Abstract
Background
Parkinson’s disease (PD) is the fastest growing neurological condition worldwide. Recent theories suggest that symptoms of PD may arise due to spread of Lewy-body pathology where the process begins in the gut and propagate transynaptically via the vagus nerve to the central nervous system. In PD, gait impairments are common motor manifestations that are progressive and can appear early in the disease course. As therapies to mitigate gait impairments are limited, novel interventions targeting these and their consequences, i.e., reducing the risk of falls, are urgently needed. Non-invasive vagus nerve stimulation (nVNS) is a neuromodulation technique targeting the vagus nerve. We recently showed in a small pilot trial that a single dose of nVNS improved (decreased) discrete gait variability characteristics in those receiving active stimulation relative to those receiving sham stimulation. Further multi-dose, multi-session studies are needed to assess the safety and tolerability of the stimulation and if improvement in gait is sustained over time.
Design
This will be an investigator-initiated, single-site, proof-of-concept, double-blind sham-controlled randomised pilot trial in 40 people with PD. Participants will be randomly assigned on a 1:1 ratio to receive either active or sham transcutaneous cervical VNS. All participants will undergo comprehensive cognitive, autonomic and gait assessments during three sessions over 24 weeks, in addition to remote monitoring of ambulatory activity and falls, and exploratory analyses of cholinergic peripheral plasma markers. The primary outcome measure is the safety and tolerability of multi-dose nVNS in PD. Secondary outcomes include improvements in gait, cognition and autonomic function that will be summarised using descriptive statistics.
Discussion
This study will report on the proportion of eligible and enrolled patients, rates of eligibility and reasons for ineligibility. Adverse events will be recorded informing on the safety and device tolerability in PD. This study will additionally provide us with information for sample size calculations for future studies and evidence whether improvement in gait control is enhanced when nVNS is delivered repeatedly and sustained over time.
Trial registration
This trial is prospectively registered at www.isrctn.com/ISRCTN19394828. Registered August 23, 2021.
Similar content being viewed by others
Background
Prevention of immobility and falls in older people is a public health priority. In ageing and age-associated conditions such as Parkinson’s disease (PD), gait disorders and their consequences – most notably falls – are common manifestations. The impact of gait dysfunction and falls in PD is such that people with Parkinson’s (PwP) voted both as the top research priority in a recent James Lind Alliance and Parkinson’s UK priority setting partnership [1].
Gait impairments in PD are evident at the time of diagnosis and respond only selectively to treatment [2]. They have significant consequences, as discrete gait characteristics predict the time to first fall in a cohort of newly diagnosed PwP [3]. Progression of discrete gait impairments such as step time variability and step length variability is evident in early disease despite optimal dopaminergic treatment [4]. This reflects the contemporary view of PD as a complex multisystem disorder in which gait impairments are underpinned by deficits in multiple cortical and subcortical networks as well as age-related neurodegeneration. Novel interventions targeting dopamine-resistant gait impairments and their consequences are urgently needed.
Cognitive impairment contributes to early gait deficits [5] and conversely, gait impairments predict cognitive decline in early PD [6]. There is evidence that the loss of cholinergic neurones from the nucleus basalis of Meynert (nbM) in the basal forebrain and their projections to the frontal cortex is a major contributor to the functional decline in gait, cognition and increased risk of falls [7,8,9,10]. For example, we have recently shown that nbM volumes predict future disease-specific gait decline over three years in early PD [10]. In this study, gait pace and variability characteristics were most closely linked with nbM neurodegeneration. Furthermore, a metabolic temporal variability gait covariance network in PD was recently reported to correlate with variability of step and swing time. This network was characterised by predominantly decreased brain glucose metabolism (associated with increased step and swing time variability) in the caudate nucleus, hippocampus, and red nucleus, which all receive cholinergic input from cell groups within the basal forebrain and the pedunculopontine nucleus (PPN) [11]. Research has shown that the number of acetylcholinesterase + neurons in the PPN is reduced in PD fallers relative to those patients without balance deficits and a history of falls [12]. Cholinesterase inhibitors aimed at preventing the breakdown of acetylcholine have demonstrated some promise in the management of PD-associated gait disorder and postural instability [13]. However, drugs are not without side effects and that may be more problematic in older people with multimorbidity.
An alternative approach now being trialled is to implant stimulating electrodes into the nbM [14], although this involves major neurosurgery with equivocal outcomes. A non-invasive technique that may activate the cholinergic circuitry which has gained recent traction in a number of neurological disorders is via non-invasive vagus nerve stimulation (nVNS) [15,16,17]. The exact underlying mechanisms of action of this method are poorly understood, but an indirect effect impacting the cholinergic system has been postulated [18]. Neuroimaging studies have provided some evidence with increased neural activity observed in multiple regions of the forebrain containing cholinergic neurons including the caudate nucleus and thalamus [19]. Additional corroboration is provided through studies demonstrating attenuation of VNS following the application of the muscarinic agonist scopolamine in a rat model [20] and after lesioning the nbM [15]. More recently, VNS improved locomotion in a rat model of PD [21] and recent randomized controlled trials have additionally demonstrated the feasibility of VNS combined with rehabilitation therapy in improving upper limb motor function in stroke patients [22]. Based on this, adjunctive VNS may be an attractive non-pharmacological approach to improve gait and balance impairments in PD [23].
Research from our group recently assessed the effect of a single dose of nVNS on dopaminergic-resistant gait characteristics (step time variability and step length variability) [4] in a pilot study in 30 PwP [24]. Gait was measured both pre- and post-intervention during a two-minute continuous walk at a comfortable walking pace. All participants were assessed while in an ‘on’ motor state. Participants were randomised to receive either a single dose of active nVNS stimulation or sham stimulation that does not activate the vagus nerve (VN) between the pre- and post-assessments with gammaCore® handheld device, which was applied to the left side of the neck. In the active group, step time variability and step length variability decreased whereas an increase was observed in the sham group, with step length variability reaching statistical significance (-5.6 vs 25.4% change for active vs control group, p = 0.045). Decrements in these characteristics represent an improvement; limiting the manifold of gait variability increases gait stability [25].
This exploratory study provides data suggesting that dopamine-resistant gait characteristics improve with nVNS. It is now imperative to ascertain if the effect is enhanced when nVNS is delivered repeatedly and sustained over time. Therefore, to establish a signal of efficacy and feasibility to deliver in the home, a multi-dose study is now needed.
The aim of the Adjunctive Vagus Nerve Stimulation for Improving Neural Control of Gait in Parkinson’s Disease (AdVaNSING-PD) project is to establish the safety, feasibility, and proof of concept of a non-pharmacological, low-cost, simple-to-use, home-delivered electroceutical approach to boost cholinergic function, improve gait parameters and ultimately reduce falls risk.
Materials and methods
Study design
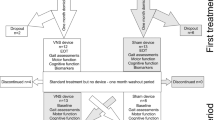
The study is a single-site, proof-of-concept, double-blind sham-controlled randomised pilot trial in patients with PD. Our trial will follow the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) guidelines [26, 27]. The study comprises a baseline assessment visit (t1) followed by a 12-week treatment phase that includes remote monitoring of ambulatory activity and falls with monthly follow-up calls (t2 and t3). All participants will be invited for two follow-up visits at 12 weeks (t4) and 24 weeks (t5) with the latter to assess any long-term retention. At the end of the study, a subset of participants will be invited to contribute to a focus group discussion, to facilitate the shape of a future trial. Table 1 illustrates the SPIRIT flow diagram summarising all study procedures and outcome measures, randomisation, assessments, and interventions for each phase of the study.
Study setting
This trial is carried out at the Clinical Ageing Research Unit on the Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne.
Participants and eligibility criteria
We aim to recruit 40 PwP in total. This study will be used to establish proof-of-concept and feasibility allowing power calculation for a larger definitive clinical trial. Participants will be over 18 years of age but younger than 76 years of either sex and of any racial or ethnic origin. The age cut-off will be used due to the lack of safety data in older or younger participants. All participants will have a diagnosis of PD, as per the UK Brain Bank Criteria [28] and participants may be Hoehn and Yahr stages I to III [29]. All participants must be on stable medication for the preceding month and anticipated over the next three months, able to walk independently without aid for a minimum of two minutes without rest and able to provide informed consent. Our exclusion criteria include:
-
Parkinson’s disease dementia [30] or significant cognitive impairment as determined by a score of < 21 on the Montreal Cognitive Assessment (MoCA);
-
History of stroke, traumatic brain injury, intracranial aneurysm, intracranial haemorrhage, brain tumour or atypical parkinsonian disorder;
-
An unstable medical condition in the last six months or planned surgeries that may involve implants within the next six months;
-
Prescribed centrally acting anticholinergics (e.g., amitriptyline) or cholinesterase inhibitors;
-
Severe orthopaedic or neurological (excluding PD) pathology that will adversely affect gait;
-
Pain at the nVNS treatment site (e.g., dysesthesia, neuralgia, cervicalgia);
-
Previous use of nVNS stimulator device, including previous participation in nVNS research;
-
Women of childbearing potential or who are pregnant or lactating;
-
Active participation in another interventional trial or exposure to an experimental drug or intervention;
-
Lesion (including lymphadenopathy), previous surgery (including carotid endarterectomy or vascular neck surgery) or abnormal anatomy at the treatment site (open wound, rash, infection, swelling, cut, sore, drug patch, surgical scar[s]);
-
Known or suspected severe atherosclerotic cardiovascular disease, severe carotid artery disease (e.g., bruits or history of TIA or stroke), congestive heart failure, known severe coronary artery disease or prior myocardial infarction;
-
Abnormal baseline electrocardiogram (ECG) within the last year (e.g., second or third-degree heart block, prolonged QT interval, atrial fibrillation, atrial flutter, history of ventricular tachycardia or ventricular fibrillation);
-
Uncontrolled high blood pressure (systolic > 160 mmHg, diastolic > 100 mmHg) after 3 measurements within 24 h;
-
Previous unilateral or bilateral vagotomy;
-
Implanted metal cervical spine hardware, other metallic implants or implantable medical devices such as deep brain stimulator, hearing aid implant, pacemaker, implanted cardioverter defibrillator, cranial aneurysm and/or cranial aneurysm clips, history of facial/orbital/metallic fragments, implanted electronic device, neurostimulator, valve replacements/stents, metallic implants/prostheses) near the stimulation site such as a bone plate or bone screw;
-
History of syncope or seizures (within the last 2 years);
-
Patients with active cancer or are in a recent remission of cancer;
-
Clinically significant hypotension, bradycardia, or tachycardia;
-
Insufficient comprehension of the English language.
The primary objectives are to develop and administer the study protocol, to establish feasibility indicators of the study and to assess compliance, safety, and tolerability of multi-dose nVNS in participants with PD. Key secondary objectives include assessing the impact of multi-dose nVNS treatment on (1) gait as proof of concept. We will be focussing on changes in dopaminergic-resistant gait characteristics including step length variability and step time variability [4] in addition to other discrete (microstructural) characteristics according to an a priori model of gait [31]; (2) changes in macrostructural gait characteristics including volume, variability pattern of walking bouts and steps as measured using seven-day free living monitoring (Axivity monitor); (3) cognition as proof of concept. Particular attention will be paid to changes in cognitive domains relating to attention and fluctuating attention and changes in executive and memory function; and, (4) falls.
Trial design
The AdVaNSING-PD project is designed as a single-site randomised double-blind sham-controlled feasibility trial with two parallel groups and a primary endpoint of safety and tolerability after 12 weeks of self-administered nVNS with additional follow-up at 24 weeks to assess any carryover effects.
Randomization and blinding
Patients who provide informed consent to participate and fulfil the eligibility criteria will be equally randomised using a covariate-adaptive randomisation process with a minimisation method [32] into two groups to receive either active nVNS or sham nVNS. This is achieved using the variance minimisation procedure recently proposed [33]. Allocation to groups will be stratified by age, sex, global cognition (MoCA scores) and motor severity (scored using the MDS-UPDRS-III). Both patients and researchers performing the assessments and analyses will be blinded to the intervention.
Intervention
Patients allocated to the active group will receive nVNS using the gammaCore® handheld nVNS device (CE 571,753). This is a handheld device that indirectly stimulates the cervical branch of the VN within the carotid sheath. Two flat stimulation contact surfaces emit a low-voltage electrical signal burst which permeates the skin and subcutaneous structures. The electrical signal consists of a 5 kHz sinusoidal wave burst lasting 1 ms that repeats once every 39 ms (25 Hz frequency). The stimulation surface is placed on one side of the neck and the stimulus is applied for 120 s (see Fig. 1). Patients will be instructed to deliver two doses of stimulation 2–5 min apart on the left side, twice daily (in the morning and afternoon) for 12 weeks. The sham devices will be supplied by ElectroCore, LLC, manufacturers of the gammaCore® device. The sham device looks identical to the active nVNS device but does not stimulate the VN. Patients in the sham group will receive identical instructions to those in the active group.
Participants in both arms will be shown how to deliver the intervention at the Clinical Ageing Research Unit (Newcastle University) by an unblinded trainer after the participant’s eligibility has been confirmed and randomisation has occurred. The stimulation intensity will be controlled using the device control buttons and ramped up to a comfortable level of intensity. In addition to visual instructions, all participants receive a detailed instruction sheet on how to use the device. Participants will be phoned at weeks 4 and 8 to check progress. Following the 12-week treatment phase, participants will stop using the device, after they have additionally demonstrated their final stimulation to the assessor at the 12-week visit. This is followed by completion of the device tolerability and user questionnaire. Assessors will remain blinded.
The risk associated with the use of the gammaCore® nVNS device is minimal, and the safety profile is strong. The most common adverse events reported include local site tingling, pain, redness or itching and occur in approximately 17% of participants [34]. These adverse events are quickly resolved following discontinuation of the use of the device. Due to the theoretical risk of increased cardiac adverse events with stimulation of the right VN, participants will be asked to stimulate the left side [35]. Any adverse events (AE) will be diarised during the intervention and closely monitored until resolution, stabilisation or until it has been shown that the study procedures are not the cause. Participants who discontinue or deviate from the intervention protocol will be retained in the study as intention to treat.
Sample size
The purpose of this study is to establish proof of concept and feasibility for a larger clinical trial and the number of participants recruited for this study (n = 40) matches that of a recent feasibility trial [36]. This study will therefore establish a proof of concept and allow power calculations for a definitive trial.
Assessment procedures
All patients will be assessed while in an ‘on’ motor state, taking their medication as prescribed. Participants will undergo a baseline visit to assess study eligibility by a clinician (AJY). This will include a detailed clinical review to encompass demographic and anthropometric data, falls history and medication. This clinical review will additionally include haemodynamic and autonomic function assessment including a 12-lead electrocardiogram (ECG) recorded using a bedside monitor. The ECG will be scrutinised by a clinician (AJY) for cardiac abnormalities. Haemodynamic parameters including heart rate, systolic and diastolic blood pressure will be recorded. In addition, five minutes of artefact-free R-R (beat-to-beat) interval data will be recorded. Quantitative markers of autonomic activity – both in time and frequency domains – will be computed according to the Task Force of the European Society of Cardiology [37], including heart rate variability (HRV).
Health-related quality of life, depression, global cognition and motor evaluation will be assessed using the Parkinson’s Disease Questionnaire (PDQ-39) [38], Geriatric Depression Scale-15 (GDS-15) [39], the Montreal Cognitive Assessment (MoCA) [40] and the MDS Unified Parkinson’s Disease Rating Scale III (MDS-UPDRS III) [41] plus Hoehn and Yahr staging [29], respectively. Hand grip strength will be measured with a Jamar handheld hydraulic dynamometer (Promedics, UK) acquiring three trials bilaterally following a standard protocol [42]. Furthermore, we will employ the Clinical Frailty Scale (CFS) [43] to estimate frailty status in the two weeks before assessment; the Gastroparesis Cardinal Symptom Index (GCSI) [44] to explore the impact of nVNS on gastroparesis symptoms; the Late-Life Function & Disability Instrument (LLFDI) to measure deficits in function and disability [45]; and the Functional Assessment of Chronic Illness Therapy-Fatigue Scale (FACIT-Fatigue) [46,47,48] to monitor fatigue.
Primary outcome measures
We will primarily be examining the safety of the intervention in PwP and device tolerability by recording all adverse events reported by patients. Also, the proportion of eligible patients who agree to participate in the trial and the proportion of enrolled patients who successfully complete follow-ups will be monitored. During study visits, we will ask the participants to report any issues with using the device.
Secondary outcome measures
Gait
Gait and posture will be assessed pre- and post-intervention during a two-minute continuous walk using an instrumented walkway and a small, lightweight, sensor worn on the lower back (AX6, Axivity, York, UK; sampling frequency = 100 Hz, gravitational acceleration = ± 8 g, and gyroscope full-scale range (dps) = 2000, attached using double sided tape and secured in place using hypafix [BNS Medical Limited, Hull, UK]). The sensor is an inertial measurement unit comprising a tri-axial accelerometer and gyroscope. Participants will walk at a comfortable walking pace around a 25 m circuit inclusive of a 6.1 × 0.6 m instrumented walkway (ProtoKinetics LLC, Havertown, Pennsylvania, 120 Hz). The analysis will focus on dopa-resistant characteristics; namely, step time variability and step length variability [4, 13, 49], and other discrete characteristics representing five domains according to an a priori model of gait in PwP (see Table 2 for details) [50].
Ambulatory activity
Ambulatory activity will be measured in the real-world using a body-worn sensor on the lower back (same sensor as used in the laboratory assessment, attached using hydrogel patch [Amgel Technologies, USA] and secured in place using hypafix). Participants will be asked to wear the sensor for seven consecutive days to provide free-living data in terms of gait activity. Outcome measures will be in accordance with published work [51, 52]. Description of these measures, which can be broadly categorised into three domains including volume, pattern, and variability (macro gait characteristics), is described briefly in Table 3.
Previous work from our group indicates the sensor is well tolerated [51, 53, 54]. Macro gait characteristics will be measured following baseline (t1), the 12-week treatment phase (t4) and the 24-week assessment (t5).
Cognition
Attention and fluctuating attention will be measured using a computerised battery by assessing mean reaction time during simple reaction time (SRT), choice reaction time (CRT) and digit vigilance (DV) tests (examples of these tests are depicted in Fig. 2) [55, 56]. Power of attention (PoA) will be computed as a marker of attention and is the composite score of mean reaction time from all three tests reported in milliseconds. Fluctuating attention will be determined by the coefficient of variance (CoV) of the three tests. Also, digit vigilance accuracy will be reported as the percentage of correct targets detected.
The figure illustrates the three cognitive assessments: simple reaction time task (A), choice reaction time task (B) and Digit vigilance task (C) collectively measuring attention and fluctuating attention. Hand icon represents the participant’s hand responding to the stimuli displayed on the computer screen. For SRT and DV the dominant hand is used
Executive and memory functions will be evaluated using the computerised Cambridge Neuropsychological Test Automated Battery (CANTAB) [57]. Executive function will be assessed using the One Touch Stockings of Cambridge (OTS) and Paired Associates Learning (PAL) will be used to assess memory. The use of these two assessments is informed by previous research [56, 58]. The Trail Making Test (TMT) parts A and B will be used to assess attention and executive function, respectively.
Falls
Consistent with the recommendations of the Prevention of Falls Network Europe group falls will be defined as “an unexpected event in which the participant comes to rest on the ground, floor, or lower level” [59]. These will be recorded prospectively using standardised falls diaries, sent out on a monthly basis with a pre-paid envelope and regular telephone calls from a study team member, to verify the information [60, 61].
Cholinergic peripheral plasma markers
Approximately 14 ml of whole blood will be taken from participants pre- and post-intervention. Samples will be used to measure cholinergic peripheral markers in plasma. Metabolomic analysis of blood plasma concentrations of protein elements of the cholinergic metabolic pathway will be completed by external collaborators. Plasma will be analysed, and protein elements involved in the cholinergic (Cho) metabolic pathway will be identified and quantified. Assays will be validated for specificity, linearity, accuracy, precision, recovery, and stability according to FDA bio-analytical method validation guidelines [62].
Focus group
The primary goal of our focus group, which will be held with a subset of participants towards the end of the trial, will be to co-plan and co-design a future, multi-site nVNS trial in PwP.
Statistical analysis
The main analyses will be descriptive, to inform the design of a future trial. We will analyse the numbers of eligible patients enrolled, rate of recruitment, compliance with the intervention and completion of study assessments. We will ascertain the completeness and acceptability of nVNS and identify any potential barriers. Qualitative data analysis from the focus group will adopt a thematic approach [63] with NVivo (QSR International Pty Ltd. NVivo) software used as a coding tool. Statistical analyses will be conducted using MATLAB (The MathWorks, Inc., Natick, MA, USA), SPSS (IBM SPSS Statistics for Windows, Armonk, NY: IBM Corp) and/or R (http://www.r-project.org/). The outcome data will be summarised using descriptive statistics, including means (and medians), standard deviations, and interquartile ranges. It is expected that non-parametric statistics (e.g., Friedman test) will be used throughout due to the small number of participants in this study. To establish proof of concept for active nVNS treatment, we will compare the median change scores (post–pre assessment) of gait and cognitive outcomes between groups using Mann–Whitney U tests. An exploratory outcome will be a metabolomic analysis of cholinergic markers in blood plasma. We will explore the effects of concentrations of the various targeted small molecule Cho metabolites (Cho inclusive) in plasma on cognitive functions and discrete gait characteristics.
Discussion
nVNS is a non-pharmacological neuromodulation intervention currently approved by the FDA for the acute and preventive treatment of most forms of primary headache and received a CE mark from the Medicines and Healthcare products Regulatory Agency (MHRA) for the treatment of primary headache, as adjunctive therapy to reduce symptoms of certain anxiety and depression conditions and to reduce symptoms of gastric motility disorders and irritable bowel syndrome. nVNS furthermore has some evidence in improving symptoms in conditions including fatigue and systemic inflammation [64] which are characteristic features of PD.
In PD, previous investigations have shown that single-dose nVNS [24, 65] may improve discrete gait characteristics. However, no formal investigation of the safety and tolerability of the device has been conducted, especially in a multi-dose nVNS trial. According to findings from a large meta-analysis that synthesised all reported adverse events from studies, the safety profile of vagal stimulation is excellent [34].
In this 24-week feasibility trial, we aim to generate clinical, digital and laboratory data to assess the safety, tolerability, feasibility, and potential effectiveness of domiciliary multi-dose nVNS in people with Parkinson’s. Further outcomes from this study include metrics needed for sample size calculations for future studies and evidence that improvement in gait, cognition and autonomic function is enhanced when nVNS is delivered repeatedly and whether this is sustained over time. Critically, these study outcomes will be used to provide input to a future larger multi-centre trial with benefits seen for patients within the next few years.
Availability of data and materials
Not applicable. Data will be pseudo-anonymised using a unique identification code assigned at the first assessment and entered to a secure data system on Newcastle University computers for analysis.
Abbreviations
- CANTAB:
-
Cambridge neuropsychological test automated battery
- CFS:
-
Clinical frailty scale
- CRT:
-
Choice reaction time
- DV:
-
Digit vigilance
- ECG:
-
Electrocardiography
- FACIT-Fatigue:
-
Functional assessment of chronic illness therapy-fatigue
- GCSI:
-
Gastroparesis cardinal symptom index
- GDS:
-
Geriatric depression scale
- HRV:
-
Heart rate variability
- LLFDI:
-
Late-life function & disability instrument
- MDS-UPDRS:
-
Movement disorders society-unified parkinson's disease rating scale
- MHRA:
-
Medicines and healthcare products regulatory agency
- nbM:
-
Nucleus basalis of meynert
- OTS:
-
One touch stockings
- PAL:
-
Paired associative learning
- PD:
-
Parkinson's disease
- PDQ-39:
-
Parkinson's disease questionnaire-39
- PwP:
-
People with parkinson‘s
- REC:
-
Research ethics committee
- SRT:
-
Simple reaction time
- TAU:
-
Treatment as usual
- TMT:
-
Trail making test
- VN:
-
Vagus nerve
- VNS:
-
Vagus nerve stimulation
References
Deane KHO, Flaherty H, Daley DJ, Pascoe R, Penhale B, Clarke CE, et al. Priority setting partnership to identify the top 10 research priorities for the management of parkinson’s disease. BMJ Open. 2015;4. https://doi.org/10.1136/bmjopen-2014-006434.
Galna B, Lord S, Burn DJ, Rochester L. Progression of gait dysfunction in incident parkinson’s disease: impact of medication and phenotype. Mov Disord. 2015;30:359–67. https://doi.org/10.1002/mds.26110.
Lord S, Galna B, Yarnall AJ, Coleman S, Burn DJ, Rochester L. Predicting first fall in newly diagnosed parkinson’s disease: insights from a fall-naive cohort. Mov Disord. 2016;31:1829–36. https://doi.org/10.1002/mds.26742.
Rochester L, Galna B, Lord S, Yarnall AJ, Morris R, Duncan GW, et al. Decrease in Aβ42 predicts dopa-resistant gait progression in early parkinson disease. Neurology. 2017;88:1501–11. https://doi.org/10.1212/WNL.0000000000003840.
Lord S, Galna B, Coleman S, Yarnall AJ, Burn DJ, Rochester L. Cognition and gait show a selective pattern of association dominated by phenotype in incident parkinson’s disease. Front Aging Neurosci. 2014;6:1–9. https://doi.org/10.3389/fnagi.2014.00249.
Morris R, Lord S, Lawson RA, Coleman S, Galna B, Duncan GW, et al. Gait rather than cognition predicts decline in specific cognitive domains in early parkinson’s disease. Journals Gerontol - Ser A Biol Sci Med Sci. 2017;72:1656–62. https://doi.org/10.1093/gerona/glx071.
Rochester L, Yarnall AJ, Baker MR, David RV, Lord SS, Galna B, et al. Cholinergic dysfunction contributes to gait disturbance in early parkinson’s disease. Brain. 2012;135:2779–88. https://doi.org/10.1093/brain/aws207.
Yarnall AJ, Rochester L, Burn DJ. The interplay of cholinergic function, attention, and falls in parkinson’s disease. Mov Disord. 2011;26:2496–503. https://doi.org/10.1002/mds.23932.
Yarnall AJ, Rochester L, Baker MR, David R, Khoo TK, Duncan GW, et al. Short latency afferent inhibition: a biomarker for mild cognitive impairment in parkinson’s disease? Mov Disord. 2013;28:1285–8. https://doi.org/10.1002/mds.25360.
Wilson J, Yarnall AJ, Craig CE, Galna B, Lord S, Morris R, et al. Cholinergic basal forebrain volumes predict gait decline in parkinson’s disease. Mov Disord. 2020;36:611–21. https://doi.org/10.1002/mds.28453.
Sigurdsson HP, Yarnall AJ, Galna B, Lord S, Alcock L, Lawson RA, et al. Gait-related metabolic covariance networks at rest in parkinson’s disease. Mov Disord. 2022;37:1222–34. https://doi.org/10.1002/mds.28977.
Karachi C, Grabli D, Bernard FA, Tandé D, Wattiez N, Belaid H, et al. Cholinergic mesencephalic neurons are involved in gait and postural disorders in parkinson disease. J Clin Invest. 2010;120:2745–54. https://doi.org/10.1172/JCI42642.
Henderson EJ, Lord SR, Brodie MA, Gaunt DM, Lawrence AD, Close JCT, et al. Rivastigmine for gait stability in patients with parkinson’s disease (ReSPonD): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 2016;15:249–58. https://doi.org/10.1016/S1474-4422(15)00389-0.
Gratwicke J, Zrinzo L, Kahan J, Peters A, Beigi M, Akram H, et al. Bilateral deep brain stimulation of the nucleus basalis of meynert for parkinson disease dementia a randomized clinical trial. JAMA Neurol. 2018;75:169–78. https://doi.org/10.1001/jamaneurol.2017.3762.
Hulsey DR, Hays SA, Khodaparast N, Ruiz A, Das P, Rennaker RL, et al. Reorganization of motor cortex by vagus nerve stimulation requires cholinergic innervation. Brain Stimul. 2016;9:174–81. https://doi.org/10.1016/j.brs.2015.12.007.
Hays SA, Ruiz A, Bethea T, Khodaparast N, Carmel JB, Rennaker RL, et al. Vagus nerve stimulation during rehabilitative training enhances recovery of forelimb function after ischemic stroke in aged rats. Neurobiol Aging. 2016;43:111–8. https://doi.org/10.1016/j.neurobiolaging.2016.03.030.
Wang Y, Zhan G, Cai Z, Jiao B, Zhao Y, Li S, et al. Vagus nerve stimulation in brain diseases: therapeutic applications and biological mechanisms. Neurosci Biobehav Rev. 2021;127:37–53. https://doi.org/10.1016/j.neubiorev.2021.04.018.
Johnson RL, Wilson CG. A review of vagus nerve stimulation as a therapeutic intervention. J Inflamm Res. 2018;11:203–13. https://doi.org/10.2147/JIR.S163248.
Frangos E, Ellrich J, Komisaruk BR. Non-invasive access to the vagus nerve central projections via electrical stimulation of the external ear: FMRI evidence in humans. Brain Stimul. 2015;8:624–36. https://doi.org/10.1016/j.brs.2014.11.018.
Nichols JA, Nichols AR, Smirnakis SM, Engineer ND, Kilgard MP, Atzori M. Vagus nerve stimulation modulates cortical synchrony and excitability through the activation of muscarinic receptors. Neuroscience. 2011;189:207–14. https://doi.org/10.1016/j.neuroscience.2011.05.024.
Farrand AQ, Helke KL, Gregory RA, Gooz MB, Hinson VK, Boger HA. Vagus nerve stimulation improves locomotion and neuronal populations in a model of parkinson’s disease. Brain Stimul. 2017;10:1045–54. https://doi.org/10.1016/j.brs.2017.08.008.
Dawson J, Liu CY, Francisco GE, Cramer SC, Wolf SL, Dixit A, et al. Vagus nerve stimulation paired with rehabilitation for upper limb motor function after ischaemic stroke (VNS-REHAB): a randomised, blinded, pivotal, device trial. Lancet. 2021;397:1545–53. https://doi.org/10.1016/S0140-6736(21)00475-X.
Sigurdsson HP, Raw R, Hunter H, Baker MR, Taylor J-P, Rochester L, et al. Non-invasive vagus nerve stimulation in parkinson’s disease: current status and future prospects. Expert Rev Med Devices. 2021. https://doi.org/10.1080/17434440.2021.1969913.
Morris R, Yarnall AJ, Hunter H, Taylor JP, Baker MR, Rochester L. Noninvasive vagus nerve stimulation to target gait impairment in parkinson’s disease. Mov Disord. 2019;34:918–9. https://doi.org/10.1002/mds.27664.
Hausdorff JM. Gait variability : methods , modeling and meaning example of increased stride time variability in elderly fallers quantification of stride-to-stride fluctuations 2005;9:1–9. https://doi.org/10.1186/1743-Received.
Chan AW, Tetzlaff JM, Gøtzsche PC, Altman DG, Mann H, Berlin JA, SPIRIT, et al. Explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;2013:346. https://doi.org/10.1136/bmj.e7586.
Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža-Jerić K, SPIRIT, et al. statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;2013:158. https://doi.org/10.7326/0003-4819-158-3-201302050-00583.
Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–4. https://doi.org/10.1136/jnnp.55.3.181.
Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–42. https://doi.org/10.1212/wnl.17.5.427.
Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, et al. Clinical diagnostic criteria for dementia associated with parkinson’s disease. Mov Disord. 2007;22:1689–707. https://doi.org/10.1002/mds.21507.
Lord S, Galna B, Verghese J, Coleman S, Burn DJ, Rochester L. Independent domains of gait in older adults and associated motor and nonmotor attributes: validation of a factor analysis approach. J Gerontol A Biol Sci Med Sci. 2013;68:820–7. https://doi.org/10.1093/gerona/gls255.
Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31:103–15. https://doi.org/10.2307/2529712.
Sella F, Raz G, Cohen KR. When randomisation is not good enough: matching groups in intervention studies. Psychon Bull Rev. 2021. https://doi.org/10.3758/s13423-021-01970-5.
Redgrave J, Day D, Leung H, Laud PJ, Ali A, Lindert R, et al. Safety and tolerability of transcutaneous vagus nerve stimulation in humans; a systematic review. Brain Stimul. 2018;11:1225–38. https://doi.org/10.1016/j.brs.2018.08.010.
Brown GL, Eccles JC. The action of a single vagal volley on the rhythm of the heart beat. J Physiol. 1934;82:211–41. https://doi.org/10.1113/jphysiol.1934.sp003176.
Best KL, Miller WC, Routhier F, Eng JJ. Feasibility of the trial procedures for a randomized controlled trial of a community-based peer-led wheelchair training program for older adults. Pilot Feasibility Stud. 2018;4:1–12. https://doi.org/10.1186/s40814-017-0158-3.
North American Society of Pacing and Electrophysiology TF of the ES of C. Heart rate variability. Standards of measurement, physiological interpretation and clinical use. Eur Heart J. 1996;17:354–81.
Jenkinson C, Fitzpatrick R, Peto V, Greenhall R, Hyman N. The parkinson’s disease questionnaire (pdq-39): development and validation of a parkinson’s disease summary index score. Age Ageing. 1997;26:353–7. https://doi.org/10.1093/ageing/26.5.353.
Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. https://doi.org/10.1016/0022-3956(82)90033-4.
Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The montreal cognitive assessment, moca: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–9. https://doi.org/10.1111/j.1532-5415.2005.53221.x.
Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, et al. Movement disorder society-sponsored revision of the unified parkinson’s disease rating scale (mds-updrs): scale presentation and clinimetric testing results. Mov Disord. 2008;23:2129–70. https://doi.org/10.1002/mds.22340.
Roberts HC, Denison HJ, Martin HJ, Patel HP, Syddall H, Cooper C, et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing. 2011;40:423–9. https://doi.org/10.1093/ageing/afr051.
Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–95. https://doi.org/10.1503/cmaj.050051.
Revicki DA, Rentz AM, Dubois D, Kahrilas P, Stanghellini V, Talley NJ, et al. Gastroparesis cardinal symptom index ( GCSI ): development and validation of a patient reported assessment of severity of gastroparesis symptoms. Qual Life Res. 2004;13:833–44.
Jette AM, Haley SM, Coster WJ, Kooyoomjian JT, Levenson S, Heeren T, et al. Late life function and disability instrument: II.development and evaluation of the function component. Journals Gerontol - Ser A Biol Sci Med Sci. 2002;57:M217–22. https://doi.org/10.1093/gerona/57.4.M217.
Friedman JH, Alves G, Hagell P, Marinus J, Marsh L, Martinez-Martin P, et al. Fatigue rating scales critique and recommendations by the movement disorders society task force on rating scales for parkinson’s disease. Mov Disord. 2010;25:805–22. https://doi.org/10.1002/mds.22989.
Hagell P, Höglund A, Reimer J, Eriksson B, Knutsson I, Widner H, et al. Measuring fatigue in parkinson’s disease: a psychometric study of two brief generic fatigue questionnaires. J Pain Symptom Manage. 2006;32:420–32. https://doi.org/10.1016/j.jpainsymman.2006.05.021.
Elbers RG, Rietberg MB, Van Wegen EEH, Verhoef J, Kramer SF, Terwee CB, et al. Self-report fatigue questionnaires in multiple sclerosis, parkinson’s disease and stroke: a systematic review of measurement properties. Qual Life Res. 2012;21:925–44. https://doi.org/10.1007/s11136-011-0009-2.
Smulders K, Dale ML, Carlson-Kuhta P, Nutt JG, Horak FB. Pharmacological treatment in parkinson’s disease: Effects on gait. Park Relat Disord. 2016;31:3–13. https://doi.org/10.1016/j.parkreldis.2016.07.006.
Lord S, Galna B, Rochester L. Moving forward on gait measurement: toward a more refined approach. Mov Disord. 2013;28:1534–43. https://doi.org/10.1002/mds.25545.
Del Din S, Godfrey A, Rochester L. Validation of an accelerometer to quantify a comprehensive battery of gait characteristics in healthy older adults and parkinson’s disease: toward clinical and at home use. IEEE J Biomed Heal Informatics. 2016;20:838–47. https://doi.org/10.1109/JBHI.2015.2419317.
Hickey A, Del Din S, Rochester L, Godfrey A. Detecting free-living steps and walking bouts: validating an algorithm for macro gait analysis. Physiol Meas. 2017;38:N1-15. https://doi.org/10.1088/1361-6579/38/1/N1.
Fisher JM, Hammerla NY, Rochester L, Andras P, Walker RW. Body-worn sensors in parkinson’s disease: evaluating their acceptability to patients. Telemed e-Health. 2016;22:63–9. https://doi.org/10.1089/tmj.2015.0026.
Godfrey A, Lara J, Del Din S, Hickey A, Munro CA, Wiuff C, et al. iCap: Instrumented assessment of physical capability. Maturitas. 2015;82:116–22. https://doi.org/10.1016/j.maturitas.2015.04.003.
Nicholl CG, Lynch S, Kelly CA, White L, Simpson PM, Wesnes KA, et al. The cognitive drug research computerized assessment system in the evaluation of early dementia-is speed of the essence? Int J Geriatr Psychiatry. 1995;10:199–206. https://doi.org/10.1002/gps.930100306.
Lawson RA, Yarnall AJ, Duncan GW, Breen DP, Khoo TK, Williams-Gray CH, et al. Cognitive decline and quality of life in incident parkinson’s disease: the role of attention. Park Relat Disord. 2016;27:47–53. https://doi.org/10.1016/j.parkreldis.2016.04.009.
Robbins TW, James M, Owen AM, Sahakian BJ, McInnes L, Rabbitt P. Cambridge neuropsychological test automated battery (CANTAB): a factor analytic study of a large sample of normal elderly volunteers. Dementia. 1994;5:266–81. https://doi.org/10.1159/000106735.
Lawson RA, Williams-Gray CH, Camacho M, Duncan GW, Khoo TK, Breen DP, et al. Which neuropsychological tests? predicting cognitive decline and dementia in parkinson’s disease in the icicle-pd cohort. J Parkinsons Dis. 2021;11:1297–308. https://doi.org/10.3233/JPD-212581.
Lamb SE, Jørstad-Stein EC, Hauer K, Becker C. Development of a common outcome data set for fall injury prevention trials: the prevention of falls network europe consensus. J Am Geriatr Soc. 2005;53:1618–22. https://doi.org/10.1111/j.1532-5415.2005.53455.x.
Hunter H, Rochester L, Morris R, Lord S. Longitudinal falls data in parkinson’s disease: feasibility of fall diaries and effect of attrition. Disabil Rehabil. 2018;40:2236–41. https://doi.org/10.1080/09638288.2017.1329357.
Lord S, Galna B, Yarnall AJ, Morris R, Coleman S, Burn D, et al. Natural history of falls in an incident cohort of parkinson’s disease: early evolution, risk and protective features. J Neurol. 2017;264(11):2268–76. https://doi.org/10.1007/s00415-017-8620-y.
FDA. Bioanalytical method validation: guidance for industry. 2018:1–37. https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf.
Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3:77–101. https://doi.org/10.1191/1478088706qp063oa.
Tarn J, Legg S, Mitchell S, Simon B, Ng WF. The effects of noninvasive vagus nerve stimulation on fatigue and immune responses in patients with primary sjögren’s syndrome. Neuromodulation. 2019;22:580–5. https://doi.org/10.1111/ner.12879.
Mondal B, Choudhury S, Simon B, Baker MR, Kumar H. Noninvasive vagus nerve stimulation improves gait and reduces freezing of gait in parkinson’s disease. Mov Disord. 2019;34:917–8. https://doi.org/10.1002/mds.27662.
Acknowledgements
This work has been supported by the National Institute for Health Research (NIHR) Biomedical Research Unit based at Newcastle upon Tyne Hospitals National Health Service Foundation Trust and Newcastle University; the NIHR Newcastle BRC; and Newcastle CRF Infrastructure funding. We would like to thank Dr. Silvia Del Din for development of algorithms and codes for planned analyses of gait outcomes from wearable sensors. We thank Ann Ankers, Ann McNichol, Helen Pilkington, Tom Marshall, Liberty Emerson and Dr Rachael Raw for support.
Funding
The study is funded in full by research grants from Parkinson’s UK (ref: G-1903) and Dunhill Medical Trust (ref: RPGF1906\154) awarded to the senior author and Principal Investigator, Dr Alison J. Yarnall. The two funding bodies had no role or impact on the design of the study, data collection or writing of this manuscript. Newcastle upon Tyne NHS Hospitals Trust is the sponsor. All nVNS devices used in the study are supplied free of charge by ElectroCore, LLC (Basking Ridge, NJ, USA), who have no intellectual input to the design, analysis, and interpretation of the data.
Author information
Authors and Affiliations
Contributions
HPS contributed to the design of the work, data acquisition, data curation, wrote the original draft of the manuscript and revisions, and prepared Figs. 1–2; HH contributed to the acquisition of the data and substantively revised this manuscript; RW contributed to data acquisition and substantively revised this manuscript; LA contributed to data acquisition and substantively revised this manuscript; IP contributed to the design of the work and substantively revised this manuscript; EW contributed to the design of the work and substantively revised this manuscript; MRB contributed to the study conception, design of the study, and substantively revised this manuscript; JPT contributed to the design of the study, and substantively revised this manuscript; LR contributed to the conception and design of the study, and substantively revised this manuscript; AJY is the PI and study lead and has been involved in all aspects of the study development, implementation, drafting and revising this manuscript as well as funding acquisition and project administration. All authors have read and approved the final version of this manuscript. No other author contributed to the writing of this manuscript except those acknowledged.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval for this study was received in 2021 (the East Midlands—Derby Research Ethics Committee, REC reference: 21/EM/0177) and recruitment started in January 2022. The study has been prospectively registered with the ISRCTN registry (ISRCTN19394828, registered on August 23, 2021). This study is conducted in accordance with the Declaration of Helsinki. All participants are required to provide written informed consent by signing all study form(s), which are witnessed by an appropriately qualified and delegated member of the research team, prior to the completion of all study-specific procedures/investigations. Any protocol amendments will be approved by the appropriate research ethics committee.
Consent for publication
Not applicable.
Competing interests
HPS. has received support from Guarantors of Brain and the Newcastle Wellcome Trust Translational Partnership for attending conferences. His salary is paid by grants from Parkinson’s UK and Dunhill Medical Trust awarded to the PI AJY. HH: Salary is supported by the NIHR Newcastle clinical Research Facility (CRF) infrastructure. LA’s salary is supported by the NIHR Newcastle Clinical Research Facility (CRF) infrastructure, and LA has received grants from the Economic and Social Research Council (ESRC), Medical Research Council (MRC), Parkinson’s UK, and the Royal College of Radiologists. RW: None. IP: None. EW: None. MRB is employed by the NHS (Newcastle upon Tyne NHS Foundation Trust) and Newcastle University. His research is currently funded by the NIHR, MRC, EPSRC, LifeArc, MNDA and MyName’5 Dodie Foundation. JPT is supported by Newcastle NIHR BRC. He has received speaker fees from GE Healthcare and Bial, has received funding from Sosei-Heptares, and has consulted for Kyowa-Kirin. LR’s research program is supported in part by grants from the MRC, European Union, Parkinson’s UK, NIHR BRC, Cure Parkinson’s Trust, Health Research Council New Zealand, Dunhill Medical, and Stroke Association. AJY is supported by the Newcastle NIHR BRC plus grants from Parkinson’s UK, The Michael J. Fox Foundation, EU IMI, NIHR, Cure Parkinson’s Trust, Lewy Body Society, Intercept Pharmaceuticals, and the Weston Brain Institute. She has received honoraria from Teva-Lundbeck, GE Healthcare, Bial and Parkinson’s Academy and sponsorship from Teva-Lundbeck, UCB, GlaxoSmithKline, Genus, Britannia, and AbbVie for attending conferences.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sigurdsson, H.P., Hunter, H., Alcock, L. et al. Safety and tolerability of adjunct non-invasive vagus nerve stimulation in people with parkinson’s: a study protocol. BMC Neurol 23, 58 (2023). https://doi.org/10.1186/s12883-023-03081-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12883-023-03081-1