Abstract
Background
To determine the effects of maternal age at first cesarean on maternal complications and adverse outcomes of pregnancy with the second cesarean.
Methods
This was a multicenter, historical, cross-sectional cohort study involving singleton pregnancies ≥28 gestational weeks, with a history of 1 cesarean delivery, and who underwent a second cesarean between January and December 2017 at 11 public tertiary hospitals in 7 provinces of China. We analyzed the effects of maternal age at first cesarean on adverse outcomes of pregnancy in the second cesarean using multivariate logistic regression analysis.
Results
The study consisted of 10,206 singleton pregnancies. Women were at first cesarean between 18 and 24, 25–29, 30–34, and ≥ 35 years of age; and numbered 2711, 5524, 1751, and 220 cases, respectively. Maternal age between 18 and 24 years at first cesarean increased the risk of placenta accreta spectrum (aOR, 1.499; 95% CI, 1.12–2.01), placenta previa (aOR, 1.349; 95% CI, 1.07–1.70), intrahepatic cholestasis of pregnancy (aOR, 1.947; 95% CI, 1.24–3.07), postpartum hemorrhage (aOR, 1.505; 95% CI, 1.05–2.16), and blood transfusion (aOR, 1.517; 95% CI, 1.21–1.91) in the second cesarean compared with the reference group (aged 25–29 years). In addition, maternal age ≥ 35 years at first cesarean was a risk factor for premature rupture of membranes (aOR, 1.556; 95% CI, 1.08–2.24), placental abruption (aOR, 6.464, 95% CI, 1.33–31.51), uterine rupture (aOR, 7.952; 95% CI, 1.43–44.10), puerperal infection (aOR, 6.864; 95% CI, 1.95–24.22), neonatal mild asphyxia (aOR, 4.339; 95% CI, 1.53–12.32), severe asphyxia (aOR, 18.439; 95% CI, 1.54–220.95), and admission to a neonatal intensive care unit (aOR, 2.825; 95% CI, 1.54–5.17) compared with the reference group (aged 25–29 years).
Conclusions
Maternal age between 18 and 24 years or advanced maternal age at first cesarean was an independent risk factor for adverse maternal outcomes with the second cesarean. Advanced maternal age at the first cesarean specifically increased adverse neonatal outcomes with the second. Therefore, decisions as to whether to perform a first cesarean at a young or advanced maternal age must be critically evaluated.
Similar content being viewed by others
Background
Age plays a significant role in infertility, pregnancy-related complications, and adverse obstetric and perinatal outcomes [1]. As the average human life span increases [2], the role of age in the development and outcome of diseases [2, 3] has also changed concomitantly. An apparent trend in obstetrics worldwide is that childbearing is being postponed to later age [4, 5]. Although advanced maternal age (AMA) is currently defined as maternal age 35 years or older at the time of delivery [6], some investigators define AMA as over 40 years [7, 8]. This trend is partially attributed to women’s pursuit of higher education, desire to have successful careers, and wish to attain financial stability [7, 9]. In addition, women delay childbearing by modifications to lifestyle (delayed marriage and increased rates of divorce) or due to underlying subfertility [10]. Effective contraception, developments in assisted reproductive technology, and multiparous women have also driven a shift toward postponing motherhood or bearing an additional child at a more advanced age [11]. The risks to women and newborns associated with AMA have therefore recently undergone greater scrutiny.
The rate of cesarean delivery (CD) has risen rapidly worldwide in recent years. In China, the cesarean rate increased from 28.8% in 2008 to 34.9% in 2014 [12], considerably above the World Health Organization (WHO)-recommended rate of 10–15% of total births [13]. It also appears that AMA is occurring concomitantly with the increasing rate in cesarean delivery. A systematic review with meta-analysis has demonstrated that AMA increased the risk of obstetric interventions such as CD [14]. Due to the previous “one-child” family planning policy in China, many women requested elective CD without valid medical indications for fear of a long and painful labor, or unplanned cesarean delivery after failure of vaginal birth or pelvic floor trauma [15]. In 2016, the enactment of the 2-child policy was expected to increase the rate of CDs as related to previous cesarean history [16].
Many investigators have reported on the relationship between maternal age and obstetric complications and/or adverse outcomes in pregnancy [17, 18]. Additionally, some studies have indicated an association between short inter-pregnancy interval and poor birth outcomes in the succeeding pregnancy—including preterm birth and extremely low birth weight—in women with advanced age [19, 20]. Qin et al. reported that AMA and previous cesarean section were both risk factors for adverse outcomes of pregnancy during second pregnancies [21]. However, little is known regarding the effects of age at first delivery on the subsequent pregnancy, especially the age at the first CD. To garner more insight into possible links, we herein analyzed the association between maternal age at first CD and complications and adverse outcomes of pregnancy in the second CD using data from 11 public tertiary hospitals within 7 provinces of China.
Methods
Study design
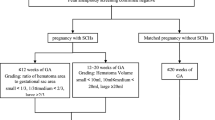
This was a multicenter, historical, cross-sectional cohort study conducted at 11 public tertiary hospitals covering 7 provinces, municipalities, and autonomous regions within China (Guangdong, Bei**g, **njiang, Shanxi, Henan, Hubei, and Chongqing). The cohort comprised 14,734 women with uterine scars who delivered again between January 2017 and December 2017. We selected women with singleton pregnancies at ≥28 gestational weeks, and with a CD history who underwent a repeat CD. Antepartum fetal death, major fetal congenital anomalies, a scarred uterus caused by myomectomy, and a history of 2 or more CDs were excluded. Women lacking their essential records—such as delivery mode or severe data loss—were also excluded. Figure 1 shows a flow diagram of the women’s enrollment process.
Research methods
Data were obtained by chart review based on electronic medical records. Maternal clinical characteristics included maternal ages at the first and second CDs, gestational weeks, gravidity, parity, nationality, mode of conception (natural vs. assisted), source of pregnant women (“referral” meant that pregnant women were referred from low-level hospitals to tertiary hospitals. “hospital” meant that pregnant women delivered in a tertiary hospital from the beginning), body mass index (BMI) before pregnancy, number of months (interval) between the 2 CDs, sex of the offspring, and indications for the 2 CDs.
We reviewed the electronic medical records with regard to complications, and the following details were recorded: premature rupture of membranes (PROM), placenta previa (PP), placenta accreta spectrum disorders (PAS), placental abruption, idiopathic thrombocytopenic purpura (ITP), abnormal amniotic fluid (oligohydramnios and polyhydramnios), hypertension disorders, diabetes mellitus (DM), thyroid diseases (hypothyroidism and hyperthyroidism), and intrahepatic cholestasis of pregnancy (ICP).
We collected adverse maternal outcomes, including any of the following: postpartum hemorrhage (PPH), severe PPH, uterine rupture, disseminated intravascular coagulation (DIC), puerperal infection, hysterectomy, bladder injury, and blood transfusion. We defined PPH as a loss of ≥1000 ml of blood after cesarean delivery and severe PPH as the loss of ≥1500 ml of blood after cesarean delivery.
Adverse neonatal outcomes included prematurity (< 37 weeks), fetal growth restriction (FGR), mild asphyxia (1-min Apgar score < 8), severe asphyxia (1-min Apgar score < 4), neonatal complication, and admission to a neonatal intensive care unit (NICU). FGR is an estimated fetal weight that is less than the 10th percentile for gestational age. Neonatal complication included neonatal asphyxia, neonatal malformation and other complications (hemolysis, jaundice, congenital heart disease, meconium aspiration pneumonia, et al.).
Information regarding maternal and neonatal diseases was classified according to the WHO’s Classification of diseases (ICD)-10.
Statistical analyses
We performed statistical analyses using SPSS v24.0 for Windows and R software (version 3.6.1). Missing values were imputed using a random forest algorithm. Missing data was listed in Supplementary Table S1. We examined whether quantitative data were normally distributed using the Kolmogorov-Smirnov test. Non-parametric continuous features are presented as medians and their corresponding interquartile ranges (IQR), and the Kruskal-Wallis test was applied for comparisons among multiple groups. Categorical variables are reported as frequencies (percentages), and the differences between groups were compared using the χ2 or Fisher exact-probability test in the case of small numbers, where appropriate. The associations between maternal age at first CD and each outcome were investigated by logistic regression analysis in 2 models: model 1 was adjusted for possible confounders—including gravidity, parity, BMI, assisted reproductive technology, and interval months; and with model 2 we explored possible explanations of the findings by adding mediating factors—i.e., maternal age at the second CD [19]. There is no official international definition of “advanced maternal age,” nor is there an “age interval” or reference group of maternal age [20]. According to most studies and guidelines, adolescent mothers have been excluded as a unique group. The rationale for our choice of a reference group (25–29 years) for this study was that the expected outcomes would be optimal for this age range, which also included the largest number of pregnancies. We also calculated crude odds ratios (ORs) and adjusted odds ratios (aORs), along with their 95% confidence intervals (CIs). Differences with P-values of < 0.05 were considered to be statistically significant.
Results
The present study consisted of 10,206 women with a history of CD who underwent a repeat CD. Women at their first CD were aged 18–24 years, 25–29 years, 30–34 years, and ≥ 35 years; and numbered 2711, 5524, 1751, and 220 cases, respectively. Figure 2 shows that the indications for the first CDs were principally pregnancy complications, fetal distress, failure of labor, abnormal fetal position, social factors, and unknown reasons. The indications for the second CDs were primarily uterine scarring, pregnancy complications, fetal distress, failure of labor, and abnormal fetal position. The compositional ratio of CD indications was similar for different age groups, and scarred uterus was the primary indication for a second CD.
Table 1 shows the general characteristics and potentially mediating factors for the women undergoing a consecutive CD—with the median values showing an increase, and the median interval between the 2 cesareans shortened in an almost continuously commensurate fashion relative to the age at first CD: 18–24 years of age (second CD at a median age of 29 years, with a median interval of 84 months); 25–29 years (33 years and 72 months); 30–34 years (36 years and 60 months); and ≥ 35 years (40 years and 48 months). In the group of women who were older than 35 years at their first CD, gravidity and parity increased; and more women underwent assisted reproductive technology prior to the second CD compared with other groups. A greater number of women between 18 and 24 years of age at their first CD tended to be either leaner (a BMI less than 18.5 kg/m2) or obese (a BMI above 30 kg/m2) with the second CD.
The effects of maternal age at first CD on maternal complications with the second CD were the principal findings of our study (model 2). A maternal age of 18–24 years at the first CD increased the risk of PAS (aOR, 1.499; 95% CI, 1.12–2.01), PP (aOR, 1.349; 95% CI, 1.07–1.70), and ICP (aOR, 1.947; 95% CI, 1.24–3.07) at the second CD compared with the reference group (aged 25–29 years). By comparison, maternal age ≥ 35 years at first CD was a risk factor for PROM (aOR, 1.556; 95% CI, 1.08–2.24) and placental abruption (aOR, 6.464; 95% CI, 1.33–31.51) (Table 2).
We further explored the effects of maternal age at first CD on adverse maternal and neonatal outcomes with the second CD (Tables 3 and 4). A maternal age of 18–24 years at the first CD increased the risk of PPH (aOR, 1.505; 95% CI, 1.05–2.16) and blood transfusion (aOR, 1.517; 95% CI, 1.21–1.91) with the second CD compared with the reference group (aged 25–29 years), while maternal age ≥ 35 years at first CD was a risk factor for uterine rupture (aOR, 7.952; 95% CI, 1.43–44.10) and puerperal infection (aOR, 6.864; 95% CI, 1.95–24.22).
AMA at the first CD increased adverse neonatal outcomes with the second CD, including mild asphyxia (aOR, 4.339; 95% CI, 1.53–12.32), severe asphyxia (aOR, 18.439; 95% CI, 1.54–220.95), and admission to the NICU (aOR, 2.825; 95% CI, 1.54–5.17) compared with the reference group (aged 25–29 years).
After adjusting for maternal age at the second CD, maternal age ≥ 35 years at first CD did not influence the incidence of DIC (model 1: aOR, 13.951; 95% CI, 1.05–185.44; model 2: aOR, 51.8; 95% CI, 0.32–8409.68). In addition, the odds of ITP (model 1: aOR, 1.565; 95% CI, 1.10–2.22; model 2: aOR, 1.057; 95% CI, 0.70–1.60), severe PPH (model 1: aOR, 1.383; 95% CI, 1.03–1.86; model 2: aOR, 1.188; 95% CI, 0.72–1.95), uterine rupture (model 1: aOR, 2.579; 95% CI, 1.06–6.25; model 2: aOR, 2.034; 95% CI, 0.75–5.49), FGR (model 1: aOR, 2.061; 95% CI, 1.48–2.86; model 2: aOR, 1.223; 95% CI, 0.70–2.14), mild asphyxia (model 1: aOR, 0.87–1.38; model 2: aOR, 0.882, 95% CI, 0.57–1.38), neonatal complications (model 1: aOR, 1.469; 95% CI, 1.12–1.93; model 2: aOR, 1.131; 95% CI, 0.72–1.79), and admission to the NICU (model 1: aOR, 1.425; 95% CI, 1.22–1.67; model 2: aOR, 1.036; 95% CI, 0.79–1.35) for women between 18 and 24 years of age were not statistically significant with the second CD after adjusting for maternal age.
Discussion
We found that maternal age at the first cesarean delivery was associated with obstetric complications and adverse outcomes of pregnancy with the second cesarean delivery. Compared with the reference group (25–29 years), maternal age between 18 and 24 years at the first CD increased the risk of PAS, PP, ICP, PPH, and blood transfusion with the second CD. In addition, maternal age ≥ 35 years at first CD was a risk factor for PROM, placental abruption, uterine rupture, puerperal infection, mild neonatal asphyxia, severe asphyxia, and admission to the NICU. We found that ages between 25 and 34 years constituted optimal times for the first CD from the perspective of outcomes with the second CD. And the study can be used for counseling AMA or young patients about possible adverse outcome of a second CD.
We noted in our study that, intriguingly, maternal age between 18 and 24 years at first CD increased the risk of PAS, PP, ICP, PPH, and blood transfusion with the second CD. In the United States, the highest rate of unintended pregnancy occurs among young adult women in their early twenties (18–24 years). Young adult women are likely to be unmarried, poorer, cohabiting, or participating in the labor force without having completed a college education; and it has been reported that unintended pregnancies are associated with adverse infant and maternal health outcomes [21, 22]. Additionally, in young women, unhealthy behaviors such as tobacco use and high body mass index were independent risks for PAS and PP [23]; and postpartum endometritis due to ignorance of postpartum care and poor postpartum recovery was associated with PAS disorders in subsequent pregnancies [23]. PAS and PP were the principal reasons for PPH and blood transfusion with the second CD [24]. A history of illicit drug use and viral hepatitis of any type increased the risk of ICP, with the recurrence rates for ICP ranging from 40 to 92% [25].
In our study, maternal age ≥ 35 years at first CD was a substantial risk factor for PROM, placental abruption, uterine rupture, and puerperal infection. A large proportion of AMA pregnancies were undesirable and unplanned—with short interpregnancy intervals, insufficient recovery of the uterus, physiologic stresses of a previous pregnancy [26], and reduced resistance—all potentially related to adverse pregnancy outcomes. Furthermore, it was alarming that maternal age ≥ 35 years at first CD was associated with a 4-fold increase in neonatal mild asphyxia, 18-fold increase in severe asphyxia, and 2-fold increase in NICU admission. Numerous investigators have reported that AMA was a risk factor for preterm delivery [27], low birthweight [28], fetal chromosomal abnormalities [29], and fetal death [30]. AMA was also associated with pre-eclampsia, gestational diabetes, PROM, venous thromboembolism, and the use of assisted conception [17, 26]—which would likely increase adverse neonatal outcomes.
In China, the rate of CD increased from 28.8% in 2008 to 34.9% in 2014 [12]. When we analyzed the indications for the 2 CDs, we found that the compositional ratios of CD indications were similar for the different age groups. The primary indications for the first CDs were pregnancy complications and social factors, while for the second CDs they were scarred uterus and pregnancy complications. In order to reduce the rate of CD, vaginal birth after 1 CD without contraindications has been recommended in China. However, the proportion of trials of vaginal birth after 1 CS was lower (9.1%) in our previous study compared with that in other countries (75.6% in Israel [31] and 70% in France) [32]. In our study, the rate of trials of vaginal birth among women between 18 and 24 years of age at their first CD was higher than for women who were ≥ 35 years of age (14.9% vs. 4.5%, respectively). Our best advice is to avoid unnecessary CDs, encourage women to have their children at an appropriate age, and propose that more women choose a trial of labor after a CD so as to decrease excess risks. This paper can be used for counseling AMA or young patients about possible adverse outcome of a second CD.
Strengths and limitations
This study possesses several strengths. First, our study was based upon the multicenter database that encompassed 11 public tertiary hospitals; and it covered 7 provinces, municipalities, and autonomous regions within China in 2017, effectively avoiding the selection bias of single-center and small-sample studies. To some extent, these data can therefore be generalizable to more heterogeneous populations. Second, this was the first study of its kind on the effect of maternal age at first CD on the complications and adverse outcomes of the second CD. However, this study also has several limitations. This was a historical cohort study, and data from different centers were not always ideal and complete; some missing values were therefore imputed using the random forest algorithm. The number of women at their first CD with age ≥ 35 years was relatively small. Further repeated studies are needed.
Conclusions
In conclusion, this multicenter, historical, cross-sectional cohort study of singleton pregnancies showed that maternal age between 18 and 24 years at first CD increased the risks for PAS, PP, ICP, PPH, and blood transfusion with the second CD. Maternal age ≥ 35 years at first CD was a risk factor for PROM, placental abruption, uterine rupture, puerperal infection, neonatal mild asphyxia, severe asphyxia, and admission to a NICU. The underlying mechanism(s) governing these relationships are unclear, and therefore further studies are needed to confirm that both young and advanced maternal ages at first CD are risk factors for many serious complications and adverse pregnancy outcomes at the subsequent CD.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BMI:
-
Body mass index
- CD:
-
Cesarean delivery
- aOR:
-
Adjusted odds ratios
- CI:
-
Confidence interval
- PAS:
-
Placenta accreta spectrum
- PP:
-
Placenta previa
- ICP:
-
Intrahepatic cholestasis of pregnancy
- PPH:
-
Postpartum hemorrhage
- PROM:
-
Premature rupture of membrane
- NICU:
-
Neonatal intensive care unit
- AMA:
-
Advanced maternal age
- ITP:
-
Idiopathic thrombocytopenic purpura
- DM:
-
Diabetes mellitus
- DIC:
-
Disseminated intravascular coagulation
- FGR:
-
Fetal growth restriction
References
Brown W, Ahmed S, Roche N, Sonneveldt E, Darmstadt GL. Impact of family planning programs in reducing high-risk births due to younger and older maternal age, short birth intervals, and high parity. Semin Perinatol. 2015;39(5):338–44.
Olshansky SJ. Articulating the case for the longevity dividend. Cold Spring Harb Perspect Med. 2016;6(2):a025940.
Perkins BA, Caskey CT, Brar P, Dec E, Karow DS, Kahn AM, et al. Precision medicine screening using whole-genome sequencing and advanced imaging to identify disease risk in adults. Proc Natl Acad Sci U S A. 2018;115(14):3686–91.
Laopaiboon M, Lumbiganon P, Intarut N, et al. Advanced maternal age and pregnancy outcomes: a multicountry assessment. BJOG. 2014;121(1):49–56.
Sauer MV. Reproduction at an advanced maternal age and maternal health. Fertil Steril. 2015;103(5):1136–43.
Benli AR, Cetin Benli N, Usta AT, Atakul T, Koroglu M. Effect of maternal age on pregnancy outcome and cesarean delivery rate. J Clin Med Res. 2015;7(2):97–102.
Pinheiro RL, Areia AL, Mota Pinto A, Donato H. Advanced maternal age: adverse outcomes of pregnancy, a meta-analysis. Acta Med Port. 2019;32(3):219–26.
Klemetti R, Gissler MA-O, Sainio S, Hemminki E. At what age does the risk for adverse maternal and infant outcomes increase? Nationwide register-based study on first births in Finland in 2005-2014. Acta Obstet Gynecol Scand. 2016;95(12):1368–75.
Cohen W. Does maternal age affect pregnancy outcome? BJOG. 2014;121(3):252–4.
Guedes M, Canavarro MC. Characteristics of primiparous women of advanced age and their partners: a homogenous or heterogenous group? Birth. 2014 Mar;41(1):46–55.
Imterat M, Wainstock T, Sheiner E, Kapelushnik J, Fischer L, Walfisch A. Advanced maternal age during pregnancy and the risk for malignant morbidity in the childhood. Eur J Pediatr. 2018;177(6):879–86.
Li HT, Luo S, Trasande L, Hellerstein S, Kang C, Li JX, et al. Geographic variations and temporal trends in cesarean delivery rates in China, 2008-2014. JAMA. 2017;317(1):69–76.
Appropriate technology for birth. Lancet. 1985;2(8452):436–7.
Bayrampour H, Heaman M. Advanced maternal age and the risk of cesarean birth: a systematic review. Birth. 2010;37(3):219–26.
Candice PY, Wang WCT, Kanagalingam D, Tan HK. Why we do Caesars a comparison of the trends in caesarean section delivery. Ann Acad Med Singapore. 2013;42(8):408–12.
Zeng Y, Hesketh T. The effects of China's universal two-child policy. Lancet. 2016;388(10054):1930–8.
Goisis A, Remes H, Barclay K, Martikainen P, Myrskylä M. Advanced maternal age and the risk of low birth weight and preterm delivery: a within-family analysis using Finnish population registers. Am J Epidemiol. 2017;186(11):1219–26.
Kenny LC, Lavender T, McNamee R, O'Neill SM, Mills T, Khashan AS. Advanced maternal age and adverse pregnancy outcome: evidence from a large contemporary cohort. PLoS One. 2013;8(2):e56583.
Ihongbe TO, Wallenborn JT, Rozario S, Masho SW. Short interpregnancy interval and adverse birth outcomes in women of advanced age: a population-based study. Ann Epidemiol. 2018;28(9):605–11.
Risk of adverse pregnancy outcomes of late- and postterm pregnancies in advanced maternal age: a national cohort study. 2020; 99(8):1022–30.
Kornides ML, Kitsantas P, Lindley LL, Wu H. Factors associated with young adults' pregnancy likelihood. J Midwifery Womens Health. 2015;60(2):158–68.
Norton M, Chandra-Mouli V, Lane C. Interventions for preventing unintended, rapid repeat pregnancy among adolescents: a review of the evidence and lessons from high-quality evaluations. Glob Health Sci Pract. 2017;5(4):547–70.
Jauniaux E, Chantraine F, Siilver RM. FIGO consensus guidelines on placenta accreta spectrum disorders: epidemiology. Int J Gynaecol Obstet. 2018;140(3):265–73.
Sentilhes L, Kayem G, Chandraharan E, et al. FIGO consensus guidelines on placenta accreta spectrum disorders: conservative management. Int J Gynaecol Obstet. 2018;140(3):291–8.
Bicocca MJ, Sperling JD, Chauhan SP. Intrahepatic cholestasis of pregnancy: review of six national and regional guidelines. Eur J Obstet Gynecol Reprod Biol. 2018;231:180–7.
Oppong SA, Torto M, Beyuo T. Risk factors and pregnancy outcome in women aged over 40 years at Korle-Bu teaching Hospital in Accra, Ghana. Int J Gynaecol Obstet. 2020;149(1):56–60.
Joseph KS, Allen ACF, Dodds L, et al. The perinatal effects of delayed childbearing. Obstet Gynecol. 2005;105(6):1410–8.
Aliyu MH, Salihu HMF, Wilson RE, et al. The risk of intrapartum stillbirth among smokers of advanced maternal age. Arch Gynecol Obstet. 2008;278(1):39–45.
Waldenstrom U, Cnattingius S, Vixner L, Norman M. Advanced maternal age increases the risk of very preterm birth, irrespective of parity: a population-based register study. BJOG. 2017;124(8):1235–44.
Flenady V, Koopmans LF, Middleton P, et al. Major risk factors for stillbirth in high-income countries: a systematic review and meta-analysis. Lancet. 2011;377(9774):1331–40.
Lipschuetz M, Guedalia J, Rottenstreich A, Novoselsky Persky M, Cohen SM, Kabiri D, et al. Prediction of vaginal birth after cesarean deliveries using machine learning. Am J Obstet Gynecol. 2020;222(6):613.e1–613.e12.
Haumonte JB, Raylet M, Christophe M, Mauviel F, Bertrand A, Desbriere R, et al. French validation and adaptation of the Grobman nomogram for prediction of vaginal birth after cesarean delivery. J Gynecol Obstet Hum Reprod. 2018;47(3):127–31.
Acknowledgements
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Funding
The present study was supported by National Key R&D Program of China (No. 2016YFC1000405, 2017YFC1001402, 2018YFC1004104 and 2018YFC10029002) and the National Natural Science Foundation (No. 81830045, 81671533, 81571518 and 81971415). General program of Guangdong Natural Science Foundation (No. 2020A1515010273). The funding bodies had no role in the design of the study, the collection, analysis, or interpretation of the data, or writing the manuscript.
Author information
Authors and Affiliations
Contributions
SB and LZ1: conceptualization, methodology, software, validation, writing, review and editing. JC: investigation, review and editing. MH, LH, SZ, YL1, and YL2: investigation, data collection. JJ, SW1, YC, SW2, XX, LF, XZ, YZ, QZ, HQ, LZ2, and HL: investigation, and resources. LD, ZW and DC: supervision, project administration, and funding acquisition. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This historical, cross-sectional cohort study was approved by the Medical Ethics Committee of Guangzhou Medical University with Medical Research No. 2016 (0406); the date of approval was April 6, 2016. All of the methods were performed in accordance with the relevant guidelines and regulations, and the statements on consent for participation were signed by participants and their legally authorized representatives.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Missingness table
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Bi, S., Zhang, L., Chen, J. et al. Maternal age at first cesarean delivery related to adverse pregnancy outcomes in a second cesarean delivery: a multicenter, historical, cross-sectional cohort study. BMC Pregnancy Childbirth 21, 126 (2021). https://doi.org/10.1186/s12884-021-03608-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12884-021-03608-9