Abstract
Background
Estrogen receptor-positive and progesterone receptor-negative (ER + /PR-) breast cancer comprise a special type. More than 10% breast cancer patients belonged to ER + /PR-.
Methods
In order to better understand this patient population, we utilized a unique dataset from China, examining the clinicopathological features and genomic profiles of ER + /PR- breast cancers. Our study involved three cohorts: Cohort 1 included 2120 unselected ER-positive female patients with re-evaluated clinicopathological and survival data; Cohort 2 comprised 442 ER-positive females who underwent genetic testing; and Cohort 3 consisted of 77 ER-positive/HER2-negative females tested with MammaPrint and BluePrint.
Results
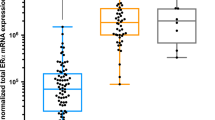
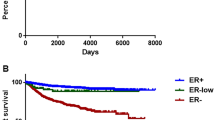
Patients were stratified into four categories based on the PR/ER ratio. Clinically, ER + /PR- tumors (PR/ER ratio = 0) showed the lowest proportion of T1 tumors (10.88%) and highest proportion of HER2-positive tumors (28.36%) than did other ER + /PR + tumors groups. The ER + /PR- group contained a higher number of underweight patients (20.20%). Independently of HER2 status, ER + /PR- patients demonstrated the poorest prognosis. Genomically, the most prevalent mutations were PIK3CA (50%) in ER + /PR + tumors and TP53 (65%) in ER + /PR- tumors. ER + /PR- tumors presented more frequent mutations in TP53, ERBB2, CDK12, SPEN, and NEB, with mutation rates of 65%, 42%, 27%, 13%, and 10%, respectively. Additionally, the Tumor Mutational Burden (TMB) was higher in the ER + /PR- group compared to the ER + /PR + group. The MammaPrint score for the ER + /PR-/HER2- group was significantly lower than that of other groups. In the BluePrint analysis, only four patients were classified as Basal-Type, all of whom were ER + /PR-/HER2-.
Conclusions
In this study, we identified the clinical and genetic characteristics of ER + /PR- breast cancer patients in China. Distinct PR statuses indicated different biological processes of ER + breast cancer and survival outcomes. Future treatment strategies may need to be tailored for ER + /PR- patients.
Similar content being viewed by others
Background
Steroid hormone receptors are crucial biomarkers in breast cancer, which including estrogen receptor (ER) and progesterone receptor (PR) [1]. Over 70% of breast cancers are hormone receptors positive [2]. PR serves as a biomarker for ER function, and its expression closely correlates with that of ER [3]. Mechanistically, PR is a downstream gene target of ER [4]. Since the expression activity of ER can regulate the expression of PR, ER and PR expression is generally consistent. However, inconsistent ER and PR expression also exist in some patients. Some ER-positive tumors have a partial loss or a complete lack of PR expression [5, 6].
Clinically, ER + /PR- breast cancer is still defined as Luminal subtype breast cancer, which recommends endocrine therapy. It is evident that ER + /PR- tumors have more aggressive biological and clinical characteristics compared to ER + /PR + tumors [7]. Tamoxifen is less effective against ER + /PR- tumors, so more aggressive treatments might be beneficial [8, 9]. Nevertheless, the genetic characteristics of ER + /PR + breast cancer and ER + /PR- breast cancer is not identical.
Some studies, based on western patients, have tried to reveal the characteristics of ER + PR- breast cancer during the last two decades [5, 6]. Currently, the significance of ER + PR- breast cancer remains unclear. Furthermore, differences in survival following a breast cancer diagnosis based on ethnicity and race, while considering the status of estrogen and progesterone receptors, have been observed [10]. However, we still lack a comprehensive understanding of the genetic landscape of ER + PR- breast cancer patients, particularly in Asian populations. We should not underestimate the contribution of racial disparities to breast cancer genomic traits. Therefore, it is of utmost importance to utilize data from Asian patients to explore the features of ER + /PR- breast cancer.
In this study, we included more than two thousand ER positive breast cancer patients in two large tertiary hospitals from China. We have examined ER + /PR- breast cancer patients with long-term follow-up, genomic data, the risk assessment of recurrence and intrinsic molecular subtypes data (MammaPrint and BluePrint). This study sought to enhance our understanding of the genetic and clinical characteristic underlying ER + /PR- breast cancer in China.
Patients and methods
Patients
This study included three cohorts. The first cohort (Cohort 1) is from Sun Yat-Sen University Cancer Centre including 2120 ER-positive unselected female patients. Those patients diagnosed of breast cancer between January 1, 2001 and December 16, 2011. Cohort 1 is comprising clinicopathological and follow-up data. Patients were followed up to April 27, 2017 or until death. The second (Cohort 2) and third cohorts (Cohort 3) are from Guangdong Provincial People's Hospital. Patients in Cohort 2 and Cohort 3 are diagnosed of breast cancer during June, 2017 to September, 2019. Cohort 2 is including 442 ER-positive female patients who had performed genetic test using a panel comprising 520 cancer-related genes. Cohort 3 is including 77 ER-positive/HER2-negative female patients who had performed MammaPrint and BluePrint test. This study was conducted in accordance with the principles of the 1964 Declaration of Helsinki. Both Sun Yat-Sen University Cancer Center Institute Research Ethics Committee (No. YB2016-002–03) and Guangdong Provincial People's Hospital Ethics Committee (No. GDREC2019497H; 2019-040H-1) approved this retrospective study. There was written informed consent from every patient enrolled.
Immunohistochemistry (IHC)
ER and PR expression was considered positive in tumors with 1% or more positively stained nuclei. All patients are ER positive in this study. In accordance with 2013 American Society of Clinical Oncology/College of American Pathologists (ASCO-CAP) guidelines, HER2 status was evaluated. When IHC result was two-plus (2 +), HER2 status was confirmed using fluorescence in situ hybridization (FISH). Ki67 expression was measured and reported as a percentage score of positive tumor cells (range 0–100%).
Next-Generation Sequencing (NGS)
Next generation targeted genomic DNA-sequencing of formalin-fixed paraffin-embedded tissue was performed using a panel covering 520 cancer related genes, spanning 1.64 megabases of the human genome, as previously described [11, 16]. However, a subset of ER-positive breast cancer patients still experiences recurrences despite undergoing endocrine therapy [17]. Two crucial molecules for evaluating breast cancer heterogeneity and the advantages of hormonal therapy are the steroid hormone receptor ER and the progesterone receptor PR. This study focuses on the clinicopathological features and genomic changes in ER + /PR- breast cancers, utilizing an exclusive patient dataset from China, which includes comprehensively annotated clinical data, survival follow-up, and genomic information.
Even though this study is retrospective, it does comprise an unselected breast cancer population without exclusions or selection biases. In ER + patients (Cohort 1), ER + /PR- patients comprise approximately 13.49% of the total. This result is similar to that reported by SEER database (15.35%) [1]. ER + /PR- breast cancers are more frequently observed in older women and underweight patients, and this group had the lowest proportion of T1 tumors and the highest proportion of HER2-positive tumors. Consistent with previous research, our study confirms that ER + /PR- tumors display more aggressive characteristics and higher HER2 expression compared to ER + /PR + tumors. The absence of PR expression may reflect hyperactive cross talk between growth factor signaling pathways and ER [3]. Previous studies have also explored whether PR expression serves as an independent prognostic variable. A European study found that the prognostic effect of PR-negativity in the ER + /HER2- group becomes most pronounced beyond 6 years of follow up [18]. Bae et al. observed that ER + /PR-/HER2- tumors were associated with worse survival outcomes than ER + /PR + /HER2- tumors, though PR negativity was not a significant prognostic factor in tumors with HER2 overexpression [6]. A SEER database study found that ER + /PR- breast cancer has a prognosis midway between that of the ER + /PR + and ER-/PR- subtypes [1]. In our ER-positive series, regardless of HER2 status, ER + /PR- patients exhibit the poorest prognosis. This phenomenon could be linked to variations in race and healthcare services across different regions. Additional studies are required to prospectively confirm these findings.
Our research has unveiled somatic mutations in ER-positive breast cancers. The most prevalent mutations in ER + /PR + tumors and ER + /PR- tumors are PIK3CA (50%) and TP53 (65%), respectively. These findings align with previous studies that have identified TP53 and PIK3CA mutations as common in breast cancer [19]. In ER + /PR- breast cancer, tumor activation of non-canonical ER-signaling leads to increased activation of the PI3K and MAPK pathways at the cellular level [20]. Furthermore, our study reveals that ER + /PR- breast tumors exhibit a higher incidence of variants in TP53, ERBB2, CDK12, SPEN, and NEB, with variant rates of 65%, 42%, 27%, 13%, and 10%, respectively. based on the TCGA dataset discovered that ER + /PR-/HER2- tumors have higher TP53 mutation rates and lower PIK3CA mutation rates compared to ER + /PR + HER2- tumors, along with a higher frequency of ZNF703 and RPS6KB1 amplification events [21]. TP53 is a tumor suppressor gene located on chromosome 17p13.1 and is frequently inactivated by mutations or deletions. Ahn et al. analyzed mutational of exons 5–9 of the TP53 in ER-positive breast cancer by PCR amplification and direct sequencing. Since they did not utilize NGS, they identified somatic TP53 mutations in only 10.3% of ER-positive tumors. But, similar to our findings, they concluded that the TP53 mutation rate was significantly higher in ER + /PR- tumors compared to ER + /PR + tumors (P = 0.039) [22]. TP53 mutations are associated with primary endocrine resistance in breast cancer [23]. Codons 273 is a hotspot for TP53 mutations found in most human cancers, including breast cancer [24]. In our study, despite the high overall TP53 mutation rate, no mutation in codon 273 was observed in the ER + /PR- group. This characteristic may impact the sensitivity of therapy in ER + /PR- patients. Previous esearch has shown that breast cancer patients with codon 273 mutations are more sensitive to chemotherapy compared with other TP53 mutant patients and TP53 wild-type patients [25].
Our investigation revealed a higher proportion of HER2-positive tumors in ER + /PR- patients. Therefore, it is reasonable that ER + /PR- patients have more ERBB2 gene amplified than other group. CDK12 is located approximately 200 kb proximal to the ERBB2 gene [26]. In breast cancer, CDK12 frequently displays co-amplification and cooperation with the ERBB2 and interaction with oncogenic pathways, such as IRS1-ErbB-PI3K signaling [27]. SPEN is recognized as a tumor-suppressor gene, and its deletion or intragenic mutation may contribute to breast cancer progression [28]. SPEN binds ERα and exerts a negative regulatory influence on the transcription of Erα target genes. It is a candidate predictive biomarker of tamoxifen response [29]. The functional roles of NEB in breast cancer have been poorly studied. In breast cancer, the median TMB significantly varies depending on the tumor subtype, with HR-/HER2- tumors exhibiting the highest TMB, followed by HER2 + and HR + /HER2- tumors [30]. Our study also observed differences in TMB between the ER + /PR- and ER + /PR + groups, with the ER + /PR- group displaying a higher TMB. Moreover, ER + /PR- tumors had a higher percentage of cases with high TMB. High TMB is indicative of genomic instability and an abundance of tumor neoantigens [31]. In a study by ** of Luminal B (HER2 negative), a better discriminator of outcome and recurrence score. Cancer Med. 2023;12(3):2493–504." href="/article/10.1186/s12885-023-11643-2#ref-CR4" id="ref-link-section-d245656332e3121">4]. By PAM50 genomic assay, about 20% ER + /PR-/HER2- tumors were defined as non-luminal-like subgroup and enriched biosynthesis, metabolism and DNA replication pathways [21]. In our study, we conducted MammaPrint and BluePrint analyses on a cohort of 77 ER + /HER2- patients.. The results revealed that 80% of ER + /PR-/HER2- patients (n = 8) had a high-risk profile. Among these 77 patients, only four patients were Basal-Type, and interestingly, all of them belonged to the ER + /PR-/HER2- subgroup. Our findings confirmed the genetic heterogeneity of ER + /PR-/HER2- tumors, and proved that the genetic characteristics of ER + /PR-/HER2- tumors are more malignant. Here, we note several limitations to our work. Firstly, due to the retrospective of this study, inherent biases may be present. Notably, detailed patient information regarding chemotherapy and radiation therapy is unavailable. Secondly, some patients are missing the FISH information of HER2 and cannot judge the final status of HER2. Thirdly, patients in Cohort 2 and Cohort 3 have not been followed up for a long time, so survival analysis cannot be carried out to link their genetic characteristics with prognosis in the future, an external validation cohort is required for our study to make the results more compelling. Fourthly, while our findings provide valuable insights into the genetic landscape of ER + /PR- breast cancer, we did not extensively investigate the mechanistic underpinnings of these observed variations. Understanding these mechanisms is pivotal for a comprehensive grasp of the disease and the development of targeted treatment strategies.
Conclusions
In this large retrospective study, we identified the clinical and genetic characteristics of ER + /PR- breast cancer patients in China. Distinct PR statuses indicated different biological processes of ER + breast cancer and survival outcomes. ER + /PR- patients might require different treatment strategies in the future.
Availability of data and materials
All data can be viewed in the National Omics Data Encyclopedia (NODE; http://www.biosino.org/node) by pasting the accession OEP001295 (http://www.biosino.org/node/project/detail/OEP001295) and OEP001992(http://www.biosino.org/node/project/detail/OEP001992). Data information available from the corresponding author on reasonable request in accordance with Chinese law for genomic data.
Abbreviations
- ASCO-CAP:
-
American Society of Clinical Oncology/College of American Pathologists
- CDK12:
-
Cyclin-dependent kinase 12
- DNA:
-
Deoxyribonucleic Acid
- ER:
-
Estrogen Receptor
- ERBB2:
-
Human Epidermal Growth Factor Receptor 2
- FISH:
-
Fluorescence in Situ Hybridization
- HER2:
-
Human Epidermal Growth Factor Receptor 2
- HR:
-
Hormone Receptor
- IHC:
-
Immunohistochemistry
- MAPK:
-
Mitogen-Activated Protein Kinase
- METABRIC:
-
Molecular Taxonomy of Breast Cancer International Consortium
- NEB:
-
Next-Generation Sequencing
- OS:
-
Overall Survival
- PIK3CA:
-
Phosphatidylinositol-4,5-Bisphosphate 3-Kinase, Catalytic Subunit Alpha
- PR:
-
Progesterone Receptor
- RPS6KB1:
-
Ribosomal Protein S6 Kinase B1
- SPEN:
-
Split Ends
- TCGA:
-
The Cancer Genome Atlas
- TP53:
-
Tumor Protein 53
- TMB:
-
Tumor Mutation Burden
- ZNF703:
-
Zinc Finger Protein 703
References
Li Y, Yang D, Yin X, Zhang X, Huang J, Wu Y, Wang M, Yi Z, Li H, Li H, et al. Clinicopathological Characteristics and Breast Cancer-Specific Survival of Patients With Single Hormone Receptor-Positive Breast Cancer. JAMA Netw Open. 2020;3(1):e1918160.
Waks AG, Winer EP. Breast Cancer Treatment: A Review. JAMA. 2019;321(3):288–300.
Cui X, Schiff R, Arpino G, Osborne CK, Lee AV. Biology of progesterone receptor loss in breast cancer and its implications for endocrine therapy. J Clin Oncol. 2005;23(30):7721–35.
Yang ZJ, Liu YX, Huang Y, Chen ZJ, Zhang HZ, Yu Y, Wang X, Cao XC. The regrou** of Luminal B (HER2 negative), a better discriminator of outcome and recurrence score. Cancer Med. 2023;12(3):2493–504.
Rakha EA, El-Sayed ME, Green AR, Paish EC, Powe DG, Gee J, Nicholson RI, Lee AH, Robertson JF, Ellis IO. Biologic and clinical characteristics of breast cancer with single hormone receptor positive phenotype. J Clin Oncol. 2007;25(30):4772–8.
Bae SY, Kim S, Lee JH, Lee HC, Lee SK, Kil WH, Kim SW, Lee JE, Nam SJ. Poor prognosis of single hormone receptor- positive breast cancer: similar outcome as triple-negative breast cancer. BMC Cancer. 2015;15:138.
Yao N, Song Z, Wang X, Yang S, Song H. Prognostic Impact of Progesterone Receptor Status in Chinese Estrogen Receptor Positive Invasive Breast Cancer Patients. J Breast Cancer. 2017;20(2):160–9.
Dunnwald LK, Rossing MA, Li CI. Hormone receptor status, tumor characteristics, and prognosis: a prospective cohort of breast cancer patients. Breast Cancer Res. 2007;9(1):R6.
Verde G, De Llobet LI, Wright RHG, Quilez J, Peiro S, Le Dily F, Beato M. Unliganded Progesterone Receptor Governs Estrogen Receptor Gene Expression by Regulating DNA Methylation in Breast Cancer Cells. Cancers (Basel). 2018;10(10):371.
John EM, McGuire V, Kurian AW, Koo J, Shariff-Marco S, Gomez SL, Cheng I, Keegan THM, Kwan ML, Bernstein L, et al. Racial/Ethnic Disparities in Survival after Breast Cancer Diagnosis by Estrogen and Progesterone Receptor Status: A Pooled Analysis. Cancer Epidemiol Biomarkers Prev. 2021;30(2):351–63.
Chen B, Zhang G, Wei G, Wang Y, Guo L, Lin J, Li K, Mok H, Cao L, Ren C, et al. Heterogeneity of genomic profile in patients with HER2-positive breast cancer. Endocr Relat Cancer. 2020;27(3):153–62.
Chen B, Zhang G, Lai J, **ao W, Li X, Li C, Mok H, Li K, Wang Y, Cao L, et al. Genetic and immune characteristics of sentinel lymph node metastases and multiple lymph node metastases compared to their matched primary breast tumours. EBioMedicine. 2021;71:103542.
Zhang G, Ren C, Li C, Wang Y, Chen B, Wen L, Jia M, Li K, Mok H, Cao L, et al. Distinct clinical and somatic mutational features of breast tumors with high-, low-, or non-expressing human epidermal growth factor receptor 2 status. BMC Med. 2022;20(1):142.
Nik-Zainal S, Davies H, Staaf J, Ramakrishna M, Glodzik D, Zou X, Martincorena I, Alexandrov LB, Martin S, Wedge DC, et al. Landscape of somatic mutations in 560 breast cancer whole-genome sequences. Nature. 2016;534(7605):47–54.
Sha D, ** Z, Budczies J, Kluck K, Stenzinger A, Sinicrope FA. Tumor Mutational Burden as a Predictive Biomarker in Solid Tumors. Cancer Discov. 2020;10(12):1808–25.
Hanker AB, Sudhan DR, Arteaga CL. Overcoming Endocrine Resistance in Breast Cancer. Cancer Cell. 2020;37(4):496–513.
Saatci O, Huynh-Dam KT, Sahin O. Endocrine resistance in breast cancer: from molecular mechanisms to therapeutic strategies. J Mol Med (Berl). 2021;99(12):1691–710.
Purdie CA, Quinlan P, Jordan LB, Ashfield A, Ogston S, Dewar JA, Thompson AM. Progesterone receptor expression is an independent prognostic variable in early breast cancer: a population-based study. Br J Cancer. 2014;110(3):565–72.
Schrijver W, Selenica P, Lee JY, Ng CKY, Burke KA, Piscuoglio S, Berman SH, Reis-Filho JS, Weigelt B, van Diest PJ, et al. Mutation Profiling of Key Cancer Genes in Primary Breast Cancers and Their Distant Metastases. Cancer Res. 2018;78(12):3112–21.
Braun L, Mietzsch F, Seibold P, Schneeweiss A, Schirmacher P, Chang-Claude J, Peter Sinn H, Aulmann S. Intrinsic breast cancer subtypes defined by estrogen receptor signalling-prognostic relevance of progesterone receptor loss. Mod Pathol. 2013;26(9):1161–71.
Liu XY, Ma D, Xu XE, ** X, Yu KD, Jiang YZ, Shao ZM. Genomic Landscape and Endocrine-Resistant Subgroup in Estrogen Receptor-Positive, Progesterone Receptor-Negative, and HER2-Negative Breast Cancer. Theranostics. 2018;8(22):6386–99.
Ahn SG, Yoon CI, Lee JH, Lee HS, Park SE, Cha YJ, Cha C, Bae SJ, Lee KA, Jeong J. Low PR in ER(+)/HER2(-) breast cancer: high rates of TP53 mutation and high SUV. Endocr Relat Cancer. 2019;26(2):177–85.
Mueller S, Grote I, Bartels S, Kandt L, Christgen H, Lehmann U, Gluz O, Graeser M, Kates R, Harbeck N, et al. p53 Expression in Luminal Breast Cancer Correlates With TP53 Mutation and Primary Endocrine Resistance. Mod Pathol. 2023;36(4):100100.
Xu-Monette ZY, Wu L, Visco C, Tai YC, Tzankov A, Liu WM, Montes-Moreno S, Dybkaer K, Chiu A, Orazi A, et al. Mutational profile and prognostic significance of TP53 in diffuse large B-cell lymphoma patients treated with R-CHOP: report from an International DLBCL Rituximab-CHOP Consortium Program Study. Blood. 2012;120(19):3986–96.
Noor H, Briggs NE, McDonald KL, Holst J, Vittorio O. TP53 Mutation Is a Prognostic Factor in Lower Grade Glioma and May Influence Chemotherapy Efficacy. Cancers (Basel). 2021;13(21):5362.
Yanai Y, Kosaka T, Nakamura K, Aimono E, Matsumoto K, Morita S, Mikami S, Nishihara H, Oya M. CDK12 and HER2 coamplification in two urothelial carcinomas with rapid and aggressive clinical progression. Cancer Sci. 2020;111(12):4652–5.
Filippone MG, Gaglio D, Bonfanti R, Tucci FA, Ceccacci E, Pennisi R, Bonanomi M, Jodice G, Tillhon M, Montani F, et al. CDK12 promotes tumorigenesis but induces vulnerability to therapies inhibiting folate one-carbon metabolism in breast cancer. Nat Commun. 2022;13(1):2642.
Legare S, Chabot C, Basik M. SPEN, a new player in primary cilia formation and cell migration in breast cancer. Breast Cancer Res. 2017;19(1):104.
Legare S, Cavallone L, Mamo A, Chabot C, Sirois I, Magliocco A, Klimowicz A, Tonin PN, Buchanan M, Keilty D, et al. The Estrogen Receptor Cofactor SPEN Functions as a Tumor Suppressor and Candidate Biomarker of Drug Responsiveness in Hormone-Dependent Breast Cancers. Cancer Res. 2015;75(20):4351–63.
Barroso-Sousa R, Jain E, Cohen O, Kim D, Buendia-Buendia J, Winer E, Lin N, Tolaney SM, Wagle N. Prevalence and mutational determinants of high tumor mutation burden in breast cancer. Ann Oncol. 2020;31(3):387–94.
Lai JZ, Zhu YY, Liu Y, Zhou LL, Hu L, Chen L, Zhang QY. Abscopal Effects of Local Radiotherapy Are Dependent on Tumor Immunogenicity. Front Oncol. 2021;11:690188.
**e P, An R, Yu S, He J, Zhang H. A novel immune subtype classification of ER-positive, PR-negative and HER2-negative breast cancer based on the genomic and transcriptomic landscape. J Transl Med. 2021;19(1):398.
Acknowledgements
We thank all the patients and their families for participation in our study.
Funding
This work was supported by funds from the Science and Technology Program of Guangzhou (202201011427, Bo Chen; 2023A04J0527, Danian Dai), the Guangdong Basic and Applied Basic Research Foundation (2022A1515011599, Bo Chen), the Excellent Young Talent Program of Guangdong Provincial People’s Hospital (KY012021190, Bo Chen), the National Natural Science Foundation of China (82272998, 81902828 Bo Chen) and the High-level Hospital Construction Project (DFJH201921, Bo Chen). The funding agencies had no role in the design of the study; the collection, analysis, and interpretation of data; or the writing of the manuscript.
Author information
Authors and Affiliations
Contributions
All authors participated in the planning and execution of this study or analysis of the study data. BC designed the study. DD, HW, HZ, CL and RC participated in sample collection, sample processing, collection of the clinical information and data analysis. BC, DD and HZ performed the statistical analyses. BC, DD and HW drafted the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Sun Yat-sen University Cancer Center (No. YB2016-002–03) and Guangdong Provincial People's Hospital Ethics Committee (No. GDREC2019497H; 2019-040H-1). There was written informed consent from every patient enrolled. All experiments in this study were conducted following the corresponding regulations and guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Supplemental Table S1. Baseline characteristics of patients in Cohort 2.
Additional file 2:
Supplemental Table S2. Mutations in the ER+/PR- group.
Additional file 3:
Supplemental Table S3. Mutations in the ER+/PR+ group.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Dai, D., Wu, H., Zhuang, H. et al. Genetic and clinical landscape of ER + /PR- breast cancer in China. BMC Cancer 23, 1189 (2023). https://doi.org/10.1186/s12885-023-11643-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-11643-2