Abstract
Background
To investigate the effects of two different exercise interventions during pregnancy on gestational weight gain (GWG) and obstetric and neonatal outcomes compared to standard care. Additionally, we aimed to improve standardization of GWG measurements by develo** a model to estimate GWG for a standardized pregnancy period of 40 weeks and 0 days accounting for individual differences in gestational age (GA) at delivery.
Methods
In a randomized controlled trial we compared the effects of structured supervised exercise training (EXE) three times per week throughout pregnancy versus motivational counselling on physical activity (MOT) seven times during pregnancy with standard care (CON) on GWG and obstetric and neonatal outcomes. Uniquely, to estimate GWG for a standardized pregnancy period, we developed a novel model to predict GWG based on longitudinally observed body weights during pregnancy and at admission for delivery. Observed weights were fitted to a mixed effects model that was used to predict maternal body weight and estimate GWG at different gestational ages. Obstetric and neonatal outcomes, among them gestational diabetes mellitus (GDM) and birth weight, were obtained after delivery. GWG and the investigated obstetric and neonatal outcomes are secondary outcomes of the randomized controlled trial, which might be underpowered to detect intervention effects on these outcomes.
Results
From 2018–2020, 219 healthy, inactive pregnant women with median pre-pregnancy BMI of 24.1 (21.8–28.7) kg/m2 were included at median GA 12.9 (9.4–13.9) weeks and randomized to EXE (n = 87), MOT (n = 87) or CON (n = 45). In total 178 (81%) completed the study. GWG at GA 40 weeks and 0 days did not differ between groups (CON: 14.9 kg [95% CI, 13.6;16.1]; EXE: 15.7 kg [14.7;16.7]; MOT: 15.0 kg [13.6;16.4], p = 0.538), neither did obstetric nor neonatal outcomes. For example, there were no differences between groups in the proportions of participants develo** GDM (CON: 6%, EXE: 7%, MOT: 7%, p = 1.000) or in birth weight (CON: 3630 (3024–3899), EXE: 3768 (3410–4069), MOT: 3665 (3266–3880), p = 0.083).
Conclusions
Neither structured supervised exercise training nor motivational counselling on physical activity during pregnancy affected GWG or obstetric and neonatal outcomes compared to standard care.
Trial registration
ClinicalTrials.gov; NCT03679130; 20/09/2018.
Similar content being viewed by others
Background
It has been suggested that prenatal maternal exercise reduces gestational weight gain (GWG) [1,2,3,4,5] and incidence of other pregnancy and delivery-related complications, including gestational diabetes mellitus (GDM), preeclampsia, gestational hypertension, preterm delivery, caesarean section and odds of instrumental delivery [4, 6,7,8]. Further, prenatal exercise can reduce duration of labor in some pregnant populations [9] and has been associated with optimization of offspring birth weight into a healthy range [7, 10, 11].
Yet, it remains to be investigated which maternal exercise approaches are most effective for improving health of the mother and her offspring [6]. Structured supervised exercise training and motivational counselling on physical activity constitute two exercise interventions widely used [3, 12]. Both approaches have been applied separately in pregnant women with normal weight [13,14,15,16,17,18,19] and overweight or obesity [20,21,22,23,24,25,26,27], but a direct comparison of the effectiveness on improving GWG and obstetric and neonatal outcomes has not been conducted.
The Institute of Medicine has presented ideal and practical methods for measurement and acquisition of body weight data required to determine GWG [28]. Ideally, body weights used to calculate GWG should be pre-pregnancy weight measured at a preconceptional visit and the last measured available weight abstracted from clinical records (ideally at delivery). If not practically feasible to measure maternal weight before conception and at delivery, pre-pregnancy weight and last available weight can be recalled (self-reported) by the women as soon as possible, for example at the first prenatal visit and after delivery, using standardized questions [28]. However, such data will most likely be less precise than objectively measured weights during hospital visits. For practical reasons, most studies calculate GWG based on a weight measured at the last pregnancy visit to the clinic [2, 3, 29], which may vary significantly both between women within a study and between studies, and only few studies have measured weight at delivery and hence reported GWG for the entire pregnancy period [30, 31]. Standardization of GWG measurements at specific gestational age (GA) timepoints during pregnancy can potentially improve comparison of GWG effect sizes between studies.
In this paper, which reports prespecified secondary outcomes of a randomized controlled trial [32], we aimed to investigate the effects of structured supervised exercise training (EXE) or motivational counselling on physical activity (MOT) during pregnancy on GWG and obstetric and neonatal outcomes compared to standard care (CON). Obstetric outcomes included GDM, gestational hypertensive disorders, induction of labor, epidural analgesia, oxytocin augmentation, duration of labor, mode of delivery, rupture degree 3 and 4, and postpartum haemorrhage. Neonatal outcomes included GA at delivery, premature delivery (GA < 37 + 0 weeks), birth weight, birth length, birth weight z-score, small for gestational age (SGA), large for gestational age (LGA), and Apgar score (5 min). Additionally, we aimed to improve standardization of GWG measurements by develo** a new model to estimate GWG at specific timepoints during pregnancy and for the entire pregnancy period and account for missing weight measurements during pregnancy as well as individual differences in GA at delivery. Our hypotheses were that GWG would be lower in EXE compared to MOT, and in MOT compared to CON. The remaining investigations in this paper were explorative.
Methods
Participants and study procedures
The FitMum study was a randomized controlled trial conducted in 2018–2021 at Copenhagen University Hospital – North Zealand, Hilleroed, Denmark. The study design is described in detail elsewhere [32]. Healthy (without pre-existing or ongoing obstetric or medical complications), inactive (structured exercise at moderate-to-vigorous intensity < 1 h/week during early pregnancy) women with GA ≤ 15 + 0 weeks were eligible for inclusion. The primary objective was to investigate the effect of the two different exercise interventions (EXE and MOT) on moderate-to-vigorous-intensity physical activity during pregnancy compared to CON [33], whereas this paper reports secondary outcomes of the study. Demographic information was obtained at inclusion and pre-pregnancy BMI (kg/m2) was calculated based on self-reported pre-pregnancy weight and height. Physical activity, including moderate-to-vigorous-intensity physical activity (min per week), steps (per day), and active kilocalories (per day), was measured continuously from inclusion to delivery by a wrist-worn activity tracker (Garmin Vivosport). Randomization (n = 219) in a 1:2:2 pattern to either CON, EXE, or MOT, respectively, occurred after a one-week baseline period (GA ≤ 16 + 0 weeks). Interventions ran from randomization until delivery. Participants in the EXE intervention were offered one-hour supervised exercise training at moderate intensity three times per week, including two exercise sessions in a gym and one in a swimming pool. The gym sessions consisted of a combination of aerobic and resistance training with 30 min stationary bike training and 30 min of other exercise, for example, using elastic bands. In the swimming pool, participants did 15 min of swimming and 45 min of water exercises with plates, balls etc. The MOT intervention consisted of four individual and three group physical activity motivational counselling sessions of 1–2 h duration during pregnancy and a personalized text message once weekly to motivate to increased physical activity. EXE and MOT sessions were conducted by instructors with a bachelor’s or master’s degree in physiotherapy, exercise physiology or similar. During the COVID-19 pandemic, starting from March 11th, 2020, and throughout the intervention period, most exercise training sessions, motivational counselling sessions and periodically test visits (except delivery) were conducted online from home using Zoom Cloud Meetings or telephone. EXE could access to the swimming pool for three months during this period.
Outcome measurements
Gestational weight gain
Pre-pregnancy body weight was self-reported by the participants. From inclusion, all weight measurements were recorded to the nearest 0.1 kg on calibrated electronic scales (SECA799) at baseline (GA ≤ 15 + 0 weeks), GA 28 + 0–6 and 34 + 0–6 weeks (visit 2 and 3), and at delivery. During COVID-19, women were weighed at home on private scales and weights were self-reported. To estimate GWG for a standardized pregnancy period of 40 + 0 weeks (from here called Total GWG) and account for missing measurements and individual differences in GA at delivery, all observed weights (self-reported and measured) were fitted to a mixed effects model to predict the weights at specific timepoints throughout pregnancy at the participant-level. GWG was estimated at GA 12 + 0, 28 + 0, and 40 + 0 weeks as the difference between the predicted weight and predicted pre-pregnancy weight (GA = 0).
Obstetric and neonatal outcomes
Obstetric and neonatal outcomes were collected from medical records. Obstetric outcomes included pregnancy complications (GDM and gestational hypertensive disorders) and delivery-related outcomes (induction of labor, epidural analgesia, oxytocin augmentation, duration of labor, mode of delivery, rupture degree 3 and 4, postpartum haemorrhage). Gestational hypertensive disorders included gestational hypertension, preeclampsia, HELLP syndrome and eclampsia, which were defined based on recommendations from the International Society for the Study of Hypertension in Pregnancy [34] and evaluated by a physician. Total duration of labor included the time from the active phase (starting when cervix was dilated 4 cm and the woman had regular contractions) until the baby was born. The active second stage was defined as the time of active pushing. Neonatal outcomes included GA at delivery, premature delivery (GA < 37 + 0 weeks), birth weight, birth length, birth weight z-score, SGA, LGA and Apgar score (5 min). Birth weight was transformed to a z-score, and SGA (< 10th percentile) and LGA (> 90th percentile) [35] were defined for a Danish standard population and calculated from the Marsal formula [36], which includes fetal sex, birth weight and GA.
Statistical analysis
A retrospective sample size calculation performed for the prespecified secondary outcome, GWG, showed an estimated sample size of 33 participants in CON and 66 participants in each intervention group to detect a difference of 2.8 kg between CON and the intervention groups (statistical analysis plan available with trial registration at clinicaltrials.gov). Data are presented as mean ± standard deviation (SD) for approximately symmetric distributions, median and interquartile range (IQR) for asymmetric distributions, and frequency and proportion for categorical data. Estimated effect sizes are presented with 95% confidence interval [95% CI]. Statistical analyses were performed using R [37] and statistical significance was defined as a p-value below 5%.
Analysis of GWG was based on the intention-to-treat principle including all randomized participants. Trajectories of observed gestational weights during pregnancy were modelled by a mixed effects model featuring an intercept constrained to be equal across groups due to the randomized design [38]. Group-specific change-points were included in the model to allow for a piece-wise linear relationship with two different slopes over time in each of the groups. This led to a total of ten fixed effects in the model consisting of the common intercept and two different slopes and the change-point for each of the three groups. Normal distributed random effects were included at the subject level as intercepts and the two slopes with an unstructured covariance matrix. The model was implemented in Stan [39] and estimated using Markov-Chain Monte Carlo in four parallel chains each running for 10,000 iterations with half of them used for warm-up. A uniform distribution between 50 and 250 days was used as priors for the change-points. The fitted model was subsequently applied to predict individual weights at predetermined timepoints.
We used the randomized controlled trial design to investigate differences between groups in GWG and obstetric and neonatal outcomes, and an observational design combining all participants independent of group allocation to investigate associations between prenatal physical activity measures per se and GWG. Between-group comparisons of estimated GWG after each trimester were performed using analysis of variance (ANOVA). A sensitivity analysis was conducted using linear regression to investigate total GWG in each group before and during COVID-19, where the intervention groups received in-person and online interventions, respectively. Another sensitivity analysis using ANOVA included only participants, whose weight were measured at the hospital, to investigate the influence of weight measurements being obtained by the calibrated scale at the hospital versus via the participants’ home scales. For obstetric and neonatal outcomes, differences between groups were tested with Pearson’s χ2 test for categorical variables, ANOVA for symmetrically distributed variables, and Kruskal–Wallis test for asymmetrically distributed variables. Post-hoc pairwise comparisons were performed using Pearson’s χ2 test with Holm-corrected p-values, Tukey’s method, or Wilcoxon rank sum test with Holm-corrected p-values for categorical, symmetrically distributed, and asymmetrically distributed variables, respectively.
Associations between physical activity measures and total GWG among all participants were performed using linear regression. Physical activity measures (moderate-to-vigorous-intensity physical activity (min per week), steps (per day), and active kilocalories (per day)) used for association analyses were the average values from randomization to delivery day for participants who delivered at GA ≤ 40 + 0 weeks, and from randomization to GA 40 + 0 weeks for participants who were lost to follow-up before delivery or delivered at GA > 40 + 0 weeks.
Results
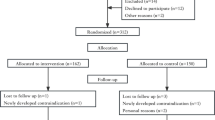
We included 220 participants from GA 6 + 1–15 + 0 weeks. One participant was lost to follow-up before randomization and hence, 219 women with median pre-pregnancy BMI of 24.1 (21.8–28.7) kg/m2 were randomized to CON (n = 45), EXE (n = 87) and MOT (n = 87) (Fig. 1). Maternal baseline characteristics are presented in Table 1. All 219 participants were included in the analysis of GWG. From randomization to delivery, 19% of the participants were lost to follow-up, thus data from 178 participants (CON: n = 34; EXE: n = 74; MOT: n= 70) were included in the analyses of obstetric and neonatal outcomes. Lost to follow-up rate did not differ between groups. Adverse and serious adverse events did not differ between groups (data not shown) and the interventions did not seem to negatively influence the mother or offspring. The average weekly moderate-to-vigorous-intensity physical activity from randomization to delivery was 35.4 min [19.4;51.4] in CON, 53.5 min [42.0;65.0] in EXE, and 43.1 min [31.6;54.6] in MOT. The average daily steps from randomization to delivery was 6896 [6408;7383] in CON, 6680 [6331;7028] in EXE, and 6792 [6441;7143] in MOT. The average daily active kilocalories from randomization to delivery was 562 [504;619] in CON, 560 [518;601] in EXE, and 583 [541;625] in MOT [33]. Adherence to the interventions in EXE and MOT was on average 1.3 [1.1;1.5] out of 3 sessions per week and 5.2 [4.7;5.7] out of 7 pregnancy counselling sessions, respectively [33].
Inclusion, randomization, allocation and completion of the FitMum study reported in accordance with the CONSORT (Consolidated Standards of Reporting Trials) guidelines [40]. CON; Control, EXE; Structured supervised exercise training, MOT; Motivational counselling on physical activity. The figure was created with BioRender.com
Gestational weight gain after each trimester
Estimated GWG did not differ between groups at GA 12 + 0 weeks (p = 0.310) or GA 28 + 0 weeks (p = 0.396) (Fig. 2A-B). Total GWG (GWG at GA 40 + 0 weeks) was 14.9 kg [13.6;16.1] in CON, 15.7 kg [14.7;16.7] in EXE and 15.0 kg [13.6;16.4] in MOT and did not differ between groups (p = 0.538) (Fig. 2C). Pairwise comparisons of total GWG showed no differences in total GWG between either MOT and EXE (-0.7 kg [-2.6;1.3], p = 0.710), MOT and CON (0.2 kg [-2.0;2.3], p = 0.985), or EXE and CON (0.8 kg [-1.1;2.7], p = 0.562). Figure 2D-F illustrates the estimated relationships between self-reported and measured body weight observations (dots) and predicted body weights by the mixed effects model (lines) for all individuals in the three groups. A complete case analysis of GWG calculated traditionally as weight measured at delivery (available for n = 131) minus self-reported pre-pregnancy weight showed no difference between groups (p = 0.612) (Figure S.1). Sensitivity analyses showed higher total GWG (4.7 kg [1.6;7.8], p = 0.003) in MOT during COVID-19 compared with before COVID-19, but no differences were found in EXE (1.6 kg [-0.8;4.0], p = 0.184) and CON (-1.2 kg [-4.3;1.9], p = 0.425). We found no differences between the three study groups in total GWG among participants, whose weights were measured at the hospital only (n = 167, p = 0.537).
Gestational weight gain (GWG) for all participants (n = 219) after first trimester/GA 12 + 0 weeks (A), second trimester/GA 28 + 0 weeks (B), and third trimester/GA 40 + 0 weeks (total GWG) (C). Self-reported and measured weights (dots) and predicted weights by mixed effects model (lines) for all individuals throughout pregnancy in the three groups (D-F). ANOVA was used for A-C and showed no differences between groups at GA 12 + 0 weeks (p = 0.310), GA 28 + 0 weeks (p = 0.396) and GA 40 + 0 weeks (p = 0.538). CON; Control, EXE; Structured supervised exercise training, MOT; Motivational counselling on physical activity, GWG; Gestational weight gain, GA; Gestational age
Associations between prenatal physical activity per se and GWG
We investigated if physical activity per se was associated with total GWG independently of group allocation. We found no associations between any of the physical activity measures and total GWG (moderate-to-vigorous-intensity physical activity: slope -0.01 [-0.02;0.01], p = 0.363; steps: slope 0.14∙10–3 [-0.03∙10–2;0.06∙10–2], p = 0.537; active kilocalories: slope -0.09∙10–2 [-0.05∙10–1;0.03∙10–1], p = 0.637) (Figure S.2A-C).
Obstetric and neonatal outcomes
Overall, none of the obstetric and neonatal outcomes differed between groups (Tables 2 and 3) apart from GA at delivery (p = 0.048). Holm-corrected post-hoc pairwise comparisons showed that EXE had higher GA at delivery compared to MOT (EXE: 40.6 weeks (39.9–41.3), MOT: 40.0 weeks (39.3–40.9), p = 0.038) (Table 3).
Discussion
We found no effect of either EXE or MOT during pregnancy on GWG and obstetric and neonatal outcomes in healthy pregnant women compared to CON. Hence, our predefined hypotheses that GWG would be lower in EXE compared to MOT, and in MOT compared to CON were rejected. Overall, the GWG and incidences of obstetric and neonatal outcomes were within the Institute of Medicine’s recommendations for pregnant women with normal weight [28] and corresponded to the general incidences in Denmark [41, 42], respectively. Notably, the interventions did not seem to influence the mother or offspring negatively, which is in line with previous studies [43].
It is noteworthy that the interventions did not reduce GWG compared to CON. These findings contrast with several studies showing reduced GWG compared to standard care after prenatal exercise in healthy [3] and normal weight women [44]. However, some studies also found no effect of exercise on GWG in women with normal weight [19, 45]. Moreover, our findings of no effects of the interventions on obstetric and neonatal outcomes contradict most studies reporting a protective effect of prenatal exercise on GDM and hypertensive disorders [3] and reduced preterm delivery, SGA and LGA [11] in women with normal weight, overweight and obesity. However, similar to the present study, other studies found no effects of prenatal exercise on GDM, preeclampsia, preterm delivery and birth weight [45,46,47]. The literature is inconsistent regarding the effects of prenatal exercise on mode of delivery, induction of labor and use of epidural analgesia [6, 48, 49]. Given the importance of achieving a certain amount of physical activity to obtain beneficial effects [50, 51], the lack of effect from interventions in the present study might be explained by a low adherence rate and low moderate-to-vigorous-intensity physical activity level among participants in EXE and MOT. In both EXE and MOT the moderate-to-vigorous-intensity physical activity level was below one hour per week and thus markedly lower than the recommended physical activity level by the Danish Health Authorities of 210 min per week at moderate intensity [51]. COVID-19 did not seem to affect physical activity level negatively in our study. Moderate-to-vigorous-intensity physical activity did not differ between participants included before the COVID-19 pandemic (physical intervention only) and during the COVID-19 pandemic (online intervention only) in any of the three study groups [33]. The online intervention attendance rate in EXE during COVID-19 was significantly higher (women participating in more exercise sessions per week) compared to the physical EXE intervention [33].
However, even if assuming that our interventions would be effective on reducing GWG and improving obstetric and neonatal outcomes had adherence rate and moderate-to-vigorous-intensity physical activity level been higher, the present study might still be underpowered to detect effects on GWG and the investigated obstetric and neonatal outcomes. Despite that we fulfilled the sample size estimated from our sample size calculation for GWG, this calculation might have been based on too optimistic estimates [17] compared to effect sizes of lifestyle and physical activity interventions on GWG generally reported in the literature [1, 4, 6, 44].
Another possible explanation of the negative findings on health outcomes in the present study could be that the results might be biased by the Hawthorne effect since it is uncertain to what extent CON interacted with the activity tracker and improved physical activity level as a result hereof. This could challenge detection of possible differences between intervention groups and CON. However, none of the physical activity measures were per se associated with GWG.
Regarding health status of our overall study population, the prevalence of overweight or obesity [52] and the level of insufficient PA [53] are lower among Danish women in general compared to women in other western countries such as the United States and the United Kingdom. This might reduce the potential for exercise to induce beneficial health effects on GWG and obstetric and neonatal outcomes in our study population compared to pregnant populations in other countries with a higher overweight and obesity burden among pregnant women, for example the United States [54]. Thus, intervention effects of EXE and MOT on reduced GWG and improved obstetric and neonatal outcomes would probably have been more evident among populations with overweight or obesity.
The GWG data included in this paper do not take the maternal body composition into account. Since exercise-induced improvement of body composition has been shown in both pregnant [55] and non-pregnant populations [56, 57], further investigation on whether maternal body composition might be improved in EXE or MOT compared to CON is needed. Furthermore, a limitation of the study is that dietary intake was not monitored. Diet interventions have been found to result in lower GWG than physical activity interventions when compared to control [4]. Thus, at least intervening on dietary intake seems to influence GWG more than physical activity interventions, and possible differences in dietary intake in our study might have blurred potential effects of exercise.
A strength of the present study is that the last weight measurement is obtained at delivery enabling us to develop a novel model to estimate GWG for a standardized pregnancy period of 40 + 0 weeks accounting for missing weight measurements and individual differences in GA at delivery. Using this model, we showed a good fit between observed weights and model-predicted weights for all individuals in all three groups, and a complete-case analysis including only participants with weight measurements at delivery showed similar results of GWG as the results of estimated GWG by the model. Thus, this novel method can be used to precisely estimate GWG at specific timepoints throughout pregnancy, for example GWG for the entire pregnancy period. Further, this method allowed us to take GA into account, which can vary up to five weeks within term-deliveries and thus likely influence GWG markedly. Moreover, missing data could be predicted by the model allowing us to report mean GWG at GA 40 + 0 weeks for all 219 study participants even though we only obtained weight data on 131 women at delivery. Thus, this model can be used to standardize measurements of GWG at specific GA timepoints during pregnancy, which can improve comparison of GWG effect sizes between studies. Including self-reported pre-pregnancy weights in the model constitutes a limitation but is justified by the finding that the weight gain slopes are visually comparable between participants in all three groups with no significant outliers.
Conclusions
The present randomized controlled study compared two different exercise intervention strategies during pregnancy and found no effect of either structured supervised exercise training or motivational counselling on physical activity on GWG and obstetric and neonatal outcomes compared to standard care. The study proposes a novel method to estimate GWG for a standardized pregnancy period of 40 + 0 weeks, which may contribute to advance state-of-the-art in the obstetric research field.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due to confidentiality but are available from the corresponding author on reasonable request. We can transfer individual participant data when we have obtained approval from the Danish Data Protection Authority according to the Data Protection Act and completed a SCC (Standard Contractual Clause) to ensure the legal basis of the transfer.
Abbreviations
- BMI:
-
Body mass index
- CON:
-
Standard care
- EXE:
-
Structured supervised exercise training
- GA:
-
Gestational age
- GDM:
-
Gestational diabetes mellitus
- GWG:
-
Gestational weight gain
- IQR:
-
Interquartile range
- LGA:
-
Large for gestational age
- MOT:
-
Motivational counselling on physical activity
- SD:
-
Standard deviation
- SGA:
-
Small for gestational age
References
Fair F, Soltani H. A meta-review of systematic reviews of lifestyle interventions for reducing gestational weight gain in women with overweight or obesity. Obes Rev. 2021;22(5):1–21.
Ruchat SM, Mottola MF, Skow RJ, et al. Effectiveness of exercise interventions in the prevention of excessive gestational weight gain and postpartum weight retention: a systematic review and meta-analysis. Br J Sports Med. 2018;52(21):1347–56.
Díaz-Burrueco JR, Cano-Ibáñez N, Martín-Peláez S, Khan KS, Amezcua-Prieto C. Effects on the maternal-fetal health outcomes of various physical activity types in healthy pregnant women. A systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2021;262:203–15.
Teede HJ, Bailey C, Moran LJ, et al. Association of antenatal diet and physical activity–based interventions with gestational weight gain and pregnancy outcomes. JAMA Intern Med. 2022;182(2):106–114.
Hamann V, Deruelle P, Enaux C, Deguen S, Kihal-Talantikite W. Physical activity and gestational weight gain: a systematic review of observational studies. BMC Public Health. 2022;22(1):1–15.
Hayes L, McParlin C, Azevedo L, et al. The effectiveness of smoking cessation, alcohol reduction, diet and physical activity interventions in improving maternal and infant health outcomes: a systematic review of meta-analyses. Nutrients. 2021;13(1036):1–31.
Bhattacharjee J, Mohammad S, Adamo KB. Does exercise during pregnancy impact organs or structures of the maternal-fetal interface? Tissue Cell. 2021;72:1–14.
Martínez-Vizcaíno V, Sanabria-Martínez G, Fernández-Rodríguez R, et al. Exercise during pregnancy for preventing gestational diabetes mellitus and hypertensive disorders: an umbrella review of randomised controlled trials and an updated meta-analysis. BJOG. 2023;130(3):264–75.
Masoud AT, AbdelGawad MM, Elshamy NH, et al. The effect of antenatal exercise on delivery outcomes: a systematic review and meta-analysis of randomized controlled trials. J Gynecol Obstet Hum Reprod. 2020;49:1–9.
Bennett CJ, Walker RE, Blumfield ML, et al. Attenuation of maternal weight gain impacts infant birthweight: systematic review and meta-analysis. J Dev Orig Health Dis. 2019;10(4):387–405.
Chen Y, Ma G, Hu Y, et al. Effects of maternal exercise during pregnancy on perinatal growth and childhood obesity outcomes: a meta-analysis and meta-regression. Sport Med. 2021;51(11):2329–47.
Heslehurst N, Hayes L, Jones D, et al. The effectiveness of smoking cessation, alcohol reduction, diet and physical activity interventions in changing behaviours during pregnancy: a systematic review of systematic reviews. PLoS ONE. 2020;15(5):e0232774.
Ruiz JR, Perales M, Pelaez M, Lopez C, Lucia A, Barakat R. Supervised exercise-based intervention to prevent excessive gestational weight gain: A randomized controlled trial. Mayo Clin Proc. 2013;88(12):1388–97.
Leung Hui A, Back L, Ludwig S, et al. Effects of lifestyle intervention on dietary intake, physical activity level, and gestational weight gain in pregnant women with different pre-pregnancy body mass index in a randomized control trial. BMC Pregnancy Childbirth. 2014;14(331):1–9.
Backhausen MG, Tabor A, Albert H, Rosthøj S, Damm P, Hegaard HK. The effects of an unsupervised water exercise program on low back pain and sick leave among healthy pregnant women - a randomised controlled trial. PLoS One. 2017;12(9):1–16.
Barakat R, Perales M, Cordero Y, Bacchi M, Mottola MF. Influence of land or water exercise in pregnancy on outcomes: a cross-sectional study. Med Sci Sports Exerc. 2017;49(7):1397–403.
Haakstad LAH, Bø K. Effect of regular exercise on prevention of excessive weight gain in pregnancy: a randomised controlled trial. Eur J Contracept Reprod Heal Care. 2011;16(2):116–25.
Tomic V, Sporis G, Tomic J, Milanovic Z, Zigmundovac-Klaic D, Pantelic S. The effect of maternal exercise during pregnancy on abdominal fetal growth. Croat Med J. 2013;54:362–8.
Brik M, Fernández-Buhigas I, Martin-Arias A, Vargas-Terrones M, Barakat R, Santacruz B. Does exercise during pregnancy impact on maternal weight gain and fetal cardiac function? A randomized controlled trial. Ultrasound Obstet Gynecol. 2019;53(5):583–9.
Poston L, Bell R, Croker H, et al. Effect of a behavioural intervention in obese pregnant women (the UPBEAT study): a multicentre, randomised controlled trial. Lancet Diabetes Endocrinol. 2015;3(10):767–77.
Harrison CL, Lombard CB, Strauss BJ, Teede HJ. Optimizing healthy gestational weight gain in women at high risk of gestational diabetes: a randomized controlled trial. Obesity. 2013;21(5):904–9.
Renault KM, Nørgaard K, Nilas L, et al. The Treatment of Obese Pregnant Women (TOP) study: a randomized controlled trial of the effect of physical activity intervention assessed by pedometer with or without dietary intervention in obese pregnant women. Am J Obstet Gynecol. 2014;210(2):134.e1-134.e9.
Wang C, Wei Y, Zhang X, et al. A randomized clinical trial of exercise during pregnancy to prevent gestational diabetes mellitus and improve pregnancy outcome in overweight and obese pregnant women. Am J Obstet Gynecol. 2017;216(4):340–51.
Oostdam N, Van Poppel MNM, Wouters MGAJ, et al. No effect of the FitFor2 exercise programme on blood glucose, insulin sensitivity, and birthweight in pregnant women who were overweight and at risk for gestational diabetes: results of a randomised controlled trial. BJOG. 2012;119(9):1098–107.
Seneviratne SN, Jiang Y, Derraik JGB, et al. Effects of antenatal exercise in overweight and obese pregnant women on maternal and perinatal outcomes: a randomised controlled trial. BJOG. 2016;123(4):588–97. https://doi.org/10.1111/1471-0528.13738.
Vinter CA, Jensen DM, Ovesen P, Beck-Nielsen H, Jørgensen JS. The LiP (Lifestyle in Pregnancy) study: a randomized controlled trial of lifestyle intervention in 360 obese pregnant women. Diabetes Care. 2011;34:2502–7.
Garnæs KK, Mørkved S, Salvesen K, Salvesen Ø, Moholdt T. Exercise training during pregnancy reduces circulating insulin levels in overweight/obese women postpartum: secondary analysis of a randomised controlled trial (the ETIP trial). BMC Pregnancy Childbirth. 2018;18(1):1–11.
IOM (Institute of Medicine) and NRC (National Research Council). Weight gain during pregnancy: reexamining the guidelines. 2009.
Sandborg J, Söderström E, Henriksson P, et al. Effectiveness of a smartphone app to promote healthy weight gain, diet, and physical activity during pregnancy (HealthyMoms): randomized controlled trial. JMIR mHealth uHealth. 2021;9(3):e26091.
Garnæs KK, Mørkved S, Salvesen Ø, Moholdt T. Exercise training and weight gain in obese pregnant women: a randomized controlled trial (ETIP Trial). PLoS Med. 2016;13(7):1–18.
Ronnberg AK, Ostlund I, Fadl H, Gottvall T, Nilsson K. Intervention during pregnancy to reduce excessive gestational weight gain - a randomised controlled trial. BJOG. 2015;122(4):537–44.
Roland CB, de Knudsen SP, Alomairah SA, et al. Structured supervised exercise training or motivational counselling during pregnancy on physical activity level and health of mother and offspring: FitMum study protocol. BMJ Open. 2021;11(3):1–11.
Knudsen SDP, Alomairah SA, Roland CB, et al. Effects of structured supervised exercise training or motivational counseling on pregnant women’s physical activity level: FitMum randomized controlled trial. J Med Internet Res. 2022;24(7):1–13.
Brown MA, Magee LA, Kenny LC, et al. Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension. 2018;72(1):24–43.
Feig DS, Corcoy R, Jensen DM, et al. Diabetes in pregnancy outcomes: a systematic review and proposed codification of definitions. Diabetes Metab Res Rev. 2015;31:680–90.
Maršál K, Persson PH, Larsen T, Lilja H, Selbing A, Sultan B. Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr Int J Paediatr. 1996;85(7):843–8.
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. 2020. https://www.r-project.org/.
Liang K, Zeger SL. Longitudinal data analysis of continuous and discrete responses for pre-post designs. Sankhyā Indian J Stat. 2000;62(Series B):134–48.
Stan Development Team. Stan modeling language users guide and reference manual, 2.28. https://mc-stan.org. Published 2022.
Consort - transparent reporting of trials. http://www.consort-statement.org/.
Hoegh S, Thellesen L, Christensen KB, Bergholt T, Hedegaard M, Sorensen JL. Incidences of obstetric outcomes and sample size calculations: a Danish national registry study based on all deliveries from 2008 to 2015. Acta Obstet Gynecol Scand. 2020;99(1):34–41.
Juhl M, Rydahl E. Birth statistics MIPAC 2020: births in Denmark. 2021.
Meah VL, Davies GA, Davenport MH. Why can’t i exercise during pregnancy? Time to revisit medical “absolute” and “relative” contraindications: Systematic review of evidence of harm and a call to action. Br J Sports Med. 2020;54(23):1395–404.
Ming WK, Ding W, Zhang CJP, et al. The effect of exercise during pregnancy on gestational diabetes mellitus in normal-weight women: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2018;18(1):440.
da Silva SG, Hallal PC, Domingues MR, et al. A randomized controlled trial of exercise during pregnancy on maternal and neonatal outcomes: Results from the PAMELA study. Int J Behav Nutr Phys Act. 2017;14(1):1–11.
Barakat R, Perales M, Bacchi M, Coteron J, Refoyo I. A program of exercise throughout pregnancy. is it safe to mother and newborn? Am J Heal Promot. 2014;29(1):2–8.
Stafne SN, Salvesen KÅ, Romundstad PR, Eggebø TM, Carlsen SM, Mørkved S. Regular exercise during pregnancy to prevent gestational diabetes: a randomized controlled trial. Obstet Gynecol. 2012;119(1):29–36.
Carrascosa M del C, Navas A, Artigues C, et al. Effect of aerobic water exercise during pregnancy on epidural use and pain: a multi-centre, randomised, controlled trial. Midwifery. 2021;103:1-8.
Walasik I, Szymusik I, Kwiatkowska K, Kaczyńska KK. Physical activity patterns among 9000 pregnant women in poland: a cross-sectional study. Int J Environ Res Public Health. 2020;17(5):1–12.
Bull FC, Al-Ansari SS, Biddle S, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020;54(24):1451–62.
The Danish Health Authorities. Recommendations for pregnant women. https://sst.dk/en/English/Responsibilities-and-tasks/Health-promotion/Focus-areas/Physical-activity. Published 2021.
WHO. Global Health Observatory. Prevalence of overweight among adults, BMI ≥ 25, age-standardized estimates by country. https://apps.who.int/gho/data/view.main.2430A?lang=en. Published 2017.
Guthold R, Stevens GA, Riley LM, Bull FC. Worldwide trends in insufficient physical activity from 2001 to 2016: a pooled analysis of 358 population-based surveys with 1·9 million participants. Lancet Glob Heal. 2018;18:1–10.
Chen C, Xu X, Yan Y. Estimated global overweight and obesity burden in pregnant women based on panel data model. PLoS ONE. 2018;13(8):1–15.
Ferrari N, Bae-Gartz I, Bauer C, et al. Exercise during pregnancy and its impact on mothers and offspring in humans and mice. J Dev Orig Health Dis. 2018;9(1):63–76.
Blond MB, Rosenkilde M, Gram AS, et al. How does 6 months of active bike commuting or leisure-time exercise affect insulin sensitivity, cardiorespiratory fitness and intra-abdominal fat? A randomised controlled trial in individuals with overweight and obesity. Br J Sports Med. 2019;53(18):1183–92.
Mandrup CM, Egelund J, Nyberg M, et al. Effects of high-intensity training on cardiovascular risk factors in premenopausal and postmenopausal women. Am J Obstet Gynecol. 2017;216(4):384.e1-384.e11.
Acknowledgements
The authors would like to acknowledge and thank all the participants for signing up for the project and delivering important data. We also thank the students, research assistants, and staff at Department of Gynaecology and Obstetrics, Copenhagen University Hospital – North Zealand, Hilleroed, who contributed to conduct the intervention activities and data collection. Additionally, we would like to thank the technical staff, especially Susanne Månsson and Charlotte Pietraszek, from the Clinical Research Unit, Department of Clinical Research, Copenhagen University Hospital – North Zealand, Hilleroed, for their contribution to planning practicalities and collecting data.
Funding
The Independent Research Fund Denmark (8020-00353B), TrygFonden (128509), Copenhagen Center for Health Technology (061017), Beckett-Fonden (17–2-0883), Aase and Ejnar Danielsens Fond (10–002052) and Familien Hede Nielsens Fond (2017–1142). Financial support was also provided by the University of Copenhagen and Copenhagen University Hospital – North Zealand, Hilleroed. The grants awarded by the Independent Research Fund Denmark and TrygFonden included external peer review for scientific quality. None of the external funding bodies interfered with the design of the study and collection, analysis, and interpretation of data, and eventual reporting of the study.
Author information
Authors and Affiliations
Contributions
B.S. initiated and directed the FitMum study. C.B.R., S.dP.K., J.B., T.D.C., S.M., S.A.A., E.L and B.S. developed the study protocol. C.B.R., S.dP.K., A.D.A., N.B. and I.K.B.J. conducted intervention activities and collected data assisted by S.A.A., research assistants and master students. E.L. is the clinical trial manager and supervised the clinical part of the study in collaboration with J.B., T.D.C., S.M., and B.S. T.D.C and E.L. supervised analysis of data and writing of the manuscript, and A.K.J. performed and supervised statistical analyses. C.B.R. analyzed data and drafted the manuscript. All authors read, contributed to, and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Written informed consent was obtained from all participants. The study was approved by the Danish National Committee on Health Research Ethics (August 30, 2018, #H-18011067) and the Danish Data Protection Agency (September 12, 2018, #P-2019–512). The study adheres to the principles of the Helsinki declaration.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S.1.
Complete case analysis of gestational weight gain at delivery including participants with available weight measurements from delivery only (n=131). ANOVA showed no difference between groups (p=0.612). CON; Control, EXE; Structured supervised exercise training, MOT; Motivational counselling on physical activity.
Additional file 2: Figure S.2.
Associations between moderate-to-vigorous-intensity physical activity (min/week) and total gestational weight gain (GWG) (A), steps per day and total GWG (B), and active kilocalories per day and total GWG (C) for all participants (n=219). Data points are visualized based on average moderate-to-vigorous-intensity physical activity, steps and active kilocalories of 25 imputed data sets. A linear regression analysis showed no associations between moderate-to-vigorous-intensity physical activity (p=0.363), steps(p=0.537), active kilocalories (p=0.637) and total GWG, respectively. GWG; Gestational weight gain.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Roland, C.B., Knudsen, S.d., Alomairah, S.A. et al. Effects of prenatal exercise on gestational weight gain, obstetric and neonatal outcomes: FitMum randomized controlled trial. BMC Pregnancy Childbirth 23, 214 (2023). https://doi.org/10.1186/s12884-023-05507-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12884-023-05507-7