Abstract
Purpose
Detecting tumor progression of glioma continues to pose a formidable challenge. The role of fibroblast activation protein (FAP) in gliomas has been demonstrated to facilitate tumor progression. Glioma-circulating biomarkers have not yet been used in clinical practice. This study seeks to evaluate the feasibility of glioma detection through the utilization of a serum FAP marker.
Methods
We adopted enzyme-linked immunosorbent assay (ELISA) technique to quantify the relative FAP level of serum autoantibodies in a cohort of 87 gliomas. The correlation between preoperative serum autoantibody relative FAP levels and postoperative pathology, including molecular pathology was investigated. A series of FAP tests were conducted on 33 cases of malignant gliomas in order to ascertain their efficacy in monitoring the progression of the disease in relation to imaging observations. To validate the presence of FAP expression in tumors, immunohistochemistry was conducted on four gliomas employing a FAP-specific antibody. Additionally, the investigation encompassed the correlation between postoperative tumor burden, as assessed through volumetric analysis, and the relative FAP level of serum autoantibodies.
Results
A considerable proportion of gliomas exhibited a significantly increased level of serum autoantibody relative FAP level. This elevation was closely associated with both histopathology and molecular pathology, and demonstrated longitudinal fluctuations and variations corresponding to the progression of the disease The correlation between the rise in serum autoantibody relative FAP level and tumor progression and/or exacerbation of symptoms was observed.
Conclusions
The measurement of serum autoantibody relative FAP level can be used to detect the disease as a valuable biomarker. The combined utilization of its detection alongside MR imaging has the potential to facilitate a more accurate and prompt diagnosis.
Similar content being viewed by others
Introduction
Glioma as the most common primary malignant brain tumor in adults, is regarded as one of the leading causes of cancer death worldwide [1, 2]. Despite notable advances in therapy, patients with glioma, particularly those with high-grade glioma, persistently experience an unfavorable prognosis. According to a multicentric data, the median overall survival of glioblastoma (GBM) patients is approximately 15 months, with a 5-year survival rate of < 10% [1, 3].
The resected tumor or biopsy tissue allows direct access to genetic information or immunohistochemical biomarkers in glioma. Multiple molecular biomarkers have been identified from the tumor, including isocitrate dehydrogenase 1 and 2 (IDH1/2), codeletion of chromosome arms 1p and 19q (1p/19q codeletion), and O-6-methylguanine-DNA methyltransferase (MGMT), which play important roles in patient stratification, delineation of risk groups, and prognostication of treatment response, among other aspects [4]. Tissue specimens acquired via a highly invasive procedure present substantial clinical risk. Furthermore, the implementation of repeated tumor tissue sampling in clinical practice is deemed entirely impractical. The assessment following treatment is currently predicated exclusively on the consecutive analysis of magnetic resonance imaging (MRI), which is assessed using the modified RANO criteria [5, 6]. Therefore, in contrast to other types of tumors, the incorporation of verified circulating biomarkers into the diagnosis and treatment of glioma has not yet been achieved. MRI serves as a conventional modality for glioma imaging and demonstrates effectiveness; however, it possesses the potential to yield misleading results due to hysteresis and pseudoprogression. The monitoring of early-stage glioma relapse through exclusive reliance on MRI-based detection is challenging. Therefore, there is an urgent need to develop a widely accessible and minimally invasive method for tracking glioma. The monitoring of glioma progression should incorporate the utilization of tumor-based circulating biomarkers as an adjunctive parameter. In certain circumstances, when the likelihood of tumor recurrence is uncertain, the inclusion of supplementary detection would be highly advantageous in facilitating clinical decision-making.
FAP is a membrane protease in cancer-associated stromal fibroblasts and contributes to tumor progression but is absent or insignificant in most normal tissues [7,8,9]. The findings from immunohistochemical analyses conducted on extracranial tumor tissues indicate that elevated FAP expression is indicative of an unfavorable prognosis, suggesting a significant involvement of FAP in tumorigenesis [10]. Histopathological studies revealed that FAP expression was elevated in gliomas, particularly in mesenchymal subtypes [11]. Although FAP has been extensively investigated as a biomarker in various cancer types, there is currently a lack of studies reporting on the longitudinal monitoring of glioma progression using sequential serum FAP.
In the present study, we conducted an investigation to identify the presence of the serum marker FAP and assess its viability as a means of monitoring the progression of glioma. Based on our findings, it can be inferred that the integration of serum autoantibody relative FAP level and MRI examination has the potential to enhance the precision of tumor progression monitoring in a clinical setting.
Materials and methods
Patients
From February 2020 to May 2021, 87 glioma patients (47 males and 40 females, median age 48.2 years, range 18–74 years) were recruited for this study at the Affiliated Tumor Hospital of ** effective blood-based methods for tracking glioma. Though molecular and histological pathology based on tissue samples could provide accurate diagnosis and distinguish tumor markers for prognostic prediction, fluid-based tumor markers provide a minimally invasive approach for monitoring glioma without sampling tumors, despite its heterogeneity and evolution [16, 17]. In the current study, the potential diagnostic value of serum FAP detection as a marker was investigated in conjunction with tumor images. FAP is produced by human cancer-associated fibroblasts (CAFs) in tumors such as glioma. It, a transmembrane serine protease, is highly expressed in many tumors but completely absent in normal tissues [18, 19]. FAP has been identified as an independent biomarker associated with a poor prognosis in a growing number of cancers [20,21,22,23]. The presence of proangiogenic FAP in CAFs has been reported which is consistent with our findings [9, 12, 24].
CAFs cause the accumulation of FAP within tumors, consequently leading to an elevation in the level of FAP in the bloodstream. A group of researchers have identified a notable elevation in FAP levels among patients diagnosed with glioma [25]. According to the literature, the FAP expression in grade 2 gliomas is generally lower than that of patients with grade 3 and grade 4 gliomas, indicating that high-grade gliomas are associated with a high level of FAP expression [26]. To the best of our best knowledge, the utilization of the dynamic serum FAP test as a diagnostic tool for glioma detection has not been documented in existing literature. Due to the limited amount of research conducted on blood FAP for glioma trace, it is imperative to explore the potential of dynamic monitoring of tumor markers for clinical utilization. In comparison to an MRI examination, blood FAP test is less invasive, more accessible, inexpensive, and more convenient. In this regard, it would be extremely interesting for future studies to continuously track gliomas using serum FAP.
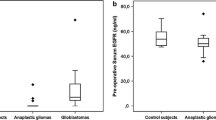
In order to explore the correlation between serum FAP expression and tumor characteristics, we conducted an analysis of serum FAP levels and their association with imaging observations. Our study demonstrates serum FAP levels in preoperative gliomas are significantly higher than those in postoperative patients, suggesting a positive correlation between serum FAP levels and tumor burden. The findings suggest that patients with tumor progression exhibit significantly elevated FAP levels compared to those without recurrent glioma, thereby highlighting the potential of blood tumor markers for glioma as a sensitive tool for early diagnosis. FAP expression in gliomas promotes tumor progression [24], though serum FAP levels vary. FAP-positive cells in immunohistochemical tests are spindle-shaped, fibroblast-like cells, which is consistent with our findings in gliomas [27]. Multiple studies conducted on stromal cells, specifically CAFs, within gliomas have revealed the presence of FAP expression in neoplastic glial cells [28]. In the majority of human solid cancers, the expression of FAP is observed in a selective manner among cancer-associated fibroblasts (CAFs) and pericytes, while tumor cells do not exhibit this expression [29]. The direct observation revealed the presence of prominent FAP staining in fibroblasts surrounding the tumor cells, while minimal or absent expression was observed in adjacent normal tissue. Due to its highly selective distribution in tumors, FAP served as a biomarker of reactive CAFs [29, 30]. Based on our research findings, there exists a positive correlation between the level of serum FAP and both the grade and molecular state of glioma. Multiple studies conducted on different types of cancers have revealed a strong correlation between elevated levels of FAP and the presence of cancer [31, 32]. The observed phenomenon was construed as a systemic reaction to the progression of the tumor [17, 33]. The occurrence of the homologous phenomenon was not documented in glioma.
Following surgical intervention and/or in conjunction with subsequent chemoradiotherapy, the evaluation of the disease predominantly relies on MRI, posing challenges in accurately discerning tumor progression from radiation necrosis within specific timeframes. While tissue biopsies are essential for precise diagnosis and molecular profiling, their limitations lie in their ability to solely capture a fixed moment in time, unable to consistently depict changes in the mutational spectrum, microenvironment, and heterogeneity evolution. The correlation between tumor volume and blood FAP levels suggests potential utility in guiding treatment strategy selection. The promotion of posttreatment glioma invasive growth by FAP suggests the existence of actively proliferating tumor cells [10]. Even if no obvious mass is visible on the MR image, blood FAP of glioma patient may serve as a tumor tracer.
Until now, the utilization of craniocerebral MRI scans has been suggested as a conventional diagnostic approach for post-treatment evaluation of gliomas. The utilization of MRI examination aids in the confirmation of the underlying cause responsible for the upregulation of FAP expression in gliomas. Investigating the origin of FAP detected in blood samples will contribute to a more comprehensive comprehension of its role as a protein biomarker. The dynamic serum FAP effectively addresses the limitations of MRI in differentiating between radiation necrosis and tumor progression. The integration of serial serum FAP test results with neuroimaging enhances the precision of early glioma recurrence detection, underscoring the potential of combining tumor markers with imaging as a viable approach in the clinical diagnosis of glioma. The early detection of glioma recurrence still remains challenging. In the present study, it was observed that serum FAP exhibited a progressive elevation in conjunction with the augmentation of tumor volume. This finding suggests that various cellular components implicated in glioma progression, including parenchymal cells, mesenchymal cells, and endothelial cells, might contribute to the synthesis of this protein.
In the present study, we conducted an analysis to determine the levels of FAP in the serum of patients diagnosed with glioma, and subsequently compared these levels with the assessments of tumor burden obtained through MRI imaging. The results of our study indicate a significant elevation in serum FAP levels as tumor progression occurs, suggesting that serum FAP has potential as a valuable tool for disease monitoring and as a marker for tumor progression. Additionally, our research reveals substantial variations in serum FAP levels among gliomas, with a notable elevation observed in a considerable proportion of high-grade gliomas compared to low-grade gliomas. Concurrently, a notable reduction in serum FAP level was observed in patients who did not experience tumor recurrence subsequent to successful treatment. The serum levels of FAP exhibited a significant increase in the presence of recurrent tumor, whereas the serum levels of FAP displayed fluctuations in accordance with the condition of the tumor. The utilization of longitudinal variations in serum FAP level in our analyses has led to the confirmation that serum FAP is a reliable indicator for evaluating the status of the disease. Another intriguing finding is that serum FAP level has a suggestive role in the molecular pathological subtypes of glioma. However, the association between serum FAP levels and MGMT promoter methylation status appears to be less definitive compared to other molecular statuses such as IDH and 1p/19q. We did not search for a direct link between MGMT status and blood FAP level in the literature. Our study found that serum FAP levels fluctuated in some cases while MRI assessments were stable. This phenomenon may be attributed to the constrained sensitivity of MRI in discerning minute masses within tumor dimensions, thereby requiring additional validation within a more extensive sample group.
Collectively, we conducted an investigation into the potential of serum-derived FAP obtained from patients with glioma to function as a biomarker for the disease. The results of our study unequivocally demonstrate that the dynamic detection of serum FAP serves as a straightforward approach to ascertain treatment response and evaluate tumor status. These findings suggest that serum FAP may be a potentially reliable biomarker for disease monitoring in the context of glioma, which is critical for the timely and accurate assessment of therapeutic effects. The incorporation of serum-derived FAP obtained from glioma patients, in conjunction with MRI evaluation, substantially enhances the precision of disease diagnosis.
Data Availability
The data presented in this study are available on request from the corresponding author.
References
Ostrom QT, Cioffi G, Gittleman H, Patil N, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS Statistical Report: primary brain and other Central Nervous System tumors diagnosed in the United States in 2012–2016. Neuro Oncol. 2019;21(Suppl 5):v1–v100.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Tan AC, Ashley DM, Lopez GY, Malinzak M, Friedman HS, Khasraw M. Management of glioblastoma: state of the art and future directions. CA Cancer J Clin. 2020;70(4):299–312.
Karsy M, Neil JA, Guan J, Mahan MA, Colman H, Jensen RL. A practical review of prognostic correlations of molecular biomarkers in glioblastoma. Neurosurg Focus. 2015;38(3):E4.
Weller M, van den Bent M, Preusser M, Le Rhun E, Tonn JC, Minniti G, Bendszus M, Balana C, Chinot O, Dirven L, et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol. 2021;18(3):170–86.
Weller M, van den Bent M, Tonn JC, Stupp R, Preusser M, Cohen-Jonathan-Moyal E, Henriksson R, Le Rhun E, Balana C, Chinot O, et al. European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 2017;18(6):e315–29.
Rettig WJ, Chesa PG, Beresford HR, Feickert HJ, Jennings MT, Cohen J, Oettgen HF, Old LJ. Differential expression of cell surface antigens and glial fibrillary acidic protein in human astrocytoma subsets. Cancer Res. 1986;46(12 Pt 1):6406–12.
Park JE, Lenter MC, Zimmermann RN, Garin-Chesa P, Old LJ, Rettig WJ. Fibroblast activation protein, a dual specificity serine protease expressed in reactive human Tumor stromal fibroblasts. J Biol Chem. 1999;274(51):36505–12.
Busek P, Balaziova E, Matrasova I, Hilser M, Tomas R, Syrucek M, Zemanova Z, Krepela E, Belacek J, Sedo A. Fibroblast activation protein alpha is expressed by transformed and stromal cells and is associated with mesenchymal features in glioblastoma. Tumour Biol. 2016;37(10):13961–71.
Mentlein R, Hattermann K, Hemion C, Jungbluth AA, Held-Feindt J. Expression and role of the cell surface protease seprase/fibroblast activation protein-α (FAP-α) in astroglial tumors. Biol Chem. 2011;392(3):199–207.
Liu F, Qi L, Liu B, Liu J, Zhang H, Che D, Cao J, Shen J, Geng J, Bi Y, et al. Fibroblast activation protein overexpression and clinical implications in solid tumors: a meta-analysis. PLoS ONE. 2015;10(3):e0116683.
Röhrich M, Loktev A, Wefers AK, Altmann A, Paech D, Adeberg S, Windisch P, Hielscher T, Flechsig P, Floca R, et al. IDH-wildtype glioblastomas and grade III/IV IDH-mutant gliomas show elevated tracer uptake in fibroblast activation protein-specific PET/CT. Eur J Nucl Med Mol Imaging. 2019;46(12):2569–80.
Li M, Li G, Kiyokawa J, Tirmizi Z, Richardson LG, Ning J, Das S, Martuza RL, Stemmer-Rachamimov A, Rabkin SD, et al. Characterization and oncolytic virus targeting of FAP-expressing tumor-associated pericytes in glioblastoma. Acta Neuropathol Commun. 2020;8(1):221.
Pandya DN, Sinha A, Yuan H, Mutkus L, Stumpf K, Marini FC, Wadas TJ. Imaging of fibroblast activation protein alpha expression in a Preclinical Mouse Model of Glioma using Positron Emission Tomography. Molecules 2020, 25(16).
Shi Y, Kong Z, Liu P, Hou G, Wu J, Ma W, Cheng X, Wang Y. Oncogenesis, Microenvironment Modulation and clinical potentiality of FAP in Glioblastoma: lessons learned from other solid tumors. Cells 2021, 10(5).
Quail DF, Joyce JA. Microenvironmental regulation of Tumor progression and Metastasis. Nat Med. 2013;19(11):1423–37.
Persano L, Rampazzo E, Basso G, Viola G. Glioblastoma cancer stem cells: role of the microenvironment and therapeutic targeting. Biochem Pharmacol. 2013;85(5):612–22.
Pure E, Blomberg R. Pro-tumorigenic roles of fibroblast activation protein in cancer: back to the basics. Oncogene. 2018;37(32):4343–57.
Fitzgerald AA, Weiner LM. The role of fibroblast activation protein in health and malignancy. Cancer Metastasis Rev. 2020;39(3):783–803.
Liao Y, Ni Y, He R, Liu W, Du J. Clinical implications of fibroblast activation protein-α in non-small cell Lung cancer after curative resection: a new predictor for prognosis. J Cancer Res Clin Oncol. 2013;139(9):1523–8.
Moreno-Ruiz P, Corvigno S, Te Grootenhuis NC, La Fleur L, Backman M, Strell C, Mezheyeuski A, Hoelzlwimmer G, Klein C, Botling J, et al. Stromal FAP is an Independent poor prognosis marker in non-small cell lung adenocarcinoma and associated with p53 mutation. Lung Cancer. 2021;155:10–9.
Li M, Cheng X, Rong R, Gao Y, Tang X, Chen Y. High expression of fibroblast activation protein (FAP) predicts poor outcome in high-grade serous Ovarian cancer. BMC Cancer. 2020;20(1):1032.
Calvete J, Larrinaga G, Errarte P, Martin AM, Dotor A, Esquinas C, Nunes-Xavier CE, Pulido R, Lopez JI, Angulo JC. The coexpression of fibroblast activation protein (FAP) and basal-type markers (CK 5/6 and CD44) predicts prognosis in high-grade invasive urothelial carcinoma of the bladder. Hum Pathol. 2019;91:61–8.
Balaziova E, Vymola P, Hrabal P, Mateu R, Zubal M, Tomas R, Netuka D, Kramar F, Zemanova Z, Svobodova K et al. Fibroblast Activation Protein Expressing Mesenchymal Cells Promote Glioblastoma Angiogenesis. Cancers (Basel) 2021, 13(13).
Hamson EJ, Keane FM, Tholen S, Schilling O, Gorrell MD. Understanding fibroblast activation protein (FAP): substrates, activities, expression and targeting for cancer therapy. Proteom Clin Appl. 2014;8(5–6):454–63.
Clavreul A, Etcheverry A, Chassevent A, Quillien V, Avril T, Jourdan ML, Michalak S, Francois P, Carre JL, Mosser J, et al. Isolation of a new cell population in the glioblastoma microenvironment. J Neurooncol. 2012;106(3):493–504.
Abbas O, Richards JE, Mahalingam M. Fibroblast-activation protein: a single marker that confidently differentiates morpheaform/infiltrative basal cell carcinoma from desmoplastic trichoepithelioma. Mod Pathol. 2010;23(11):1535–43.
Liu R, Li H, Liu L, Yu J, Ren X. Fibroblast activation protein: a potential therapeutic target in cancer. Cancer Biol Ther. 2012;13(3):123–9.
Solano-Iturri JD, Beitia M, Errarte P, Calvete-Candenas J, Etxezarraga MC, Loizate A, Echevarria E, Badiola I, Larrinaga G. Altered expression of fibroblast activation protein-α (FAP) in colorectal adenoma-carcinoma sequence and in lymph node and liver metastases. Aging. 2020;12(11):10337–58.
Miao Y, Deng Y, Liu J, Wang J, Hu B, Hao S, Wang H, Zhang Z, ** Z, Zhang Y, et al. Anti-cancer effect of targeting fibroblast activation protein alpha in glioblastoma through remodeling macrophage phenotype and suppressing Tumor progression. CNS Neurosci Ther. 2023;29(3):878–92.
Solano-Iturri JD, Errarte P, Etxezarraga MC, Echevarria E, Angulo J, López JI, Larrinaga G. Altered tissue and plasma levels of fibroblast activation Protein-α (FAP) in renal tumours. Cancers 2020, 12(11).
Lee KN, Jackson KW, Christiansen VJ, Lee CS, Chun J-G, McKee PA. Antiplasmin-cleaving enzyme is a soluble form of fibroblast activation protein. Blood. 2006;107(4):1397–404.
Liao Y, **ng S, Xu B, Liu W, Zhang G. Evaluation of the circulating level of fibroblast activation protein α for diagnosis of esophageal squamous cell carcinoma. Oncotarget. 2017;8(18):30050–62.
Acknowledgements
Not applicable.
Funding
This study was funded by Natural Science Foundation of Guangdong Province, grant number 2022A1515012539.
Author information
Authors and Affiliations
Contributions
XSY, PFC, ZQZ, JZ; Writing—original draft preparation: PZ; Formal analysis: RXX; Investigation: HL, XMC; Methodology: XMP, JQL; Data curation: HBL; Validation: QYY; Supervision, project administration, funding acquisition and writing—review and editing: JZ; All authors approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical approval and consent to participate
The study was conducted according to the guidelines of the Declaration of Helsinki and was approved by the Institutional research ethics board of Sun Yat-Sen University Cancer Center (SL-B2022-567-01). Written informed consent has been obtained from the patients or their family numbers to publish this paper.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yang, Xs., zhu, P., **e, RX. et al. Tracking tumor alteration in glioma through serum fibroblast activation protein combined with image. BMC Cancer 23, 1012 (2023). https://doi.org/10.1186/s12885-023-11544-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-11544-4