Abstract
Background
Patients with complex febrile seizures (CFS) often display abnormal laboratory results, unexpectedly prolonged seizures, and/or altered consciousness after admission. However, no standardized values have been established for the clinical and laboratory characteristics of CFS in the acute phase, making the management of CFS challenging. This study aimed to determine the clinical and laboratory characteristics of children with CFS during the acute phase. In particular, the duration of impaired consciousness and the detailed distribution of blood test values were focused.
Methods
We retrospectively reviewed medical records of a consecutive pediatric cohort aged 6–60 months who were diagnosed with CFS and admitted to Kobe Children’s Hospital between October 2002 and March 2017. During the study period, 486 seizure episodes with confirmed CFS were initially reviewed, with 317 seizure episodes included in the analysis. Detailed clinical and laboratory characteristics were summarized.
Results
Among 317 seizure episodes (296 children with CFS), 302 required two or fewer anticonvulsants to be terminated. In 296 episodes showing convulsive seizures, median seizure duration was 30.5 min. The median time from onset to consciousness recovery was 175 min. Impaired consciousness lasting > 6, 8, and 12 h was observed in 13.9%, 7.6%, and 1.9% patients with CFS, respectively. Additionally, the distribution of aspartate aminotransferase, lactate dehydrogenase, creatinine, and glucose were clarified with 3, 10, 50, 90, and 97 percentile values.
Conclusion
This study detailed the clinical and laboratory findings of acute-phase CFS using the data of the largest 15-year consecutive cohort of children with CFS. These results provide important information for appropriate acute management of CFS.
Similar content being viewed by others
Background
Febrile seizures (FS) are the most common seizure disorder in childhood, affecting 2–5% of children in the United States and 3.4–9.3% in Japan [1,2,3,4,5]. As defined by the American Academy of Pediatrics, FS occur in the absence of intracranial infection, metabolic disturbance, or a history of afebrile seizures, and are classified as simple or complex [6].
Simple FS (SFS) are isolated, brief, and generalized seizures [7]. Conversely, complex FS (CFS) are defined as those with focal onset, prolonged duration (greater than 10–15 min), or those that occur more than once within the same febrile illness [7, 8]. Approximately 35% of FS cases are classified as CFS [9].
Children with SFS usually do not require hospitalization. However, patients with CFS often have prolonged seizures and/or impaired consciousness or have an accompanying serious infection or infection whose etiology cannot be rapidly determined. Therefore, hospitalization is recommended for observation. In fact, several international guidelines also recommend routine admission for observation of all patients presenting with CFS [10, 11]; in our hospital, we also hospitalize patients with CFS.
The acute management of CFS depends on the seizure status and level of consciousness of the patient upon arrival at the hospital and on admission [4]. Recent literature suggests that routine lumbar puncture and urgent neuroimaging are not essential for all CFS cases [12, 13]. Therefore, physicians often perform routine laboratory tests and close observation during the acute management of CFS.
However, no standard values have been established for the clinical and laboratory characteristics of CFS in the acute phase. Therefore, appropriate acute management of CFS is difficult in the absence of reliable information when patients with CFS display abnormalities in laboratory results, prolonged seizures, or persistent altered consciousness at an early stage.
Accordingly, this study aimed to describe the basic epidemiological data regarding clinical and laboratory characteristics among children with CFS during the acute phase.
Methods
Patients
This study is a retrospective analysis based on a database review. All methods were performed in accordance with the relevant guidelines and regulations. We created a database of patients aged 1 month to 15 years who were admitted for fever and seizures to Kobe Children’s Hospital, a tertiary referral hospital where children with CFS are routinely admitted. We assessed consciousness using the Glasgow Coma Scale (GCS) in children recovering from seizures at least at hourly intervals. In the original cohort, we retrospectively reviewed the consecutive case records of children aged 6–60 months who were diagnosed with CFS between October 2002 and March 2017. Patients with incomplete medical data and a history of neurological disorders were excluded. Patients who needed intensive care, such as the use of continuous anticonvulsants or treatment with targeted temperature management, were also excluded because consciousness could not be evaluated.
Definitions of seizures
FS are defined as seizures accompanied by fever (temperature ≥ 38.0 °C) without central nervous system infection that occur in infants or children aged 6–60 months. Patients with a prior history of epilepsy were excluded from the FS category. CFS are defined as FS with one or more of the following three features: focal manifestations, prolonged (≥15 min) duration, and recurrence within 24 h according to the new Japanese guidelines for the management of FS [14]. Patients with temperatures of ≥38.0 °C within 24 h before or after seizure onset were treated as febrile cases and were included in this study, even if their temperature was lower than 38.0 °C upon admission.
Clinical and laboratory data
The following information was collected from patient records: age, sex, history of FS, body temperature at admission, presence of convulsive seizures, classification of CFS, total number and type of anticonvulsants used, duration of convulsive seizures, duration of impaired consciousness, and blood examination results after seizure onset.
The timing of seizure onset was determined based on the interviews with parents or observers, emergency transport records, referrals, and/or medical records. In cases with multiple seizures, the data of the final seizure that determined hospitalization were chosen and extracted.
In children who presented with convulsive seizures, the duration of the seizures was calculated as the time from the onset to the end of convulsive movement. When sequential convulsive seizures without full recovery of consciousness were observed, the duration of the seizures was determined from the beginning of the first convulsion to the end of the last convulsion. The duration of impaired consciousness was calculated as the time between seizure onset and a GCS score of 15 was confirmed by history or observation.
Blood tests included the following: white blood cell count, hemoglobin, platelet count, albumin, aspartate aminotransferase (AST), alanine aminotransferase, lactate dehydrogenase (LDH), creatine kinase, creatinine (Cr), sodium, glucose (Glu), C-reactive protein, pH, base excess, lactate, and prothrombin time. When multiple values were available for blood data, the first values recorded after the onset of seizures, which determined hospitalization, were selected for analysis.
Results
During the study period, 486 episodes of seizures with confirmed CFS were recorded. We excluded the following number of seizure episodes: 22 with incomplete data, 68 with a history of neurological disorders, 45 with continuous anticonvulsant administration, and 34 with targeted temperature management. The remaining 317 seizure episodes that occurred in 296 children were included in the analysis (Fig. 1). During the study period, 278 children, 17 children, and one child experienced CFS once, twice, and five times, respectively.
The patient’s clinical characteristics are summarized in Table 1. Convulsive seizures were observed in 277 patients, with 296 seizure episodes (93.4%). As for anticonvulsants, 302 (95.3%) seizure episodes required two or fewer anticonvulsants to terminate the seizures. Intravenous or suppository diazepam was administered for 205 seizure episodes (64.7%) and intravenous midazolam was administered for 66 seizure episodes (20.8%). Intravenous fosphenytoin was used for 23 seizure episodes (7.3%). Few children were treated with intravenous phenytoin, phenobarbital, thiamylal, buccal midazolam, or oral carbamazepine, all of which in total were administered for only 16 seizure episodes (5.0%). Figure 2 shows the distribution of the duration of convulsive seizures, with a median duration of 30.5 (range: 1–410) min. Of the 296 convulsive seizure episodes, 148 episodes (50%) terminated within 30 min, 234 (79.1%) terminated within 1 h, and 276 (93.2%) terminated within 2 h.
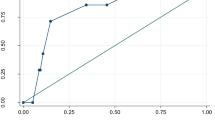
Figure 3 shows the relationship between the time from seizure onset and the cumulative percentage of patients with persistent impaired consciousness, demonstrating that the median time from seizure onset to full recovery of consciousness was 175 (range: 1–1260) min, and impaired consciousness lasting > 6, 8, and 12 h was observed in 13.9%, 7.6%, and 1.9% of the patients, respectively.
Relationship between time from seizure onset to full recovery of consciousness and cumulative percentage of patients with persistent impaired consciousness in patients with complex febrile seizures. Vertical axis shows the cumulative percentage of patients with persistent impaired consciousness. Horizontal axis shows time from seizure onset to full recovery of consciousness and the number of patients with persistent impaired consciousness
Table 2 shows the laboratory characteristics of patients with CFS. Our findings clarified the percentile distribution of multiple laboratory parameters as well as the mean and standard deviation. This information indicates that abnormal laboratory values are common in patients with CFS and reveals the actual percentage and extent of deviations from normal values.
Discussion
In the present study, we described the clinical and laboratory characteristics of CFS that required hospitalization. Although large studies on clinical features of febrile seizures have recently been published [15, 16], to the best of our knowledge, this is the first report to describe the detailed characteristics of acute-phase CFS.
Our study showed that the median duration of convulsive seizures was 30.5 min and the median time from seizure onset to full recovery of consciousness was 175 min in patients with CFS. This differs from the findings of previous reports on seizure duration and recovery time. Okumura et al. [17] examined the clinical features of prolonged unconsciousness and delirious behavior in children with FS and showed that the duration of seizures was < 5 min in 90.2% and the duration of unconsciousness was < 30 min in 93% of the seizures, leading to the conclusion that prolonged unconsciousness is rare in children with FS. In prior studies of epileptic seizures conducted by Allen et al. [18] and McKenny-Fick et al. [19], the median duration and recovery time of FS were 2.5 and 18 min, respectively. The discrepancy between our study and previous studies can be explained by the difference in the study participants. Patients with SFS were not included in our study, while the previous studies included patients with SFS. In addition, the hospital setting may have influenced this difference. While the previous reports mentioned above were conducted in hospitals providing secondary-level pediatric care, our hospital is a tertiary referral hospital for children. Therefore, there may be a tendency for more severe cases to be referred to our hospital instead of to other institutes.
In a previous study on febrile status epilepticus (FSE), which was defined as FS without full recovery lasting ≥ 30 min, Shinnar et al. [20] reported the consequences of 119 cases of FSE, showing that seizures lasted for a median of 68 min, 24% of the seizures lasted > 2 h, and 87% of the seizures did not stop spontaneously and were treated with benzodiazepines. Our study demonstrated that convulsive seizures of CFS lasted a median of 30.5 min and 6.8% of the convulsive seizures lasted > 2 h, and two-thirds of the seizures required anticonvulsants. In addition, half of the participants had FSE. In our study, the severity of seizures was milder than that reported by Shinnar et al. [20] because our study also included patients who had seizures lasting < 30 min. However, it is particularly worth noting that half of the patients with CFS had FSE. Berg et al. [9] reported that CFS and FSE accounted for approximately 35% and 5% of all FS, respectively. Therefore, our patients with CFS had more FSE than previously reported. We may have encountered only some of the more severe cases of CFS.
The most notable aspect of our study is its detailed focus on the duration of impaired consciousness and the distribution of blood test results. Prolonged impaired consciousness and abnormal laboratory values are expected to be more common in patients with CFS and FSE than in those with SFS. However, almost all previous reports on CFS and/or FSE, including that of Shinnar et al. [20], did not mention the details of the duration of impaired consciousness or blood test values. Therefore, these points could be considered the greatest novelty of this study.
The definitive diagnosis of CFS in the acute phase is sometimes challenging because patients with both CFS and acute encephalitis/encephalopathy (AEE) exhibit impaired consciousness with or without seizures as an initial manifestation. There is a well-established consensus on the clinical case definition and diagnostic methods for encephalitis [21]. However, in some cases of AEE, there may be no significant cerebrospinal fluid pleocytosis or no demonstrable neuroimaging abnormalities [21]. Therefore, complete differentiation between CFS and AEE at an early stage can sometimes be impossible in practice and often confounds clinicians, especially when symptoms during the acute phase are severe. Our previous report on the risk factors related to poor outcomes in patients with fever and seizures showed that refractory status epilepticus, consciousness disturbance or hemiplegia at 6 h from onset, or AST > 90 IU/L within 6 h of onset were associated with poor outcomes, leading to the final diagnosis of AEE [22, 23]. In addition, several reports have suggested that prolonged impaired consciousness and abnormal laboratory findings such as elevation of AST, LDH, Glu, and/or Cr might predict poor outcomes or AEE in children with seizures [24,25,26,27,28,29,30]. These abnormalities are sometimes observed in CFS, but their details in CFS have remained unclear. Our study is the first report to describe this information of acute-phase CFS, showing that impaired consciousness lasting > 6, 8, and 12 h was observed in 13.9%, 7.6%, and 1.9% of CFS cases, and 97 percentile values of AST, LDH, Cre, and Glu were 74 IU/L, 468 IU/L, 0.40 mg/dL, and 266 mg/dL, respectively. This information may be useful in that data outside of these ranges should alert an alternative diagnosis other than CFS at an early stage. Therefore, our findings may provide important information for appropriate acute management of CFS.
The present study has several limitations. First, this study was based on data from a single tertiary care institution; hence, our hospital is more likely to encounter more severe cases than other institutions. Second, since severe cases requiring intensive care were excluded, only mild cases may have been extracted, resulting in some selection bias. Third, even though we presented detailed data on the recovery time of consciousness, we did not identify independent factors that affected it. Previous reports have shown that the use of antiepileptic drugs significantly increased recovery time [18, 19]. In our report, two-thirds of patients were treated with one or more anticonvulsants. Therefore, drug administration may have resulted in a longer time to complete recovery of consciousness. However, factors other than drug type that affect recovery time need to be considered, such as the actual drug dose administered and the time taken from onset to the start of treatment. The present study design did not provide sufficient information on the drug dose and time from onset to treatment initiation, and thus it was not possible to examine factors that independently affected recovery time. Finally, this study only described the detailed characteristics of acute-phase CFS and did not compare the findings with those of patients with related disorders such as SFS or AEE. Therefore, the findings of this study do not allow us to differentiate CFS from these conditions. To confirm the clinical validity of the findings of this study as reliable information for the differential diagnosis of CFS, comparative studies with SFS and AEE are needed.
Conclusion
The present study describes the detailed clinical and laboratory findings of CFS in an acute setting by reviewing the largest 15-year consecutive data of children with CFS. These findings may provide important information for appropriate acute management of CFS.
Availability of data and materials
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- FS:
-
Febrile seizures
- SFS:
-
Simple febrile seizures
- CFS:
-
Complex febrile seizures
- GCS:
-
Glasgow Coma Scale
- AST:
-
Aspartate aminotransferase
- LDH:
-
Lactate dehydrogenase
- Cr:
-
Creatinine
- Glu:
-
Glucose
- FSE:
-
Febrile status epilepticus
- AEE:
-
Acute encephalitis/encephalopathy
References
Hauser WA. The prevalence and incidence of convulsive disorders in children. Epilepsia. 1994;35:1–6.
Baumann RJ, Duffner PK. Treatment of children with simple febrile seizures: the AAP practice parameter. American Academy of Pediatrics. Pediatr Neurol. 2000;23:11–7.
Tsuboi T. Epidemiology of febrile and afebrile convulsions in children in Japan. Neurology. 1984;34:175–81.
Sugai K. Current management of febrile seizures in Japan: an overview. Brain Dev. 2010;32:64–70.
Nishiyama M, Yamaguchi H, Ishida Y, Tomioka K, Takeda H, Nishimura N, et al. Seizure prevalence in children aged up to 3 years: a longitudinal population-based cohort study in Japan. BMJ Open. 2020;0(9):e035977.
Steering Committee on Quality Improvement and Management Subcommittee on Febrile Seizures American Academy of Pediatrics. Febrile seizures: clinical practice guideline for the long-term management of the child with simple febrile seizures. Pediatrics. 2008;121:1281–6.
Shinnar S, Glauser TA. Febrile seizures. J Child Neurol. 2002;17:44–52.
Waruiru C, Appleton R. Febrile seizures: an update. Arch Dis Child. 2004;89:751–6.
Berg AT, Shinnar S. Complex febrile seizures. Epilepsia. 1996;37:126–33.
Armon K, Stephenson T, Macfaul R, Hemingway P, Werneke U, Smith S. An evidence and consensus based guideline for the management of a child after a seizure. Emerg Med J. 2003;20:13–20.
Capovilla G, Mastrangelo M, Romeo A, Vigevano F. Recommendations for the management of “febrile seizures”: Ad Hoc Task Force of LICE Guidelines Commission. Epilepsia. 2009;50:2–6.
Kimia A, Ben-Joseph EP, Rudloe T, Capraro A, Sarco D, Hummel D, et al. Yield of lumbar puncture among children who present with their first complex febrile seizure. Pediatrics. 2010;126:62–9.
Kimia AA, Ben-Joseph E, Prabhu S, Rudloe T, Capraro A, Sarco D, et al. Yield of emergent neuroimaging among children presenting with a first complex febrile seizure. Pediatr Emerg Care. 2012;28:316–21.
Natsume J, Hamano SI, Iyoda K, Kanemura H, Kubota M, Mimaki M, et al. New guidelines for management of febrile seizures in Japan. Brain Dev. 2017;39:2–9.
Rivas-García A, Ferrero-García-Loygorri C, Carrascón González-Pinto L, Mora-Capín AA, Lorente-Romero J, Vázquez-López P. Simple and complex febrile seizures: is there such a difference? Management and complications in an emergency department. Neurologia (Engl Ed). 2022;37:317–24.
Elshana H, Özmen M, Uzunhan TA, Uzunhan O, Ünüvar E, Kiliç A, et al. A tertiary care center’s experience with febrile seizures: evaluation of 632 cases. Minerva Pediatr. 2017;69:194–9.
Okumura A, Uemura N, Suzuki M, Itomi K, Watanabe K. Unconsciousness and delirious behavior in children with febrile seizures. Pediatr Neurol. 2004;30:316–9.
Allen JE, Ferrie CD, Livingston JH, Feltbower RG. Recovery of consciousness after epileptic seizures in children. Arch Dis Child. 2007;92:39–42.
McKenny-Fick NM, Ferrie CD, Livingston JH, Taylor JC, Allen JE. Prolonged recovery of consciousness in children following symptomatic epileptic seizures. Seizure. 2009;18:180–3.
Shinnar S, Hesdorffer DC, Nordli DR Jr, Pellock JM, O’Dell C, Lewis DV, et al. Phenomenology of prolonged febrile seizures: results of the FEBSTAT study. Neurology. 2008;71:170–6.
Venkatesan A, Tunkel AR, Bloch KC, Lauring AS, Sejvar J, et al. Case definitions, diagnostic algorithms, and priorities in encephalitis: consensus statement of the international encephalitis consortium. Clin Infect Dis. 2013;57:1114–28.
Nagase H, Nakagawa T, Aoki K, Fujita K, Saji Y, Maruyama A, et al. Therapeutic indicators of acute encephalopathy in patients with complex febrile seizures. Pediatr Int. 2013;55:310–4.
Sasaki K, Nagase H, Maruyama A, Fujita K, Nishiyama M, Tanaka T, et al. Clinical prediction rule for neurological sequelae due to acute encephalopathy: a medical community-based validation study in Harima, Japan. BMJ Open. 2017;7(11):e016675.
Tada H, Takanashi J, Okuno H, Kubota M, Yamagata T, Kawano G, et al. Predictive score for early diagnosis of acute encephalopathy with biphasic seizures and late reduced diffusion (AESD). J Neurol Sci. 2015;358:62–5.
Yokochi T, Takeuchi T, Mukai J, Akita Y, Nagai K, Obu K, et al. Prediction of acute encephalopathy with biphasic seizures and late reduced diffusion in patients with febrile status epilepticus. Brain Dev. 2016;38:217–24.
Maegaki Y, Kurozawa Y, Tamasaki A, Togawa M, Tamura A, Hirao M, et al. Early predictors of status epilepticus-associated mortality and morbidity in children. Brain Dev. 2015;37:478–86.
Fukuyama T, Yamauchi S, Amagasa S, Hattori Y, Sasaki T, Nakajima H, et al. Early prognostic factors for acute encephalopathy with reduced subcortical diffusion. Brain Dev. 2018;40:707–13.
Oba C, Kashiwagi M, Tanabe T, Nomura S, Ogino M, Matsuda T, et al. Prognostic factors in the early phase of acute encephalopathy. Pediatr Int. 2018;60:270–5.
Morita H, Hosoya M, Kato A, Kawasaki Y, Suzuki H. Laboratory characteristics of acute encephalopathy with multiple organ dysfunctions. Brain Dev. 2005;27:477–82.
Hosoya M, Kawasaki Y, Katayose M, Sakuma H, Watanabe M, Igarashi E, et al. Prognostic predictive values of serum cytochrome c, cytokines, and other laboratory measurements in acute encephalopathy with multiple organ failure. Arch Dis Child. 2006;91:469–72.
Acknowledgements
We would like to thank Editage (www.editage.jp) for English language editing.
Funding
This study was supported by a Grant-in-Aid for Scientific Research (KAKENHI) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (Subject ID: 21K09047 to Hiroaki Nagase) and a Grant-in-Aid for Research on Measures for Intractable Diseases (21FC1005) from the Ministry of Health, Labor, and Welfare, Japan.
Author information
Authors and Affiliations
Contributions
T.T., A.M., and H.N. contributed to the conception and design of this study; T.T., H.Y., Y.I., K.T., M.N., D.T., A.M., and H.N. collected the data; T.T. performed data analysis and drafted the manuscript; H.Y., K.T., M.N., A.M., H.T., H.K., R.T., K.N., and H.N. critically reviewed the manuscript. All authors meet the ICMJE authorship criteria. All authors have read and approved the final manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committees of the Hyogo Prefectural Kobe Children’s Hospital (approval number 29–33) and Kobe University Hospital (Medical Research Ethics Committee of Kobe University Graduate School of Medicine, approval number 170019). The need for informed consent was waived due to the observational nature of the study, which was approved by the Medical Research Ethics Committee of Kobe University Graduate School of Medicine.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Tanaka, T., Yamaguchi, H., Ishida, Y. et al. Clinical and laboratory characteristics of complex febrile seizures in the acute phase: a case-series study in Japan. BMC Neurol 23, 28 (2023). https://doi.org/10.1186/s12883-023-03051-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12883-023-03051-7