Abstract
Background
Nomogram is a graphic representation containing the expressed factor of the mathematical formula used to define a particular phenomenon. We aim to build and internally validate a nomogram to predict overall survival (OS) in patients diagnosed with lung cancer (LC).
Methods
We included 1200 LC patients from a single institution registry diagnosed from 2013 to 2021. The independent prognostic factors of LC patients were identified via cox proportional hazard regression analysis. Based on the results of multivariate cox analysis, we constructed the nomogram to predict the OS of LC patients.
Results
We finally included a total of 1104 LC patients. Age, medical urgency at diagnosis, performance status, radiotherapy, and surgery were identified as prognostic factors, and integrated to build the nomogram. The model performance in predicting prognosis was measured by receiver operating characteristic curve. Calibration plots of 6-, 12-, and 24- months OS showed optimal agreement between observations and model predictions.
Conclusion
We have developed and validated a unique predictive tool that can offer patients with LC an individual OS prognosis. This useful prognostic model could aid doctors in making decisions and planning therapeutic trials.
Similar content being viewed by others
Background
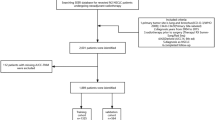
Lung cancer (LC) remains the most lethal type of cancer worldwide and in Morocco as well, accounting for 85% of all diagnosed Non-Small Cell Lung Cancer (NSCLC) and 13%-14% of Small Cell Lung Cancer (SCLC), with 1% of other histology types [1]. Growing evidence suggests that smoking is the major risk factor related to lung cancer, causing deregulated molecular pathways and or a specific type of mutations in a specific genome.
For early LC stages, including stage I, II, and III, the standard curative treatment is chemotherapy in association with radiotherapy, and if indicated, the patient may undergo local or radical resection. Patients with non-metastatic LC are categorized on the basis of tumor size, and invasion as well as the level of lymph node involvement, according to the eighth edition of the American Joint Committee on Cancer TNM classification [2]. Patients with the same stage of cancer have a wide range of survival rates. It is thought that various stages of LC are influenced by different prognostic factors such as age, smoking status, gender, histological type, invasion of tumor size, nodal status, and treatment-related factors, all of which could significantly play a role in individualized prediction survival [3, 4]. Indeed, because evidence suggests that tumor size and N stage are strongly related to the biological characteristic of the tumor, and because they are based on tumor depth invasion, they remain the most important tumor characteristics, and are therefore considered a robust risk factor for LC survival [5,6,7, A single-institution registry consisting of 1200 patients has been diagnosed with Lung Cancer between January 2013 and December 2021 from the Medical Oncology Department of the Mohammed VI University Hospital of Marrakesh, Morocco was established. To retrieve all essential data, standardized LC patients’ confirmed pathological characteristics including Age at diagnosis, Gender, Tabaco status, Cannabis status, Alcohol, Body Weight, Performance Status; Presence or absence of urgency at diagnosis including Superior Vena Cava Syndrome (SVCS) or Pleurisy syndrome; Comorbidities; Clinic-pathologic data including Pathological T, N, M categories, presence or absence of Liver Metastasis, Adrenal Metastasis, Bone Metastasis, and Brain Metastasis, Stage at diagnosis; EGFR, ALK, PDL-1; treatment-related data including Surgery, Chemotherapy, Radiotherapy; hematological toxicities reported during treatment including Anemia, Neutropenia, Thrombocytopenia; form was established. All patients' follow-up information was extracted from their most recent medical review, which included a clinical examination and/or a review of computed tomography images. The eighth edition of the American Joint Committee on Cancer TNM classification system was used to determine pathological staging. Age and weight, as continuous variables, were transformed into a categorical variables based on quartiles. Weight is defined as body mass at diagnosis and is reflected by the unit of kilograms (Kg). We define tobacco consumption as smoking cigarettes, whereas smoking marijuana is the definition of cannabis consumption. Due to the retrospective nature of the study, the exact quantity is not mentioned in all patient medical records, thus we could not define either patient is a heavy or light smoker. Variables with more than 20% of missing values were excluded. In addition, patients were also excluded from the subsequent analysis if they missing important detail information such date of biopsy or survival date, information on treatments such as radiotherapy, chemotherapy, or surgery. Finally, 1104 eligible identified LC cases were selected for the study. The main objective element of this paper was OS, which was defined as the interval time between the biopsy day to death without specific cause. All LC patients were randomly assigned (n=730) for training and (n=374) for validation cohorts with a 2:1 ratio. Categorical variables were expressed as percentages. In the training cohort, a univariate cox analysis was performed to determine the variables related to prognosis. Then, the independent prognostic variables related to the OS of LC patients were determined using multivariate cox analysis, where only factors with a p-value less than 0.05 are considered statistically significant and were therefore incorporated to develop the nomogram. Due to the necessity to test the reliability of the model, four key elements were established to assess the results performance of prediction probabilities for 6, 12, and 24 months. First, a 300 bootstrap resampling method was adopted to internally validate the nomogram. Second, the calibration curve was plotted to compare the consistency of projected clinical responses probability versus actual response proportion, which should be close to 45 degrees. Third, the area under the time-dependent receiver operating characteristic (ROC) was adopted to assess the discrimination. Fourth, the C-Index was used to judge the model’s prediction accuracy, given the closer C-index to value 1, the greater precision is [16]. Survival curves for sex, age at diagnosis, medical urgency at diagnosis, PS, radiotherapy, and surgery values were generated using the Kaplan-Meier estimates. The log-rank test was adopted to compare the subgroups of these variables, as reflected by the p-value; the smaller the p-value, the greater the difference. All statistical analyses to identify the independent prognostic factors and to build the model were performed using R-software version 4.1.3. Available from: http://www.r-project.org) with “survival”, survminer”, and “rms” [17] packages. Based on selected criteria, the 1104 enrolled LC patients’ characteristics cases, divided into training (n=730) and validation (n=374) cohorts, are summarized in Table 1. We should note the significant absence of differences among these cohorts. In the training set, the vast majority of patients were male (n=654), diagnosed above 66 years old, and most of them died during treatment. Meanwhile, in terms of tumor characteristics, LC patients were often diagnosed at advanced T4, and N2 stages, M1b and (27.3%) with bone metastasis, followed by brain, adrenal, and liver metastasis at diagnosis. Most of the patients were diagnosed at late stage IVA ( n = 448, 61,4%) and IVB (n = 212, 29%). Moreover, adenocarcinoma was the most appearing histological type (49.1%), and SVCS (5.3%) was the most present urgency at diagnosis. As for treatment, most of patients had not received radiation therapy (86.6%), and surgery (95.2%), but most of them received chemotherapy (57.1%). Regarding hematological toxicities reported during treatment, most patients did not report anemia, neutropenia, or thrombocytopenia with (21.3%), (35.5%), and (39.9%), respectively. Figure 1 presents the differences in survival between the subgroups, involving radiotherapy, age at diagnosis, urgency at diagnosis, and surgery. The median OS for the entire cohort was 934 (95% CI: 634, 1176) days. In total, 291 deaths were registered. The following variables have been subjected to univariate Cox analysis (UNCA): sex, age, Tabaco smoking, cannabis smoking, alcohol, comorbidities, histology type, T stage, N stage, M stage, liver metastasis, adrenal metastasis, bone metastasis, brain metastasis, medical urgency at diagnosis, PS, weight, chemotherapy, radiotherapy, surgery, anemia, neutropenia, and thrombocytopenia. The results of UVCA showed that age, comorbidities, M stage, brain metastasis, medical urgency at diagnosis, PS, weight, radiotherapy, chemotherapy, surgery, and anemia were prognostic factors for LC patients (Table 2). These UVCA results were subsequently interred in a multivariate Cox analysis (MVCA). Finally, 5 factors were identified as independent prognostic ones including: age, medical urgency at diagnosis, PS, radiotherapy, and surgery. The independent prognostic factors derived from the MVCA were used to build the nomogram to predict the OS for LC patients (Fig. 2). As shown in Fig. 2, performance status and medical urgency at diagnosis have the greatest contribution to prognosis, followed by radiotherapy, and surgery with the same moderate impact on prognosis, while age at diagnosis has the minimal effect on prognosis. Each variable subtype assigned a score on the point scale. We were easily able to draw a straight line down to determine the expected likelihood of survival at each time point by adding up the total score and projecting it onto the total point scale. The ROC plots showed that the AUC of the clinical predictive model for 6-, 12-, and 24- months OS scored 0.97, 0.93, 0.92 in the training set, and 0.91, 0.91, 0.81, in the validation set respectively, demonstrating a better discriminative ability (Fig. 3). Furthermore, the calibration plots for 6-, 12-, and 24- months OS showed an excellent agreement in both, the primary and validation cohorts between observed probabilities and nomogram predicted probabilities (Fig. 4). Stratification into different subgroups demonstrates a distinction between Kaplan-Meier curves for LC patients’ prognosis. Due to the heterogeneity related to individual LC patients [18], predicting survival using demographic, clinic, biologic, and pathologic characteristics is imprecise. Several prognostic models have been developed and discussed based on a specific cohort and outcome, but no nomogram has been constructed based on a purely well-defined African cohort. Thus, we sought to establish a convenient predictive model based on 1104 enrolled cases with 5 independent prognostic factors identified by Cox regression analysis to predict 6-, 12-, and 24- months OS of LC patients. The data were extracted and collected manually from the registry of a single public institution. This institution is the only leading public medical center representing central and southern Morocco, and contains all the standard technical care accepted in the kingdom. We found, in this research, through a subsequent multivariate Cox analysis that age, medical urgency at diagnosis, performance status, surgery, and radiotherapy were the prognostic factors related to progression, which were consistence with previously reported results [19,20,21,22,23,24,25,26,27]. The integration of various clinical, pathological, and biological characteristics related to each patient into a mathematical model could be holistic in terms of probability prediction based on the primary outcome [28, 29]. Differences in median OS depend on the population studied, the stage diagnosed and the treatments received. In our case, the median OS obtained was 934 (95% CI: 694, 1176) days. Based on German data, Hardtstock et al. [30] found that the median OS of NSCLC patients was 351 days. Meanwhile, David et al. [31] found that the median OS for LC patients who had undergone surgery was 9.1 months and 4.2 months for those who had not. Depending on age group stratification; Wu et al. [32] and Torre et al. [33] proved that patients diagnosed over 60 years of age were more likely to be associated with worse survival, which is somewhat contradictory to our results, as the division of age into categories was based on quartile and not risk group stratification. We should note that not all LC patients can benefit from surgery [34], but the majority of those who do, have undergone radiotherapy, and chemotherapy [35]. Interestingly, however, chemotherapy is not found to be an independent prognostic factor (p = 0.8) indicating its little effect on prognosis. For the past 30 years, and based on natural compounds, chemotherapy has been considered as an essential therapy for appropriate LC patients [36], with no proven benefits when is used alone or in patients with fourth stage of the disease, but it may adduce benefits when used in concomitant with radiotherapy, surgery, [37] - and targeted therapy. Performance status (PS), as a subjective composite to evaluate the patient’s wellness, is a key factor reflecting the patient’s ability to carry on normal activities. Several previous studies have reported the role of PS as a prognostic signature impacting the survival rate in different age categories [38,39,40,41]. Regarding medical urgency associated with late diagnosis of advanced disease, we found that SVCS, as well as pleurisy syndrome, were all associated with poor survival in patients at the different stage categories of the disease. In a retrospective study conducted by Fahem et al, [42] they concluded that SVCS was a predictive factor for mortality in broncho-pulmonary cancer in addition to pleurisy syndrome. Furthermore, pleurisy syndrome had also an impact on survival when it was associated or developed as a sign of non-response to treatment or simply progression. Even though literature recognizes the importance of the histological type signature in terms of disease prognostication and impacting survival, [43, 44] we did not find any convergence to with the literature when differentiating the disease categories by dividing into epidermoid cancinoma, neuroendocrine carcinoma, adenocarcinoma, adenosquamous carcinoma, and small cell carcinoma, and taking adenocarcinoma as the reference. Based on the IASLC paper, which indicates among all the histological subtypes of LC, adenocarcinoma remains the more favorable prognostic predictor than the other subtypes [45]. Furthermore, several studies have found, based on different types of analyses, depending on the objective element of the study, that histology type is an independent prognostic predictor and have therefore been integrated to construct the nomogram [35, 46]. We decided to exclude both clinical M category, and comorbidity variables from subsequent MVCA because they would have a bad impact on the total assigned model by being biased, even if they were significant in the results and had been declared independent prognostic factors. We did not add stage at diagnosis into the Cox analysis for the straightforward reason that stage is mirrored by the combination of T, N, and M categories, and when it is included in the analysis, it results in a substantial bias in the model without any relevance due to information redundancy. To the best of our knowledge, this is the first nomogram for predicting survival for patients diagnosed with LC based on a North African cohort and long-term follow-up, reflecting the characteristics of the African population in terms of disease response and survival. However, the creation of clinical prediction models is more significant for enhancing patient prognosis when compared to the analysis of independent risk factors. More importantly, all of the indicators used in this study can be acquired and determined clinically. As a result, the model has improved prediction capabilities and increased dependability, making it a useful tool for clinical decision-making, risk assessment, and patient consultation. This scoring system should make it easier for doctors to deal with these problems. Additionally, this tool might offer data for patient categorization in clinical research design, thereby improving comparability between study arms. Compared to the TNM staging system and certain previous prognostic models, we believe the developed nomogram provides more accurate results. We should note that this study contains certain limitations. First, this tool needs to be externally validated by an African cohort to make sure the prognostic factors are the same across the continent. Second, due to lack of access to emerging technologies, some molecular aberrations such as EGFR mutation, ALK-EML4 fusion, PDL-1, ROS1, mTOR, are not included in the study as they are not routinely requested until the end of thelast year (2021). Third, our model is still limited by the nature of retrospective data and inability to extract convenient parameters such as vascular invasion, perineural invasion, and lymphatic permeation. Fourth, the patient’s medical records do not contain information on systemic treatments, including type of surgery and radiation dose. To enhance this model, extra work should be done on prospective data gathering, patient follow-up, expanding the recruitment area, and inclusion of additional variables. In conclusion, we built a clinical prediction model to determine each LC patient’s unique prognosis. With this tool, clinicians can more precisely predict individual patient survival rates, and treatments strategy. We seek to further develop personalized treatment by conducting quantitative analysis of prognostic-related parameters.Methods
Patients’ selection and data elements
Statistical analysis
Results
Patients’ characteristics
Survival analysis
Independent prognostic factors
Prognostic nomogram for OS
Evaluation of nomogram
Discussion
Conclusion
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- OS:
-
Overall Survival
- LC:
-
Lung Cancer
- NSCLC:
-
Non-Small Cell Lung Cancer
- SCLC:
-
Small Cell Lung Cancer
- UVCA:
-
Univariate Cox Analysis
- MVCA:
-
Multivariate Cox Analysis
- SVCS:
-
Superior Vena Cava Syndrome
- AUC:
-
Area Under Curve
- ROC:
-
Receiver Operating Characteristic
References
Zheng D, Wang Y, Li Y, Sun Y, Chen H. Predicting prognosis of post-chemotherapy patients with resected IIIA non-small cell lung cancer. J Thorac Dis. 2018;10(7):4186–94. Available from: http://jtd.amegroups.com/article/view/22525/17441. cited 2022 Nov 9.
Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WEE, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Grou**s in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol. 2016;11(1):39–51. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1556086415000179. cited 2022 Nov 9.
Chansky K, Sculier JP, Crowley JJ, Giroux D, Van Meerbeeck J, Goldstraw P. The International Association for the Study of Lung Cancer Staging Project: Prognostic Factors and Pathologic TNM Stage in Surgically Managed Non-small Cell Lung Cancer. J Thorac Oncol. 2009;4(7):792–801.
Kawaguchi T, Takada M, Kubo A, Matsumura A, Fukai S, Tamura A, et al. Performance Status and Smoking Status Are Independent Favorable Prognostic Factors for Survival in Non-small Cell Lung Cancer: A Comprehensive Analysis of 26,957 Patients with NSCLC. J Thorac Oncol. 2010;5(5):620–30. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1556086415321353. cited 2022 Nov 9.
Mao A, Zhou X, Liu Y, Ding J, Miao A, Pan G. KLF8 is associated with poor prognosis and regulates glycolysis by targeting GLUT4 in gastric cancer. J Cell Mol Med. 2019;23(8):5087–97. Available from: https://onlinelibrary.wiley.com/doi/10.1111/jcmm.14378. cited 2022 Nov 9.
Sun L, Chen G, Sun A, Wang Z, Huang H, Gao Z, et al. BAG2 Promotes Proliferation and Metastasis of Gastric Cancer via ERK1/2 Signaling and Partially Regulated by miR186. Front Oncol. 2020;10:31. Available from: https://www.frontiersin.org/article/10.3389/fonc.2020.00031/full. cited 2022 Nov 9.
Wang WJ, Guo CA, Li R, Xu ZP, Yu JP, Ye Y, et al. Long non-coding RNA CASC19 is associated with the progression and prognosis of advanced gastric cancer. Aging. 2019;11(15):5829–47. Available from: https://www.aging-us.com/lookup/doi/10.18632/aging.102190. cited 2022 Nov 9.
**e J, Pang Y, Li X, Wu X. The log odds of negative lymph nodes/T stage: a new prognostic and predictive tool for resected gastric cancer patients. J Cancer Res Clin Oncol. 2021;147(8):2259–69. Available from: https://springer.longhoe.net/10.1007/s00432-021-03654-y. cited 2022 Nov 9.
Valentini V, van Stiphout RGPM, Lammering G, Gambacorta MA, Barba MC, Bebenek M, et al. Nomograms for Predicting Local Recurrence, Distant Metastases, and Overall Survival for Patients With Locally Advanced Rectal Cancer on the Basis of European Randomized Clinical Trials. J Clin Oncol. 2011;29(23):3163–72. Available from: https://ascopubs.org/doi/10.1200/JCO.2010.33.1595.
Chen QY, Hong ZL, Zhong Q, Liu ZY, Huang XB, Que SJ, et al. Nomograms for pre- and postoperative prediction of long-term survival among proximal gastric cancer patients: A large-scale, single-center retrospective study. World J Clin Cases. 2019;7(21):3419–35. Available from: https://www.wjgnet.com/2307-8960/full/v7/i21/3419.htm. cited 2022 Nov 9.
Karakiewicz PI, Briganti A, Chun FKH, Trinh QD, Perrotte P, Ficarra V, et al. Multi-Institutional Validation of a New Renal Cancer-Specific Survival Nomogram. J Clin Oncol. 2007;25(11):1316–22. Available from: https://ascopubs.org/doi/10.1200/JCO.2006.06.1218. cited 2022 Nov 9.
Thomlinson RH, Gray LH. The Histological Structure of Some Human Lung Cancers and the Possible Implications for Radiotherapy. Br J Cancer. 1955;9(4):539–49. Available from: http://www.nature.com/articles/bjc195555. cited 2022 Nov 9.
Wang Y, Li J, **a Y, Gong R, Wang K, Yan Z, et al. Prognostic Nomogram for Intrahepatic Cholangiocarcinoma After Partial Hepatectomy. J Clin Oncol. 2013;31(9):1188–95. Available from: https://ascopubs.org/doi/10.1200/JCO.2012.41.5984. cited 2022 Nov 10.
Yang G, Luo B, Yang Q, Chen M, Yang X, Sun J. Construction of nomograms for nasopharyngeal carcinoma containing primary tumor size and SEER stage. Transl Cancer Res. 2020;9(11):6939–54. Available from: http://tcr.amegroups.com/article/view/46267/html. cited 2022 Nov 10.
Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16(4):e173-80. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1470204514711167. cited 2022 Nov 10.
Harrell FE Jr. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. Springer Series in Statistics. New York: Springer; 2001.
Frank E, Harrell Jr. Rms: Regression Modeling Strategies. Available from: http://www.r-project.org/.
Nesbitt JC, Putnam JB, Walsh GL, Roth JA, Mountain CF. Survival in early-stage non-small cell lung cancer. Ann Thorac Surg. 1995;60(2):466–72. Available from: https://linkinghub.elsevier.com/retrieve/pii/000349759500169L. cited 2022 Nov 30.
Adam AK, Soubani AO. Outcome and prognostic factors of lung cancer patients admitted to the medical intensive care unit. Eur Respir J. 2008;31(1):47–53. Available from: http://erj.ersjournals.com/cgi/doi/10.1183/09031936.00031607. cited 2022 Nov 30.
Chansky K, Sculier JP, Crowley JJ, Giroux D, Van Meerbeeck J, Goldstraw P. The International Association for the Study of Lung Cancer Staging Project: Prognostic Factors and Pathologic TNM Stage in Surgically Managed Non-small Cell Lung Cancer. J Thorac Oncol. 2009;4(7):792–801. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1556086415324163. cited 2022 Nov 30.
Paesmans M. Prognostic and predictive factors for lung cancer. Breathe. 2012;9(2):112–21. Available from: http://breathe.ersjournals.com/lookup/doi/10.1183/20734735.006911. cited 2022 Nov 30.
Firat S, Bousamra M, Gore E, Byhardt RW. Comorbidity and KPS are independent prognostic factors in stage I non-small-cell lung cancer. Int J Radiat Oncol. 2002;52(4):1047–57. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0360301601027419. cited 2022 Nov 30.
Nakahashi H, Yasumoto K, Ishida T, Nagashima A, Nishino T, Oka T, et al. Results of Surgical Treatment of Patients with T3 Non—Small Cell Lung Cancer. Ann Thorac Surg. 1988;46(2):178–81. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0003497510658924. cited 2022 Nov 30.
Hirayama N, Tabata C, Tabata R, Maeda R, Yasumitsu A, Yamada S, et al. Pleural effusion VEGF levels as a prognostic factor of malignant pleural mesothelioma. Respir Med. 2011;105(1):137–42. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0954611110004373. cited 2022 Nov 30.
Hata A, Suzuki H, Nakajima T, Tanaka K, Fujiwara T, Wada H, et al. Concomitant Interstitial Lung Disease Is a Risk Factor for Pleural Invasion in Lung Cancer. Ann Thorac Surg. 2017;103(3):967–74. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0003497516311420. cited 2022 Nov 30.
Liu Y, Bai YP, Zhou ZF, Jiang CR, Xu Z, Fan XX. Preoperative anemia as a prognostic factor in patients with lung cancer: a systematic review and meta-analysis of epidemiological studies. J Cancer. 2019;10(9):2047–56. Available from: http://www.jcancer.org/v10p2047.htm. cited 2022 Nov 30.
Chen C, Song Z, Wang W, Zhou J. Baseline anemia and anemia grade are independent prognostic factors for stage IV non-small cell lung cancer. Mol Clin Oncol. 2021;14(3):59. Available from: http://www.spandidos-publications.com/10.3892/mco.2021.2221. cited 2022 Nov 30.
Wang ZX, Qiu MZ, Jiang YM, Zhou ZW, Li GX, Xu RH. Comparison of prognostic nomograms based on different nodal staging systems in patients with resected gastric cancer. J Cancer. 2017;8(6):950–8. Available from: http://www.jcancer.org/v08p0950.htm. cited 2022 Dec 1.
Qu K, Dai L, Wang W, Liu Q, **a T, Wang Q, et al. Development and validation of prognostic nomogram for lung cancer patients below the age of 45 years. Bosn J Basic Med Sci. 2020 Oct 14 [cited 2022 Dec 1]; Available from: https://www.bjbms.org/ojs/index.php/bjbms/article/view/5079.
Hardtstock F, Myers D, Li T, Cizova D, Maywald U, Wilke T, et al. Real-world treatment and survival of patients with advanced non-small cell lung Cancer: a German retrospective data analysis. BMC Cancer. 2020;20(1):260. Available from: https://bmccancer.biomedcentral.com/articles/10.1186/s12885-020-06738-z. cited 2022 Dec 1.
David EA, Andersen SW, Beckett LA, Melnikow J, Kelly K, Cooke DT, et al. A Model to Predict the Use of Surgical Resection for Advanced-Stage Non-Small Cell Lung Cancer Patients. Ann Thorac Surg. 2017;104(5):1665–72. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0003497517308172. cited 2022 Dec 1.
Wu CY, Fu JY, Wu CF, Hsieh MJ, Liu YH, Wu YC, et al. Survival Prediction Model Using Clinico-Pathologic Characteristics for Nonsmall Cell Lung Cancer Patients After Curative Resection. Medicine (Baltimore). 2015;94(45):e2013. Available from: https://journals.lww.com/00005792-201511110-00053. cited 2022 Dec 3.
Torre LA, Siegel RL, Jemal A. Lung Cancer Statistics. In: Ahmad A, Gadgeel S, editors. Lung Cancer and Personalized Medicine. Cham: Springer International Publishing; 2016 [cited 2022 Dec 3]. 1–19. (Advances in Experimental Medicine and Biology; vol. 893). Available from: http://springer.longhoe.net/https://doi.org/10.1007/978-3-319-24223-1_1.
Zhu S, Ge T, Hu J, Jiang G, Zhang P. Prognostic value of surgical intervention in advanced lung adenocarcinoma: a population-based study. J Thorac Dis. 2021;13(10):5942–53. Available from: https://jtd.amegroups.com/article/view/56295/html. cited 2022 Dec 3.
Huang Z, Hu C, Tong Y, Fan Z, Zhao C. Construction of a nomogram to predict the prognosis of non-small-cell lung cancer with brain metastases. Medicine (Baltimore). 2020;99(31):e21339. Available from: https://journals.lww.com/10.1097/MD.0000000000021339. cited 2022 Dec 3.
Salloum RG, Smith TJ, Jensen GA, Lafata JE. Using claims-based measures to predict performance status score in patients with lung cancer. Cancer. 2011;117(5):1038–48. Available from: https://onlinelibrary.wiley.com/doi/10.1002/cncr.25677. cited 2022 Dec 4.
Huang CY, Ju DT, Chang CF, Muralidhar Reddy P, Velmurugan BK. A review on the effects of current chemotherapy drugs and natural agents in treating non–small cell lung cancer. BioMedicine. 2017;7(4):23. Available from: http://biomedicine.edp-open.org/10.1051/bmdcn/2017070423. cited 2022 Dec 3.
Andreano A, Russo AG. Administrative healthcare data to predict performance status in lung cancer patients. Data Brief. 2021;39:107559. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2352340921008350. cited 2022 Dec 4.
Friedlaender A, Liu SV, Passaro A, Metro G, Banna G, Addeo A. The Role of Performance Status in Small-Cell Lung Cancer in the Era of Immune Checkpoint Inhibitors. Clin Lung Cancer. 2020;21(6):e539-43. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1525730420301054. cited 2022 Dec 4.
Jang RW, Caraiscos VB, Swami N, Banerjee S, Mak E, Kaya E, et al. Simple Prognostic Model for Patients With Advanced Cancer Based on Performance Status. J Oncol Pract. 2014;10(5):e335-41. Available from: https://ascopubs.org/doi/10.1200/JOP.2014.001457. cited 2022 Dec 4.
Paakkola NM, Lindqvist J, Jekunen A, Sihvo E, Johansson M, Andersén H. Impact of sex and age on adherence to guidelines in non-small cell lung cancer management. Cancer Treat Res Commun. 2023;34:100675. Available from: https://www.sciencedirect.com/science/article/pii/S2468294222001666. cited 2023 Jan 27.
Fahem N, Migaou A, Ben Saad A, Jobeur S, Cheikh Mhammed S, Rouatbi N. Superior vena cava syndrome and lung cancer: survival and prognostic factors. J Lung Pulm Respir Res. 2019;6(4):81–5. Available from: https://medcraveonline.com/JLPRR/superior-vena-cava-syndrome-and-lung-cancer-survival-and-prognostic-factors.html cited 2022 Dec 5.
Vincent RG, Pickren JW, Lane WW, Bross I, Takita H, Houten L, et al. The changing histopathology of lung cancer. A review of 1682 cases. Cancer. 1977;39(4):1647–55.Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/1097-0142%28197704%2939%3A4%3C1647%3A%3AAID-CNCR2820390439%3E3.0.CO%3B2-H. cited 2023 Jan 27.
Araghi M, Fidler-Benaoudia M, Arnold M, Rutherford M, Bardot A, Ferlay J, et al. International differences in lung cancer survival by sex, histological type and stage at diagnosis: an ICBP SURVMARK-2 Study. Thorax. 2022;77(4):378–90. Available from: https://thorax.bmj.com/content/77/4/378. cited 2023 Jan 27.
Sculier JP, Chansky K, Crowley JJ, Van Meerbeeck J, Goldstraw P. The Impact of Additional Prognostic Factors on Survival and their Relationship with the Anatomical Extent of Disease Expressed by the 6th Edition of the TNM Classification of Malignant Tumors and the Proposals for the 7th Edition. J Thorac Oncol. 2008;3(5):457–66. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1556086415314532. cited 2022 Dec 13.
Liang W, Zhang L, Jiang G, Wang Q, Liu L, Liu D, et al. Development and Validation of a Nomogram for Predicting Survival in Patients With Resected Non–Small-Cell Lung Cancer. J Clin Oncol. 2015;33(8):861–9. Available from: https://ascopubs.org/doi/10.1200/JCO.2014.56.6661. cited 2023 Jan 27.
Acknowledgements
Not applicable.
Funding
None.
Author information
Authors and Affiliations
Contributions
Conception and Design: Hassan Abdelilah Tafenzi, Rhizlane Belbaraka, Ismail Essaadi. Statistical Analysis: Hassan Abdelilah Tafenzi. Data Interpretation: All Authors. Financial Support: Hassan Abdelilah Tafenzi, Rhizlane Belbaraka, Ismail Essaadi. Administrative Support: Bioscience and Health Laboratory, Faculty of Medicine and Pharmacy, Cadi Ayyad Unicersity, Marrakech, Morocco & Medical Oncology Department, Mohammed VI University Hospital, Marrakech, Morocco. Provision of Study Materials or patients: Hassan Abdelilah Tafenzi, Farah Choulli, Ganio Adjade, Anas Baladi. Drafting: Hassan Abdelilah Tafenzi. Review, Revise, and Approve the Manuscript: All Authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Marrakech Faculty of Medicine and Pharmacy's Ethical Review Committee gave its approval for the study. It was not necessary to obtain the patients' informed permission. Prior to analysis, patient records were anonymized to ensure confidentiality. All methods were performed in accordance with the relevant guidelines and regulations. The need for written informed consent was waived by The Marrakech University Hospital Ethics Committee due to retrospective nature of the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Tafenzi, H.A., Choulli, F., Adjade, G. et al. Development of a well-defined tool to predict the overall survival in lung cancer patients: an African based cohort. BMC Cancer 23, 1016 (2023). https://doi.org/10.1186/s12885-023-11355-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-11355-7