(6)a mrna methylation and gene transcription by zfp217 regulates pluripotency and reprogramming. Cell Stem Cell. 2015;17:689–704. https://doi.org/10.1016/j.stem.2015.09.005.
Bertero A, Brown S, Madrigal P, Osnato A, Ortmann D, Yiangou L, et al. The smad2/3 interactome reveals that tgfβ controls m(6)a mrna methylation in pluripotency. Nature. 2018;555:256–9. https://doi.org/10.1038/nature25784.
An S, Huang W, Huang X, Cun Y, Cheng W, Sun X, et al. Integrative network analysis identifies cell-specific trans regulators of m6a. Nucleic Acids Res. 2020;48:1715–29. https://doi.org/10.1093/nar/gkz1206.
Fish L, Navickas A, Culbertson B, Xu Y, Nguyen H, Zhang S, et al. Nuclear tarbp2 drives oncogenic dysregulation of rna splicing and decay. Mol Cell. 2019;75:967–81.e9. https://doi.org/10.1016/j.molcel.2019.06.001.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74.
Yan R, Dai W, Wu R, Huang H, Shu M. Therapeutic targeting m6a-guided mir-146a-5p signaling contributes to the melittin-induced selective suppression of bladder cancer. Cancer Lett. 2022;534:215615.
Bi X, Lv X, Liu D, et al. Mettl3 promotes the initiation and metastasis of ovarian cancer by inhibiting ccng2 expression via promoting the maturation of pri-microrna-1246. Cell Death Discov. 2021;7(1):237.
Jiang X, Yuan Y, Tang L, et al. Comprehensive pan-cancer analysis of the prognostic and immunological roles of the mettl3/lncrna-snhg1/mirna-140-3p/ube2c axis. Front Cell Dev Biol. 2021;9:765772.
Ji F, Lu Y, Chen S, et al. M(6)a methyltransferase mettl3-mediated lncrna foxd2-as1 promotes the tumorigenesis of cervical cancer. Mol Ther Oncolytics. 2021;22:574–81.
Xue L, Li J, Lin Y, et al. M(6) a transferase mettl3-induced lncrna abhd11-as1 promotes the Warburg effect of non-small-cell lung cancer. J Cell Physiol. 2021;236(4):2649–58.
Wang D, Wang X, Huang B, et al. Mettl3 promotes prostate cancer progression by regulating mir-182 maturation in m6a-dependent manner. Andrologia. 2022;54(7):1581–91.
Ma H, Zhang F, Zhong Q, Hou J. Mettl3-mediated m6a modification of kif3c-mrna promotes prostate cancer progression and is negatively regulated by mir-320d. Aging. 2021;13(18):22332–44.
Mao Y, Li W, Weng Y, et al. Mettl3-mediated m(6)a modification of lncrna malat1 facilitates prostate cancer growth by activation of pi3k/akt signaling. Cell Transplant. 2022;31:9636897221122997.
Chen B, Liu C, Long H, Bai G, Zhu Y, Xu H. N(6)-methyladenosine-induced long non-coding rna pvt1 regulates the mir-27b-3p/blm axis to promote prostate cancer progression. Int J Oncol. 2023;62(1)
Mao C, Huang C, Hu Z, Qu S. Transcription factor casz1 increases an oncogenic transcriptional process in tumorigenesis and progression of glioma cells. Medcomm (2020). 2022;3:e182. https://doi.org/10.1002/mco2.182.
Qu S, Huang C, Zhu T, Wang K, Zhang H, Wang L, et al. Olfml3, as a potential predictor of prognosis and therapeutic target for glioma, is closely related to immune cell infiltration. View. 2023;4:20220052.
Chang AJ, Autio KA, Roach M 3rd, Scher HI. High-risk prostate cancer-classification and therapy. Nat Rev Clin Oncol. 2014;11(6):308–23.
Musselman CA, Me L, Côté J, Kutateladze TG. Perceiving the epigenetic landscape through histone readers. Nat Struct Mol Biol. 2012;19(12):1218–27.
Wojtas MN, Pandey RR, Mendel M, Homolka D, Sachidanandam R, Pillai RS. Regulation of m6a transcripts by the 3’→5’ rna helicase ythdc2 is essential for a successful meiotic program in the mammalian germline. Mol Cell. 2017;68(2):374–387.e12.
Du H, Zhao Y, He J, et al. Ythdf2 destabilizes m(6)a-containing rna through direct recruitment of the ccr4-not deadenylase complex. Nat Commun. 2016;7:12626.
Wang X, Lu Z, Gomez A, et al. N6-methyladenosine-dependent regulation of messenger rna stability. Nature. 2014;505(7481):117–20.
Li Z, Qian P, Shao W, et al. Suppression of m(6)a reader ythdf2 promotes hematopoietic stem cell expansion. Cell Res. 2018;28(9):904–17.
Zhou J, Wan J, Gao X, Zhang X, Jaffrey SR, Qian SB. Dynamic m(6)a mrna methylation directs translational control of heat shock response. Nature. 2015;526(7574):591–4.
Hindupur SK, Colombi M, Fuhs SR, Matter MS, Guri Y, Adam K, et al. The protein histidine phosphatase lhpp is a tumour suppressor. Nature. 2018;555:678–82. https://doi.org/10.1038/nature26140.
Zheng J, Dai X, Chen H, Fang C, Chen J, Sun L. Down-regulation of lhpp in cervical cancer influences cell proliferation, metastasis and apoptosis by modulating akt. Biochem Biophys Res Commun. 2018;503:1108–14. https://doi.org/10.1016/j.bbrc.2018.06.127.
Eide T, Ramberg H, Glackin C, Tindall D, Taskén KA. Twist1, a novel androgen-regulated gene, is a target for nkx3-1 in prostate cancer cells. Cancer Cell Int. 2013;13:4. https://doi.org/10.1186/1475-2867-13-4.
Lei Q, Jiao J, **n L, Chang CJ, Wang S, Gao J, et al. Nkx3.1 stabilizes p53, inhibits akt activation, and blocks prostate cancer initiation caused by pten loss. Cancer Cell. 2006;9:367–78. https://doi.org/10.1016/j.ccr.2006.03.031.
Ma XX, Cao ZG, Zhao SL. M6a methyltransferase mettl3 promotes the progression of prostate cancer via m6a-modified lef1. Eur Rev Med Pharmacol Sci. 2020;24(7):3565–71.
Babaei G, Aziz SG, Jaghi N. Emt, cancer stem cells and autophagy; the three main axes of metastasis. Biomed Pharmacother. 2021;133:110909.
Suarez-Carmona M, Lesage J, Cataldo D, Gilles C. Emt and inflammation: inseparable actors of cancer progression. Mol Oncol. 2017;11(7):805–23.
Zanotelli MR, Zhang J, Reinhart-King CA. Mechanoresponsive metabolism in cancer cell migration and metastasis. Cell Metab. 2021;33(7):1307–21.
Chen Y, Pan C, Wang X, et al. Silencing of mettl3 effectively hinders invasion and metastasis of prostate cancer cells. Theranostics. 2021;11(16):7640–57.
Liang L, Li Q, Huang LY, et al. Loss of arhgdia expression is associated with poor prognosis in hcc and promotes invasion and metastasis of hcc cells. Int J Oncol. 2014;45(2):659–66.
Rucci N, Angelucci A. Prostate cancer and bone: the elective affinities. Biomed Res Int. 2014;2014:167035.
Klaassen Z, Howard LE, De Hoedt A, et al. Factors predicting skeletal-related events in patients with bone metastatic castration-resistant prostate cancer. Cancer. 2017;123(9):1528–35.
Pavlova NN, Zhu J, Thompson CB. The hallmarks of cancer metabolism: still emerging. Cell Metab. 2022;34(3):355–77.
Carvalho TM, Cardoso HJ, Figueira MI, Vaz CV, Socorro S. The peculiarities of cancer cell metabolism: a route to metastasization and a target for therapy. Eur J Med Chem. 2019;171:343–63.
Sadeghi RN, Karami-Tehrani F, Salami S. Targeting prostate cancer cell metabolism: impact of hexokinase and cpt-1 enzymes. Tumour Biol. 2015;36(4):2893–905.
Jadvar H. Pet of glucose metabolism and cellular proliferation in prostate cancer. J Nucl Med. 2016;57(suppl 3):25s–9s.
Liu J, Yuan JF, Wang YZ. Mettl3-stabilized lncrna snhg7 accelerates glycolysis in prostate cancer via srsf1/c-myc axis. Exp Cell Res. 2022;416(1):113149.
Shen C, Xuan B, Yan T, et al. M(6)a-dependent glycolysis enhances colorectal cancer progression. Mol Cancer. 2020;19(1):72.
Lin Y, Wei X, Jian Z, Zhang X. Mettl3 expression is associated with glycolysis metabolism and sensitivity to glycolytic stress in hepatocellular carcinoma. Cancer Med. 2020;9(8):2859–67.
Wang Y, Chen J, Wu Z, et al. Mechanisms of enzalutamide resistance in castration-resistant prostate cancer and therapeutic strategies to overcome it. Br J Pharmacol. 2021;178(2):239–61.
Antonarakis ES, Lu C, Wang H, et al. Ar-v7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371(11):1028–38.
Cotter KA, Gallon J, Uebersax N, et al. Map** of m(6)a and its regulatory targets in prostate cancer reveals a mettl3-low induction of therapy resistance. Mol Cancer Res. 2021;19(8):1398–411.
Boriack-Sjodin PA, Ribich S, Copeland RA. Rna-modifying proteins as anticancer drug targets. Nat Rev Drug Discov. 2018;17(6):435–53.
Bedi RK, Huang D, Eberle SA, Wiedmer L, Śledź P, Caflisch A. Small-molecule inhibitors of mettl3, the major human epitranscriptomic writer. Chemmedchem. 2020;15(9):744–8.
Moroz-Omori EV, Huang D, Kumar Bedi R, et al. Mettl3 inhibitors for epitranscriptomic modulation of cellular processes. Chemmedchem. 2021;16(19):3035–43.
Dolbois A, Bedi RK, Bochenkova E, et al. 1,4,9-triazaspiro[5.5]undecan-2-one derivatives as potent and selective mettl3 inhibitors. J Med Chem. 2021;64(17):12738–60.
Yankova E, Blackaby W, Albertella M, et al. Small-molecule inhibition of mettl3 as a strategy against myeloid leukaemia. Nature. 2021;593(7860):597–601.
Ofir-Rosenfeld Y, Vasiliauskaitė L, Saunders C, et al. Stc-15, an oral small molecule inhibitor of the rna methyltransferase mettl3, inhibits tumour growth through activation of anti-cancer immune responses associated with increased interferon signalling, and synergises with t cell checkpoint blockade. Eur J Cancer. 2022;174:s123.
Miao W, Chen J, Jia L, Ma J, Song D. The m6a methyltransferase mettl3 promotes osteosarcoma progression by regulating the m6a level of lef1. Biochem Biophys Res Commun. 2019;516:719–25. https://doi.org/10.1016/j.bbrc.2019.06.128.
Li F, Chen S, Yu J, Gao Z, Sun Z, Yi Y, et al. Interplay of m(6) a and histone modifications contributes to temozolomide resistance in glioblastoma. Clin Transl Med. 2021;11:e553. https://doi.org/10.1002/ctm2.553.
Qu S, Chen Z, Liu B, Liu J, Wang H. N6-methyladenine-related genes affect biological behavior and the prognosis of glioma. Cancer Med. 2021;10:98–108. https://doi.org/10.1002/cam4.3574.
Liu T, Yang S, Sui J, Xu SY, Cheng YP, Shen B, et al. Dysregulated n6-methyladenosine methylation writer mettl3 contributes to the proliferation and migration of gastric cancer. J Cell Physiol. 2020;235:548–62. https://doi.org/10.1002/jcp.28994.
Yue B, Song C, Yang L, Cui R, Cheng X, Zhang Z, et al. Mettl3-mediated n6-methyladenosine modification is critical for epithelial-mesenchymal transition and metastasis of gastric cancer. Mol Cancer. 2019;18:142. https://doi.org/10.1186/s12943-019-1065-4.
Deng R, Cheng Y, Ye S, Zhang J, Huang R, Li P, et al. M(6)a methyltransferase mettl3 suppresses colorectal cancer proliferation and migration through p38/erk pathways. Onco Targets Ther. 2019;12:4391–402. https://doi.org/10.2147/ott.s201052.
Hua W, Zhao Y, ** X, Yu D, He J, **e D, et al. Mettl3 promotes ovarian carcinoma growth and invasion through the regulation of axl translation and epithelial to mesenchymal transition. Gynecol Oncol. 2018;151:356–65. https://doi.org/10.1016/j.ygyno.2018.09.015.
Han J, Wang JZ, Yang X, Yu H, Zhou R, Lu HC, et al. Mettl3 promote tumor proliferation of bladder cancer by accelerating pri-mir221/222 maturation in m6a-dependent manner. Mol Cancer. 2019;18:110. https://doi.org/10.1186/s12943-019-1036-9.
**a T, Wu X, Cao M, Zhang P, Shi G, Zhang J, et al. The rna m6a methyltransferase mettl3 promotes pancreatic cancer cell proliferation and invasion. Pathol Res Pract. 2019;215:152666. https://doi.org/10.1016/j.prp.2019.152666.
Zhang Y, Kang M, Zhang B, Meng F, Song J, Kaneko H, et al. M(6)a modification-mediated cbx8 induction regulates stemness and chemosensitivity of colon cancer via upregulation of lgr5. Mol Cancer. 2019;18:185. https://doi.org/10.1186/s12943-019-1116-x.
Liu S, Li Q, Li G, Zhang Q, Zhuo L, Han X, et al. The mechanism of m(6)a methyltransferase mettl3-mediated autophagy in reversing gefitinib resistance in nsclc cells by β-elemene. Cell Death Dis. 2020;11:969. https://doi.org/10.1038/s41419-020-03148-8.
Pan X, Hong X, Li S, Meng P, **ao F. Mettl3 promotes adriamycin resistance in mcf-7 breast cancer cells by accelerating pri-microrna-221-3p maturation in a m6a-dependent manner. Exp Mol Med. 2021;53:91–102. https://doi.org/10.1038/s12276-020-00510-w.
Wang L, Hui H, Agrawal K, Kang Y, Li N, Tang R, et al. M(6) a rna methyltransferases mettl3/14 regulate immune responses to anti-pd-1 therapy. EMBO J. 2020;39:e104514. https://doi.org/10.15252/embj.2020104514.
Ni Z, Sun P, Zheng J, et al. Jnk signaling promotes bladder cancer immune escape by regulating mettl3-mediated m6a modification of pd-l1 mrna. Cancer Res. 2022;82(9):1789–802.
Song C, Zhou C. Hoxa10 mediates epithelial-mesenchymal transition to promote gastric cancer metastasis partly via modulation of tgfb2/smad/mettl3 signaling axis. J Exp Clin Cancer Res. 2021;40(1):62.
Meng W, **ao H, Mei P, et al. Critical roles of mettl3 in translation regulation of cancer. Biomolecules. 2023;13(2)
Zeng C, Huang W, Li Y, Weng H. Roles of mettl3 in cancer: mechanisms and therapeutic targeting. J Hematol Oncol. 2020;13(1):117.
Chamorro-Jorganes A, Sweaad WK, Katare R, et al. Mettl3 regulates angiogenesis by modulating let-7e-5p and mirna-18a-5p expression in endothelial cells. Arterioscler Thromb Vasc Biol. 2021;41(6):e325–37.
Zhang G, Wang T, Huang Z, et al. Mettl3 dual regulation of the stability of linc00662 and vegfa rnas promotes colorectal cancer angiogenesis. Discov Oncol. 2022;13(1):89.
Wang G, Dai Y, Li K, et al. Deficiency of mettl3 in bladder cancer stem cells inhibits bladder cancer progression and angiogenesis. Front Cell Dev Biol. 2021;9:627706.
Wang Y, Wang Y, Gu J, Su T, Gu X, Feng Y. the role of rna m6a methylation in lipid metabolism. Front Endocrinol (Lausanne). 2022;13:866116.
Cui Q, Shi H, Ye P, Li L, Qu Q, Sun G, et al. M(6)a rna methylation regulates the self-renewal and tumorigenesis of glioblastoma stem cells. Cell Rep. 2017;18:2622–34. https://doi.org/10.1016/j.celrep.2017.02.059.
Gao Q, Zheng J, Ni Z, et al. The m(6)a methylation-regulated aff4 promotes self-renewal of bladder cancer stem cells. Stem Cells Int. 2020;2020:8849218.
Zhang C, Samanta D, Lu H, et al. Hypoxia induces the breast cancer stem cell phenotype by hif-dependent and alkbh5-mediated m6a-demethylation of nanog mrna. Proc Natl Acad Sci U S A. 2016;113(14):e2047–56.
Ji G, Huang C, He S, et al. Comprehensive analysis of m6a regulators prognostic value in prostate cancer. Aging (albany ny). 2020;12(14):14863–84.
Lothion-Roy J, Haigh DB, Harris AE, et al. Clinical and molecular significance of the rna m(6)a methyltransferase complex in prostate cancer. Front Genet. 2022;13:1096071.
Cai C, He HH, Chen S, et al. Androgen receptor gene expression in prostate cancer is directly suppressed by the androgen receptor through recruitment of lysine-specific demethylase 1. Cancer Cell. 2011;20(4):457–71.
Kashyap V, Ahmad S, Nilsson EM, et al. The lysine specific demethylase-1 (lsd1/kdm1a) regulates vegf-a expression in prostate cancer. Mol Oncol. 2013;7(3):555–66.
Cai C, He HH, Gao S, et al. Lysine-specific demethylase 1 has dual functions as a major regulator of androgen receptor transcriptional activity. Cell Rep. 2014;9(5):1618–27.
Lence T, Akhtar J, Bayer M, et al. M(6)a modulates neuronal functions and sex determination in drosophila. Nature. 2016;540(7632):242–7.
Haussmann IU, Bodi Z, Sanchez-Moran E, et al. M(6)a potentiates sxl alternative pre-mrna splicing for robust drosophila sex determination. Nature. 2016;540(7632):301–4.
Ma S, Chen C, Ji X, Liu J, Zhou Q, Wang G, et al. The interplay between m6a rna methylation and noncoding rna in cancer. J Hematol Oncol. 2019;12:121. https://doi.org/10.1186/s13045-019-0805-7.
Li X, Tang J, Huang W, Wang F, Li P, Qin C, et al. The m6a methyltransferase mettl3: acting as a tumor suppressor in renal cell carcinoma. Oncotarget. 2017;8:96103–16. https://doi.org/10.18632/oncotarget.21726.
Jia R, Chai P, Wang S, Sun B, Xu Y, Yang Y, et al. M(6)a modification suppresses ocular melanoma through modulating hint2 mrna translation. Mol Cancer. 2019;18:161. https://doi.org/10.1186/s12943-019-1088-x.
Lin S, Choe J, Du P, Triboulet R, Gregory RI. The m(6)a methyltransferase mettl3 promotes translation in human cancer cells. Mol Cell. 2016;62:335–45. https://doi.org/10.1016/j.molcel.2016.03.021.
Du M, Zhang Y, Mao Y, Mou J, Zhao J, Xue Q, et al. Mir-33a suppresses proliferation of nsclc cells via targeting mettl3 mrna. Biochem Biophys Res Commun. 2017;482:582–9. https://doi.org/10.1016/j.bbrc.2016.11.077.
Keith G. Mobilities of modified ribonucleotides on two-dimensional cellulose thin-layer chromatography. Biochimie. 1995;77:142–4. https://doi.org/10.1016/0300-9084(96)88118-1.
Bodi Z, Fray RG. Detection and quantification of n (6)-methyladenosine in messenger rna by tlc. Methods Mol Biol. 2017;1562:79–87. https://doi.org/10.1007/978-1-4939-6807-7_6.
Peer E, Rechavi G, Dominissini D. Epitranscriptomics: regulation of mrna metabolism through modifications. Curr Opin Chem Biol. 2017;41:93–8. https://doi.org/10.1016/j.cbpa.2017.10.008.
Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, Jaffrey SR. comprehensive analysis of mrna methylation reveals enrichment in 3′ utrs and near stop codons. Cell. 2012;149:1635–46. https://doi.org/10.1016/j.cell.2012.05.003.
Dominissini D, Moshitch-Moshkovitz S, Salmon-Divon M, Amariglio N, Rechavi G. transcriptome-wide map** of n(6)-methyladenosine by m(6)a-seq based on immunocapturing and massively parallel sequencing. Nat Protoc. 2013;8:176–89. https://doi.org/10.1038/nprot.2012.148.
Chen K, Luo GZ, He C. High-resolution map** of n6-methyladenosine in transcriptome and genome using a photo-crosslinking-assisted strategy. Methods Enzymol. 2015;560:161–85. https://doi.org/10.1016/bs.mie.2015.03.012.
Liu N, Pan T. Probing rna modification status at single-nucleotide resolution in total rna. Methods Enzymol. 2015;560:149–59. https://doi.org/10.1016/bs.mie.2015.03.005.
Linder B, Grozhik AV, Olarerin-George AO, Meydan C, Mason CE, Jaffrey SR. single-nucleotide-resolution map** of m6a and m6am throughout the transcriptome. Nat Methods. 2015;12:767–72. https://doi.org/10.1038/nmeth.3453.
Gilbert WV, Bell TA, Schaening C. Messenger rna modifications: form, distribution, and function. Science. 2016;352:1408–12. https://doi.org/10.1126/science.aad8711.
Acknowledgments
We thank Home for Researchers editorial team (www.home-forresearchers.com) for language editing service.
Funding
This work was supported by the Jiangxi Province 2023 Postgraduate Innovation Special Funds Project (Grants no.YC2023-S940 and YC2023-S954).
Author information
Authors and Affiliations
Contributions
G.Z. conceived the manuscript, X.Z.,K.C searched publications and draft the manuscript. H.Z.,C.L. edited tables and Figs. X.Z.,J.Z. reviewed the manuscript and polished the grammar. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhou, X., Chai, K., Zhu, H. et al. The role of the methyltransferase METTL3 in prostate cancer: a potential therapeutic target. BMC Cancer 24, 8 (2024). https://doi.org/10.1186/s12885-023-11741-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-11741-1