Abstract
Background
Sleep characteristics associated with dementia are poorly defined and whether their associations vary by demographics and APOE genotype among older adults are unclear.
Methods
This population-based cross-sectional study included 4742 participants (age ≥ 65 years, 57.1% women) living in rural China. Sleep parameters were measured using the self-rated questionnaires of the Pittsburgh Sleep Quality Index and Epworth Sleepiness Scale. Global cognitive function was assessed with the Mini-Mental State Examination (MMSE). Dementia was diagnosed following the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, criteria, and the National Institute on Aging-Alzheimer’s Association criteria for Alzheimer’s disease (AD). Data were analysed using multiple logistic and general linear regression models.
Results
Dementia was diagnosed in 173 participants (115 with AD). Multivariable-adjusted odds ratio (OR) of dementia was 1.71 (95%CI, 1.07-2.72) for sleep duration ≤4 h/night (vs. > 6-8 h/night), 0.76 (0.49-1.18) for > 4-6 h/night, 1.63 (1.05-2.55) for > 8 h/night, 1.11 (1.03-1.20) for lower sleep efficiency (per 10% decrease), and 1.85 (1.19-2.89) for excessive daytime sleepiness. Very short sleep duration (≤4 h/night), lower sleep efficiency, and excessive daytime sleepiness were significantly associated with being diagnosed with AD (multivariable-adjusted OR range = 1.12-2.07; p < 0.05). The associations of sleep problems with dementia and AD were evident mainly among young-old adults (65-74 years) or APOE ε4 carriers. Among dementia-free participants, these sleep characteristics were significantly associated with a lower MMSE score.
Conclusions
Self-reported sleep problems in dementia are characterized by very short or long sleep duration, low sleep efficiency, and excessive daytime sleepiness, especially among young-old people and APOE ε4 carriers.
Trial registration
ChiCTR1800017758 (Aug 13, 2018).
Similar content being viewed by others
Background
Since the 1950s, China has experienced faster population aging than most countries in the world [1]. According to the Seventh National Population Census (2020), older people (age ≥ 65 years) accounted for 13.5% of China’s total population [2]. Along with rapid population aging, the age-related disorders such as cognitive impairment and dementia become increasingly common. For instance, a nationwide survey suggested that mild cognitive impairment and dementia affected 20.80 and 5.14%, respectively, of Chinese older adults [3]. In addition, as people age, sleep patterns change as well. The age-related changes in sleep patterns are characterized by a shorter sleep duration, longer sleep latency, lower sleep efficiency, worse sleep quality, and being more prone to daytime sleepiness compared with young adults [4]. Population-based studies showed that 57.8% of older adults reported poor sleep quality [16]. However, the population-based study of the Outcomes of Sleep Disorders in Older Men (MrOS Sleep Study) suggested that both short and long self-reported sleep durations were associated with worse global cognitive function [17]. In addition, most of the previous population-based cross-sectional studies have focused on the associations between sleep characteristics and cognitive performance rather than dementia [18, 19]. Furthermore, the majority of the previous studies have investigated the sleep characteristics of patients with dementia from clinical settings or care homes [8, 20]. Few population-based cross-sectional studies have assessed the associations of sleep characteristics with dementia and Alzheimer’s disease (AD) among older adults in China, especially those living in the rural communities with limited education and low socioeconomic status. Studying sleep features associated with dementia in rural residents is important because individuals from rural areas were more susceptible to dementia and AD [3], and more likely to have poor sleep, compared to older adults living in urban areas [21].
In addition, population-based studies have shown that poor sleep quality and dementia are more common in women than in men [3, Sleep characteristics Sleep characteristics (e.g., sleep duration, sleep quality, sleep efficiency, and sleep latency) were assessed via in-person interviews (self-report) using the validated Chinese version of the Pittsburgh Sleep Quality Index (PSQI) [30]. The PSQI is a 19-item self-rated questionnaire to assess sleep quality during the last month, which includes seven domains, i.e., subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of slee** medication, and daytime dysfunction. The score for each domain ranges from 0 to 3, and the total PSQI score ranges from 0 to 21. A higher score indicates poorer sleep quality. Poor sleep quality is defined as a total PSQI score > 5. The Chinese version of PSQI showed good internal consistency (Cronbach’s α = 0.82-0.83) and test-retest reliability (r = 0.85) [31]. The PSQI has been recommended for assessing sleep quality of people with dementia [32]. The sleep duration (hours/night) was categorized as very short (≤4), short (> 4 to 6), normal (> 6 to 8, reference), and long (> 8) sleep duration, consistent with previous studies [33, 34]. Sleep efficiency is defined as the percentage of time spent on actual sleep while in bed. Sleep latency refers to the minutes taken to fall asleep at night, and prolonged sleep latency is defined as the latency > 30 min [13]. Daytime sleepiness was assessed via the in-person interviews (self-report) using the Chinese version of the Epworth Sleepiness Scale (ESS) [35]. ESS is an 8-item Likert scale to measure the daytime sleepiness of the respondent in eight daily situations. The score of each question ranges from 0 to 3, leading to the total ESS score ranging from 0 to 24. A higher score indicates greater daytime sleepiness. EDS is defined as the total ESS score > 10. The Chinese version of the ESS showed good internal consistency (Cronbach’s α = 0.81) and test-retest reliability (r = 0.74) [36]. The ESS was also recommended for evaluating EDS among people with dementia [32]. A neuropsychological test battery, mainly including MMSE, the self-reported Ascertain Dementia 8-item Questionnaire, the Auditory Verbal Learning Test, the Category Verbal Fluency Test, the Forward and Backward Digit Span Test, and the Trail Making Test A and B, was used to assess subjective cognitive complaints, global cognitive function, and function of specific cognitive domains (e.g., memory, language, attention, and executive function). Global cognitive function was evaluated with the validated Chinese version of the MMSE [28]. MMSE score ranges from 0 to 30, with a higher score indicating better global cognitive performance. Dementia was diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV), criteria [37], following a three-step diagnostic procedure [38]. In brief, trained clinicians and interviewers conducted routine clinical examination and comprehensive assessments for each participant following the standard procedure. The assessments included medical history, a neurocognitive assessment battery, and activities of daily living (ADLs). Then, the neurologists specialized in dementia diagnosis and care reviewed all the information collected from the previous assessments, and made a preliminary judgement of dementia for participants who were suspected to have dementia. Finally, the neurologists conducted additional face-to-face interviews with those participants who were suspected to have dementia, and reassessed their medical history, cognitive status, ADLs, and whenever available, neuroimaging data. If the participants were not able to undergo the interview due to severe cognitive impairment or were not available for the face-to-face interviews (about 13%), the neurologists interviewed their family members, neighbors, or village doctors (who provide primary care services to local residents). Following all the interviews and assessments, the neurologists made the diagnosis of dementia according to the DSM-IV criteria [37]. In case of uncertainty, a senior neurologist (L.C.) was consulted, and a consensus was made on whether the participant had dementia. Dementia was further classified into AD according to the National Institute on Aging-Alzheimer’s Association (NIA-AA) criteria for probable AD [39] and vascular dementia (VaD) following the National Institute of Neurological Disorders and Stroke and the Association Internationale pour la Recherche et l’Enseignement en Neurosciences (NINDS-AIREN) criteria for probable VaD [40]. Dementia cases who could not be classified as either AD or VaD were considered other types of dementia. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in metres. BMI (kg/m2) was categorized as underweight (< 18.5), normal (18.5-23.9), overweight (24-27.9), and obese (≥28), following the criteria for the Chinese population [26]. Alcohol consumption and smoking status were categorized as current, former, and never drinking or smoking, respectively. Leisure-time physical activity was defined as doing any type of physical activity during leisure time at least once a week. Hypertension was defined as arterial blood pressure ≥ 140/90 mmHg or current use of antihypertensive agents (ATC codes C02, C03, and C07-C09). Diabetes was defined as the fasting blood glucose ≥7.0 mmol/L, or taking antidiabetic agents (ATC code A10), or a self-reported history of diabetes. Dyslipidemia was defined as TC ≥6.22 mmol/L, or TG ≥2.27 mmol/L, or LDL-C ≥ 4.14 mmol/L, or HDL-C < 1.04 mmol/L, or use of hypolipidemic agents (ATC code C10) [26]. Coronary heart disease was defined according to self-reported history or ECG examination, including angina, myocardial infarction, coronary angioplasty, and coronary artery bypass grafting. Stroke was defined according to self-reported history and neurological examination. Depressive symptoms were assessed using the 15-item Geriatric Depression Scale (GDS-15) [41]. The presence of depressive symptoms was defined as a GDS-15 score ≥ 5. Hypnotics use was defined as the use of hypnotics and sedatives (ATC code N05C). APOE genotype was dichotomized as carriers vs. non-carriers of the APOE ε4 allele. Characteristics of study participants by dementia status were compared using Mann-Whitney U test for continuous variables, and chi-square test for categorical variables. Binary logistic regression models were used to examine the associations of self-reported sleep characteristics with all-cause dementia and AD. We did not analyse VaD (n = 49) and other types of dementia (n = 9) separately owing to relatively few cases. General linear models were used to analyse the associations of self-reported sleep characteristics with MMSE score among dementia-free participants. We reported the main results from two models: Model 1 was adjusted for age, sex, and education, and Model 2 was additionally adjusted for BMI, alcohol consumption status, smoking status, leisure-time physical activity, hypertension, diabetes, dyslipidemia, coronary heart disease, stroke, depressive symptoms, hypnotics use, and APOE genotype. Missing values of each of covariates were treated as a dummy variable in order to maximize the sample size. Results for the category of missing values did not provide additional insight. We then tested the statistical interactions of self-reported sleep characteristics with age groups (< 75 vs. ≥75 years), sex, education (illiteracy vs. non-illiteracy), or APOE ε4 allele (carriers vs. non-carriers) on all-cause dementia and AD by simultaneously entering the independent variables and their cross-product term into the same model. We performed stratified analyses by demographics and APOE genotype. IBM SPSS Statistics for Windows, Version 22.0 (IBM Corp., Armonk, NY) was used for all analyses. Two-tailed p < 0.05 was considered to be statistically significant.Assessments of cognitive function, dementia, and dementia subtypes
Assessment of covariates
Statistical analysis
Results
Characteristics of study participants
Of the 4742 participants, 173 (3.6%) were diagnosed with dementia, including 115 (2.4%) with AD, 49 (1.0%) with VaD, and 9 (0.2%) with other types of dementia. The mean age of all participants was 71.27 years (standard deviation 4.99), 57.1% were women, and 38.7% were illiterate. Compared with dementia-free participants, participants with dementia were older, less educated, more likely to be women and underweight, less likely to be obese, smoke, and drink alcohol, had a higher prevalence of diabetes, dyslipidemia, coronary heart disease, stroke, and depressive symptoms, more likely to use hypnotics, had a higher PSQI score and a higher ESS score, and a lower MMSE score (p < 0.05). The two groups had no significant differences in leisure-time physical activity, hypertension, APOE ε4 status, and sleep duration (p > 0.05) (Table 1).
Associations of self-reported sleep characteristics with all-cause dementia and AD
Controlling for age, sex, and education, very short or long sleep duration, poor sleep quality, lower sleep efficiency, and EDS were significantly associated with an increased likelihood of all-cause dementia (Table 2, Model 1). When controlling for additional potential confounding factors, the associations with dementia remained statistically significant for all sleep characteristics, except poor sleep quality and prolonged sleep latency (Table 2, Model 2).
Similarly, very short sleep duration, poor sleep quality, lower sleep efficiency, prolonged sleep latency, and EDS were significantly associated with an increased likelihood of AD after controlling for age, sex, and education (Table 2, Model 1). In the fully-adjusted models, the associations between poor sleep quality and prolonged sleep latency with AD became non-significant (Table 2, Model 2). There was no significant association between long sleep duration and AD. Additionally adjusting for EDS did not alter the associations of nighttime sleep characteristics with all-cause dementia and AD (data not shown).
Associations between self-reported sleep characteristics and global cognitive function in dementia-free participants
General linear regression analysis suggested that controlling for age, sex, and education, very short or long sleep duration, poor sleep quality, lower sleep efficiency, prolonged sleep latency, and EDS were significantly associated with a lower MMSE score among participants who were free of dementia (Table 3, Model 1). In the fully-adjusted models, the associations remained statistically significant for very short and long sleep duration, lower sleep efficiency, and EDS, but not for poor sleep quality and prolonged sleep latency (Table 3, Model 2). When additionally adjusting for EDS, the associations between nighttime sleep characteristics and global cognitive function were similar to those in Model 2 (data not shown).
Interactions of demographics and APOE genotype with self-reported sleep characteristics on dementia and AD
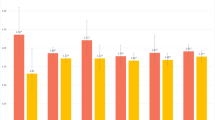
Figure 2 showed the results from stratified analyses, in which statistically significant interactions on the likelihood of dementia was detected. We reported the results from other interactive analyses and stratified analyses in the Additional file 1. These interaction and stratified analyses were summarized below.
Associations between self-reported sleep characteristics and dementia by age groups, sex, and APOE ε4 status. A Sleep duration and dementia by age groups; B Sleep efficiency and dementia by age groups; C Sleep quality and dementia by sex; D Sleep duration and dementia by APOE ε4 status; E Sleep latency and dementia by APOE ε4 status. Model 1 was adjusted for age, sex, and education. Model 2 was additionally adjusted for body mass index, alcohol consumption status, smoking status, leisure-time physical activity, hypertension, diabetes, dyslipidemia, coronary heart disease, stroke, depressive symptoms, hypnotics use, and APOE genotype. *p < 0.05, **p < 0.01, ***p < 0.001. Abbreviations: APOE apolipoprotein E gene, CI confidence interval, Ref. reference
We detected statistically significant interactions of age (< 75 vs. ≥75 years) with sleep duration (≤4 vs. > 6 to 8 h/night) and sleep efficiency (per 10% decrease) on the likelihood of dementia (p for interactions = 0.028 and 0.020, respectively). Further analyses stratified by age groups showed that, very short sleep duration and lower sleep efficiency were significantly associated with an increased likelihood of dementia among participants aged < 75 years, but not among those aged ≥75 years (Fig. 2A and B).
We examined the interactions of other sleep parameters with demographics and APOE ε4 allele on the likelihood of dementia. (1) We did not detect a statistical interaction of EDS with age groups on dementia (Supplementary Table 1, Additional file 1), but there was a statistical interaction between sex and sleep quality on dementia (p for interaction = 0.036), such that long sleep duration and EDS were associated with dementia only in women, while poor sleep quality was associated with dementia only in men (Fig. 2C and Supplementary Table 2, Additional file 1). (2) We did not detect statistical interactions between education and self-reported sleep characteristics on dementia. However, analyses stratified by education showed that the associations of lower sleep efficiency and EDS with dementia were significant only in illiterate persons, while the significant association of long sleep duration with dementia was found only in non-illiterate people (Supplementary Table 3, Additional file 1). (3) There were statistical interactions of APOE ε4 allele with sleep duration (≤4 vs. > 6 to 8 h/night) and sleep latency on the likelihood of dementia (p for interaction = 0.023 and 0.014, respectively). Analyses stratifying by APOE ε4 status suggested that extreme sleep duration, lower sleep efficiency, and prolonged sleep latency were significantly associated with dementia only in APOE ε4 allele carriers (Fig. 2D, E, and Supplementary Table 4, Additional file 1). Interactions and stratifying analyses for AD yielded results similar to those for all-cause dementia (Supplementary Tables 1-4, Additional file 1).
Discussion
In this population-based study of older adults who were living in the rural communities in western Shandong Province, China, we found that self-reported very short or long sleep duration, lower sleep efficiency, and EDS were independently associated with a higher likelihood of all-cause dementia and AD. Furthermore, among dementia-free participants, these sleep characteristics were also associated with low global cognitive performance. In addition, the associations of sleep problems with dementia and AD were evident mainly among young-old adults (age 65-74 years) and APOE ε4 allele carriers. Taken together, self-reported sleep problems among people with dementia are characterized by very short or long sleep duration, lower sleep efficiency, and EDS, especially among young-old adults and APOE ε4 allele carriers.
To the best of our knowledge, this is the first cross-sectional population-based study in China to characterize self-reported sleep characteristics associated with all-cause dementia and AD that targets older adults living in the rural communities. Previous studies showed that the prevalence of dementia was higher in rural than urban populations in China, probably due to limited education and low socioeconomic status in rural residents [3]. Moreover, compared with urban elderly residents, rural-dwelling older adults were more likely to have poor sleep [21]. Older adults in the rural areas often engage in heavy farmland labour work, have limited access to health care, are more likely to suffer from somatic disorders, and have insufficient knowledge of sleep hygiene, which in turn may be linked to poor sleep quality and dementia [21, 42]. Given that very few population-based studies have targeted rural residents, the associations between sleep characteristics and dementia among rural-dwelling older adults deserve further investigation.
We found that self-reported very short or long sleep duration, lower sleep efficiency, and EDS were independently associated with dementia or AD. Indeed, the meta-analysis of population-based cohort studies showed that both long and short sleep duration were associated with an increased risk of dementia and AD, although the association with short sleep duration was less evident [43]. Few cross-sectional population-based studies have examined the independent associations between sleep characteristics and dementia. An Italian population-based study of older adults showed that EDS was associated with dementia [44], which is in line with our findings. By contrast, the population-based case-control study in Greece showed that self-reported night sleep duration was not associated with AD after adjusting for demographic factors, BMI, depression diagnosis, and benzodiazepines use [14]. Differences in study design, ethnicities, socioeconomic status, sleep-related questionnaires, and control of potential confounders might partly contribute to the inconsistent findings across studies.
In addition, we found that self-reported very short and long sleep duration, lower sleep efficiency, and EDS were associated with worse global cognitive function in dementia-free participants. The associations of very short and long self-reported sleep duration with worse global cognitive function are in a good agreement with results from the MrOS Sleep Study [17]. However, a cross-sectional study of Spanish older adults showed that long (≥11 h), but not short (≤6 h), sleep duration was associated with worse cognitive function [15]. However, this study did not separate night and daytime sleep, which was important considering that long naps were associated with worse cognitive function [45]. A population-based study of urban-dwelling Chinese older adults also found that longer, but not shorter, sleep duration was associated with worse cognitive function [16]. However, participants in that study had much longer average sleep duration (7.96 h) and higher mean MMSE score (26.1) than those in our study. Thus, differences in socioeconomic status, educational levels, and medical conditions between rural and urban populations may partly contribute to the different results. Our findings of the associations between lower sleep efficiency and EDS with worse global cognitive function were similar to the reports from previous studies [44, 46]. Besides the self-reported sleep characteristics, the association of objectively-measured low sleep efficiency with cognitive impairment had been reported in the Study of Osteoporotic Fractures [47], which is in line with our study. Notably, the associations of nighttime sleep characteristics with all-cause dementia, AD, and low global cognitive function were present independent of EDS, suggesting that their associations could not be explained by daytime sleep features (e.g., EDS).
Several potential mechanisms may explain the cross-sectional associations of self-reported sleep characteristics with dementia and worse cognitive function. Very short sleep duration is associated with brain amyloid-β (Aβ) deposition [48], increased tau protein in cerebrospinal fluid [49], and suppression of hippocampal neurogenesis [50]. Long sleep duration may reflect a proinflammatory state [51] and has been associated with more white matter hyperintensities [52], which may be the pathways linking to dementia. In addition, lower sleep efficiency and EDS are associated with Aβ deposition [53, 54]. On the other hand, Aβ deposition and tau phosphorylation aggregation in the hypothalamus may affect brain regions that regulate sleep, and thus, may cause sleep problems in patients with dementia [55].
Few studies have explored the potential interactions of sleep characteristics with demographic and genetic factors on dementia in older adults. We found that the associations of self-reported very short sleep duration and lower sleep efficiency with dementia and AD were evident in young-old (< 75 years), but not in old-old (≥75 years), individuals. The exact reasons for the age-varying associations are unclear. Previous studies have indeed shown that older adults with dementia and poor sleep quality may have a higher risk of mortality [56, 57], which might partly contribute to the lack of cross-sectional associations of sleep duration and sleep efficiency with dementia in the old-old group. We did not find a consistent pattern of the sleep parameters-dementia associations across sexes, which was similar to a previous study [58]. Data from the Framingham Heart Study observed an interaction between sleep duration and education (lower than high school vs. high school degree and above) on incident dementia, such that long sleep duration was associated with a higher risk of incident dementia only among participants without a high school degree [59]. We did not detect statistical interactions between education and self-reported sleep characteristics on dementia. However, our data did show that the associations of lower sleep efficiency and EDS with dementia were evident mainly in illiterate individuals, while the associations between long sleep duration and dementia were present only in non-illiterate people. Differences in the study design (e.g., cross-sectional vs. longitudinal study) and demographic features of the study participants (e.g., education) might partly contribute to the different findings. Furthermore, we found that very short and long sleep duration and prolonged sleep latency were associated with dementia and AD mainly among APOE ε4 allele carriers, which was in line with the potential that extreme sleep duration and carrying APOE ε4 allele may act additively or synergistically to be linked with dementia via common pathways such as Alzheimer pathology and neuroinflammation [60]. Previous studies have yielded mixed results with regard to whether the associations between sleep problems and dementia in older adults vary by APOE ε4 allele status. For instance, a cohort study showed that better sleep consolidation could attenuate the effect of APOE ε4 allele on AD risk, which is consistent with our cross-sectional study [61]. By contrast, another longitudinal study showed that the association between sleep problems and dementia existed only among non-carriers of the APOE ε4 allele [58]. Thus, the complex interrelationships between poor sleep, genetic susceptibility, and dementia merit further exploration.
Our study targeted rural-dwelling Chinese older adults, to whom insufficient attention has been paid so far by the research community, and we performed comprehensive assessments of self-reported sleep characteristics and cognitive function. Our study also has limitations. First, the cross-sectional design prevents us from making any causal inferences regarding the associations between self-reported sleep characteristics and cognitive outcomes, and the observed cross-sectional associations may be subject to selective survival bias. Instead, our study aimed to characterize the self-reported sleep characteristics associated with dementia among rural-dwelling Chinese older adults. Second, multiple testing could increase the possibility of detecting the false positive associations, although the main analyses were driven primarily by our research hypothesis. Third, sleep characteristics were assessed retrospectively through self-report, which might be subject to recall bias, especially for people with dementia, although PSQI and ESS were recommended to assess sleep characteristics among individuals with dementia via self-report [32]. Of note, the majority (~ 93%) of dementia cases in our study were classified to have mild dementia (i.e., Clinical Dementia Rating Scale score ≤ 1). In this regard, findings from our study might not be applicable to all patients with dementia from the general rural population owing to this selection bias. In addition, we also found associations between abnormal sleep characteristics and worse cognitive performance among dementia-free participants, which partly supported the observed associations between certain sleep characteristics and dementia. Nevertheless, given that participants in our analytical sample were relatively younger and received more years of education than the target population, our results might have been affected by selection bias, which might lead to overestimations of the association between sleep problems and dementia. Fourth, due to the relatively high proportion of illiterate persons in our study participants, some dementia cases might have been misclassified. However, our comprehensive diagnostic procedure for dementia (e.g., twice face-to-face interviews) might help minimize the misclassification [38]. Finally, we did not have a standard approach to define sleep apnea, despite its strong association with dementia.
Conclusions
Our study shows that certain self-reported sleep characteristics (e.g., very short and long sleep duration and low sleep efficiency) are independently associated with dementia and poor global cognitive performance in Chinese rural older adults, in which the associations may vary by age and APOE ε4 status. This suggests that sleep characteristics associated with dementia and poor global cognitive function among Chinese rural older adults were similar to those reported from urban elderly residents. Future long-term prospective studies are needed to clarify their temporal and causal relationships, in which self-reported sleep characteristics can be integrated with those from objective assessments (e.g., polysomnography and actigraphy).
Availability of data and materials
Human genetic data from MIND-China are unsuitable for public deposition due to current regulations. However, the datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request and approval by the MIND-China Steering Committee.
Abbreviations
- AD:
-
Alzheimer’s disease
- ADLs:
-
Activities of daily living
- APOE :
-
Apolipoprotein E gene
- ATC:
-
Anatomical Therapeutic Chemical
- Aβ:
-
Amyloid β
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- DSM-IV:
-
The Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition
- ECG:
-
Electrocardiogram
- EDS:
-
Excessive daytime sleepiness
- ESS:
-
Epworth Sleepiness Scale
- GDS-15:
-
The 15-item Geriatric Depression Scale
- HDL-C:
-
High-density lipoprotein cholesterol
- LDL-C:
-
Low-density lipoprotein cholesterol
- MIND-China:
-
Multimodal Interventions to Delay Dementia and Disability in Rural China
- MMSE:
-
Mini-Mental State Examination
- OR:
-
Odds ratio
- the MrOS Sleep Study:
-
The Outcomes of Sleep Disorders in Older Men Sleep Study
- PSQI:
-
Pittsburgh Sleep Quality Index
- Ref.:
-
Reference
- TC:
-
Total cholesterol
- TG:
-
Triglycerides
References
United Nations, Department of Economic and Social Affairs, Population Division. World population prospects 2019. 2019. https://population.un.org/wpp. Accessed 5 June 2019
National Bureau of Statistics of China, Office of the Leading Group of the state Council for the Seventh National Population Census. Communiqué of the Seventh National Population Census. 2021. http://www.gov.cn/guoqing/2021-05/13/content_5606149.htm. Accessed 11 May 2021.
Jia L, Quan M, Fu Y, Zhao T, Li Y, Wei C, et al. Dementia in China: epidemiology, clinical management, and research advances. Lancet Neurol. 2020;19(1):81–92. https://doi.org/10.1016/s1474-4422(19)30290-x.
Mander BA, Winer JR, Walker MP. Sleep and human aging. Neuron. 2017;94(1):19–36. https://doi.org/10.1016/j.neuron.2017.02.004.
Wu W, Wang W, Dong Z, **e Y, Gu Y, Zhang Y, et al. Sleep quality and its associated factors among low-income adults in a rural area of China: a population-based study. Int J Environ Res Public Health. 2018;15(9):2055. https://doi.org/10.3390/ijerph15092055.
Ford ES, Cunningham TJ, Giles WH, Croft JB. Trends in insomnia and excessive daytime sleepiness among U.S. adults from 2002 to 2012. Sleep Med. 2015;16(3):372–8. https://doi.org/10.1016/j.sleep.2014.12.008.
Gulia KK, Kumar VM. Sleep disorders in the elderly: a growing challenge. Psychogeriatrics. 2018;18(3):155–65. https://doi.org/10.1111/psyg.12319.
Webster L, Costafreda Gonzalez S, Stringer A, Lineham A, Budgett J, Kyle S, et al. Measuring the prevalence of sleep disturbances in people with dementia living in care homes: a systematic review and meta-analysis. Sleep. 2020;43(4):zsz251. https://doi.org/10.1093/sleep/zsz251.
Sindi S, Kåreholt I, Johansson L, Skoog J, Sjöberg L, Wang HX, et al. Sleep disturbances and dementia risk: a multicenter study. Alzheimers Dement. 2018;14(10):1235–42. https://doi.org/10.1016/j.jalz.2018.05.012.
Lutsey PL, Misialek JR, Mosley TH, Gottesman RF, Punjabi NM, Shahar E, et al. Sleep characteristics and risk of dementia and Alzheimer's disease: the atherosclerosis risk in communities study. Alzheimers Dement. 2018;14(2):157–66. https://doi.org/10.1016/j.jalz.2017.06.2269.
Shi L, Chen SJ, Ma MY, Bao YP, Han Y, Wang YM, et al. Sleep disturbances increase the risk of dementia: a systematic review and meta-analysis. Sleep Med Rev. 2018;40:4–16. https://doi.org/10.1016/j.smrv.2017.06.010.
Xu W, Tan CC, Zou JJ, Cao XP, Tan L. Sleep problems and risk of all-cause cognitive decline or dementia: an updated systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2020;91(3):236–44. https://doi.org/10.1136/jnnp-2019-321896.
Suh SW, Han JW, Lee JR, Byun S, Kwon SJ, Oh SH, et al. Sleep and cognitive decline: a prospective nondemented elderly cohort study. Ann Neurol. 2018;83(3):472–82. https://doi.org/10.1002/ana.25166.
Basta M, Simos P, Vgontzas A, Koutentaki E, Tziraki S, Zaganas I, et al. Associations between sleep duration and cognitive impairment in mild cognitive impairment. J Sleep Res. 2019:e12864. https://doi.org/10.1111/jsr.12864.
Faubel R, López-García E, Guallar-Castillón P, Graciani A, Banegas JR, Rodríguez-Artalejo F. Usual sleep duration and cognitive function in older adults in Spain. J Sleep Res. 2009;18(4):427–35. https://doi.org/10.1111/j.1365-2869.2009.00759.x.
Auyeung TW, Lee JS, Leung J, Kwok T, Leung PC, Woo J, et al. Cognitive deficit is associated with phase advance of sleep-wake rhythm, daily nap**, and prolonged sleep duration--a cross-sectional study in 2947 community-dwelling older adults. Age (Dordr). 2013;35(2):479–86. https://doi.org/10.1007/s11357-011-9366-6.
Blackwell T, Yaffe K, Ancoli-Israel S, Redline S, Ensrud KE, Stefanick ML, et al. Association of sleep characteristics and cognition in older community-dwelling men: the MrOS sleep study. Sleep. 2011;34(10):1347–56. https://doi.org/10.5665/SLEEP.1276.
Low DV, Wu MN, Spira AP. Sleep duration and cognition in a nationally representative sample of U.S. older adults. Am J Geriatr Psychiatry. 2019;27(12):1386–96. https://doi.org/10.1016/j.jagp.2019.07.001.
Spira AP, Stone KL, Redline S, Ensrud KE, Ancoli-Israel S, Cauley JA, et al. Actigraphic sleep duration and fragmentation in older women: associations with performance across cognitive domains. Sleep. 2017;40(8):zsx073. https://doi.org/10.1001/jamaneurol.2013.4258.
Guarnieri B, Adorni F, Musicco M, Appollonio I, Bonanni E, Caffarra P, et al. Prevalence of sleep disturbances in mild cognitive impairment and dementing disorders: a multicenter Italian clinical cross-sectional study on 431 patients. Dement Geriatr Cogn Disord. 2012;33(1):50–8. https://doi.org/10.1007/s10072-014-1873-7.
Zhang YS, ** Y, Rao WW, Jiang YY, Cui LJ, Li JF, et al. Prevalence and socio-demographic correlates of poor sleep quality among older adults in Hebei province, China. Sci Rep. 2020;10(1):12266. https://doi.org/10.1038/s41598-020-68997-x.
Cunningham TJ, Ford ES, Chapman DP, Liu Y, Croft JB. Independent and joint associations of race/ethnicity and educational attainment with sleep-related symptoms in a population-based US sample. Prev Med. 2015;77:99–105. https://doi.org/10.1016/j.ypmed.2015.05.008.
Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261(5123):921–3. https://doi.org/10.1126/science.8346443.
Spira AP, An Y, Peng Y, Wu MN, Simonsick EM, Ferrucci L, et al. APOE genotype and nonrespiratory sleep parameters in cognitively intact older adults. Sleep. 2017;40(8):zsx076. https://doi.org/10.1093/sleep/zsx076.
Cong L, Ren Y, Hou T, Han X, Dong Y, Wang Y, et al. Use of cardiovascular drugs for primary and secondary prevention of cardiovascular disease among rural-dwelling older Chinese adults. Front Pharmacol. 2020;11:608136. https://doi.org/10.3389/fphar.2020.608136.
Han X, Jiang Z, Li Y, Wang Y, Liang Y, Dong Y, et al. Sex disparities in cardiovascular health metrics among rural-dwelling older adults in China: a population-based study. BMC Geriatr. 2021;21(1):158. https://doi.org/10.1186/s12877-021-02116-x.
Kivipelto M, Mangialasche F, Snyder HM, Allegri R, Andrieu S, Arai H, et al. World-wide FINGERS network: a global approach to risk reduction and prevention of dementia. Alzheimers Dement. 2020;16(7):1078–94. https://doi.org/10.1002/alz.12123.
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. https://doi.org/10.1016/0022-3956(75)90026-6.
Zhang MY, Katzman R, Salmon D, ** H, Cai GJ, Wang ZY, et al. The prevalence of dementia and Alzheimer's disease in Shanghai, China: impact of age, gender, and education. Ann Neurol. 1990;27(4):428–37. https://doi.org/10.1002/ana.410270412.
Buysse DJ, Reynolds CF III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. https://doi.org/10.1016/0165-1781(89)90047-4.
Tsai PS, Wang SY, Wang MY, Su CT, Yang TT, Huang CJ, et al. Psychometric evaluation of the Chinese version of the Pittsburgh sleep quality index (CPSQI) in primary insomnia and control subjects. Qual Life Res. 2005;14(8):1943–52. https://doi.org/10.1007/s11136-005-4346-x.
Guarnieri B, Musicco M, Caffarra P, Adorni F, Appollonio I, Arnaldi D, et al. Recommendations of the sleep study Group of the Italian Dementia Research Association (SINDem) on clinical assessment and management of sleep disorders in individuals with mild cognitive impairment and dementia: a clinical review. Neurol Sci. 2014;35(9):1329–48. https://doi.org/10.1007/s10072-014-1873-7.
Strand LB, Tsai MK, Gunnell D, Janszky I, Wen CP, Chang SS. Self-reported sleep duration and coronary heart disease mortality: a large cohort study of 400,000 Taiwanese adults. Int J Cardiol. 2016;207:246–51. https://doi.org/10.1016/j.ijcard.2016.01.044.
Kwon S, Lee H, Lee JT, Shin MJ, Choi S, Oh H. Sleep duration and mortality in Korean adults: a population-based prospective cohort study. BMC Public Health. 2020;20(1):1623. https://doi.org/10.1186/s12889-020-09720-3.
Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–5. https://doi.org/10.1093/sleep/14.6.540.
Chen N, Johns MW, Li H, Chu C, Liang S, Shu Y, et al. Validation of a Chinese version of the Epworth sleepiness scale. Qual Life Res. 2002;11(8):817–21. https://doi.org/10.1023/a:1020818417949.
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV). Washington, DC: American Psychiatric Association; 1994.
Jiang Z, Han X, Wang Y, Hou T, Cong L, Tang S, et al. Red cell distribution width and dementia among rural-dwelling older adults: the MIND-China study. J Alzheimers Dis. 2021;83(3):1187–98. https://doi.org/10.3233/JAD-210517.
McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):263–9. https://doi.org/10.1016/j.jalz.2011.03.005.
Román GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN international workshop. Neurology. 1993;43(2):250–60. https://doi.org/10.1212/wnl.43.2.250.
Almeida OP, Almeida SA. Short versions of the geriatric depression scale: a study of their validity for the diagnosis of a major depressive episode according to ICD-10 and DSM-IV. Int J Geriatr Psychiatry. 1999;14(10):858–65 https://doi.org/10.1002/(sici)1099-1166(199910)14:10<858::aid-gps35>3.0.co;2-8.
Lu L, Wang SB, Rao W, Zhang Q, Ungvari GS, Ng CH, et al. The prevalence of sleep disturbances and sleep quality in older Chinese adults: a comprehensive meta-analysis. Behav Sleep Med. 2019;17(6):683–97. https://doi.org/10.1080/15402002.2018.1469492.
Fan L, Xu W, Cai Y, Hu Y, Wu C. Sleep duration and the risk of dementia: a systematic review and meta-analysis of prospective cohort studies. J Am Med Dir Assoc. 2019;20(12):1480–7.e5. https://doi.org/10.1016/j.jamda.2019.06.009.
Merlino G, Piani A, Gigli GL, Cancelli I, Rinaldi A, Baroselli A, et al. Daytime sleepiness is associated with dementia and cognitive decline in older Italian adults: a population-based study. Sleep Med. 2010;11(4):372–7. https://doi.org/10.1016/j.sleep.2009.07.018.
Owusu JT, Wennberg AMV, Holingue CB, Tzuang M, Abeson KD, Spira AP. Nap** characteristics and cognitive performance in older adults. Int J Geriatr Psychiatry. 2019;34(1):87–96. https://doi.org/10.1002/gps.4991.
Ma XQ, Jiang CQ, Xu L, Zhang WS, Zhu F, ** YL, et al. Sleep quality and cognitive impairment in older Chinese: Guangzhou biobank cohort study. Age Ageing. 2019;49(1):119–24. https://doi.org/10.1093/ageing/afz120.
Blackwell T, Yaffe K, Ancoli-Israel S, Schneider JL, Cauley JA, Hillier TA, et al. Poor sleep is associated with impaired cognitive function in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2006;61(4):405–10. https://doi.org/10.1093/gerona/61.4.405.
Spira AP, Gamaldo AA, An Y, Wu MN, Simonsick EM, Bilgel M, et al. Self-reported sleep and β-amyloid deposition in community-dwelling older adults. JAMA Neurol. 2013;70(12):1537–43. https://doi.org/10.1001/jamaneurol.2013.4258.
Holth JK, Fritschi SK, Wang C, Pedersen NP, Cirrito JR, Mahan TE, et al. The sleep-wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Science. 2019;363(6429):880–4. https://doi.org/10.1126/science.aav2546.
Kent BA, Mistlberger RE. Sleep and hippocampal neurogenesis: implications for Alzheimer's disease. Front Neuroendocrinol. 2017;45:35–52. https://doi.org/10.1016/j.yfrne.2017.02.004.
Grandner MA, Sands-Lincoln MR, Pak VM, Garland SN. Sleep duration, cardiovascular disease, and proinflammatory biomarkers. Nat Sci Sleep. 2013;5:93–107. https://doi.org/10.2147/nss.S31063.
Ramos AR, Dong C, Rundek T, Elkind MS, Boden-Albala B, Sacco RL, et al. Sleep duration is associated with white matter hyperintensity volume in older adults: the northern Manhattan study. J Sleep Res. 2014;23(5):524–30. https://doi.org/10.1111/jsr.12177.
Ju YE, McLeland JS, Toedebusch CD, **ong C, Fagan AM, Duntley SP, et al. Sleep quality and preclinical Alzheimer disease. JAMA Neurol. 2013;70(5):587–93. https://doi.org/10.1001/jamaneurol.2013.2334.
Xu W, Tan L, Su BJ, Yu H, Bi YL, Yue XF, et al. Sleep characteristics and cerebrospinal fluid biomarkers of Alzheimer's disease pathology in cognitively intact older adults: the CABLE study. Alzheimers Dement. 2020;16:1146–52. https://doi.org/10.1002/alz.12117.
Wang C, Holtzman DM. Bidirectional relationship between sleep and Alzheimer's disease: role of amyloid, tau, and other factors. Neuropsychopharmacology. 2020;45(1):104–20. https://doi.org/10.1038/s41386-019-0478-5.
Kheirbek RE, Fokar A, Little JT, Balish M, Shara NM, Boustani MA, et al. Association between antipsychotics and all-cause mortality among community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2019;74(12):1916–21. https://doi.org/10.1093/gerona/glz045.
da Silva AA, de Mello RG, Schaan CW, Fuchs FD, Redline S, Fuchs SC. Sleep duration and mortality in the elderly: a systematic review with meta-analysis. BMJ Open. 2016;6(2):e008119. https://doi.org/10.1136/bmjopen-2015-008119.
Lysen TS, Luik AI, Ikram MK, Tiemeier H, Ikram MA. Actigraphy-estimated sleep and 24-hour activity rhythms and the risk of dementia. Alzheimers Dement. 2020;16(9):1259–67. https://doi.org/10.1002/alz.12122.
Westwood AJ, Beiser A, Jain N, Himali JJ, DeCarli C, Auerbach SH, et al. Prolonged sleep duration as a marker of early neurodegeneration predicting incident dementia. Neurology. 2017;88(12):1172–9. https://doi.org/10.1212/WNL.0000000000003732.
Hwang JY, Byun MS, Choe YM, Lee JH, Yi D, Choi JW, et al. Moderating effect of APOE ε4 on the relationship between sleep-wake cycle and brain β-amyloid. Neurology. 2018;90(13):e1167–73. https://doi.org/10.1212/WNL.0000000000005193.
Lim AS, Yu L, Kowgier M, Schneider JA, Buchman AS, Bennett DA. Modification of the relationship of the apolipoprotein E ε4 allele to the risk of Alzheimer disease and neurofibrillary tangle density by sleep. JAMA Neurol. 2013;70(12):1544–51. https://doi.org/10.1001/jamaneurol.2013.4215.
Acknowledgements
The authors would like to thank the study participants, medical staff at the Yanlou Town Hospital, and the MIND-China research group at the Shandong Provincial Hospital for their invaluable contributions to data collection and management.
Funding
MIND-China was financially supported in part by the grant from the National Key Research and Development Program of China (grant no.: 2017YFC1310100), the National Natural Science Foundation of China (NSFC, grants no.: 81861138008, and 82011530139), the Academic Promotion Program of Shandong First Medical University, and the Taishan Scholar Program of Shandong Province, China. Qiu C received grants from the Swedish Research Council (VR, grants no.: 2017-00740, 2017-05819, and 2020-01574), the Swedish Foundation for International Cooperation in Research and Higher Education (STINT, grant no.: CH2019-8320) for the China-Sweden Cooperative and Mobility Program, and Karolinska Institutet, Stockholm, Sweden. Tang S received grants from the NSFC (grant no.: 82001397), and the **an Science and Technology Bureau (grant no.: 202019187). Sindi S received research support from Alzheimerfonden, Demensförbundet, Karolinska Institutet Foundation and Funds (KI Stiftelser och Fonder), and Loo and Hans Osterman Foundation for Medical Research, Stockholm, Sweden. The funding body did not participate in the design of the study and collection, analysis and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
R.L., S.T., Y.W., Y. Du, and C.Q. designed the study. R.L., S.T., Y.W., Y. Dong, Y.R., T.H., L.C. and Y.Q. contributed to data collection. R.L., K.L., and Y. Dong analysed the data. R.L. drafted the manuscript. Y. Du and C.Q. supervised the study. All authors made critical revisions for important intellectual content and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Research within MIND-China has been conducted in accordance with the Declaration of Helsinki, and MIND-China has been approved by the Ethics Committee of Shandong Provincial Hospital in **an, Shandong. Written informed consent was obtained from all participants, or in the case of severely cognitively impaired persons, from their informants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Table 1.
Associations of self-reported sleep characteristics with all-cause dementia and Alzheimer’s disease stratified by age groups (n = 4742). Supplementary Table 2. Associations of self-reported sleep characteristics with all-cause dementia and Alzheimer’s disease stratified by sex (n = 4742). Supplementary Table 3. Associations of self-reported sleep characteristics with all-cause dementia and Alzheimer’s disease stratified by education (n = 4742). Supplementary Table 4. Associations of self-reported sleep characteristics with all-cause dementia and Alzheimer’s disease stratified by APOE genotype (n = 4599).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, R., Tang, S., Wang, Y. et al. Self-reported sleep characteristics associated with dementia among rural-dwelling Chinese older adults: a population-based study. BMC Neurol 22, 5 (2022). https://doi.org/10.1186/s12883-021-02521-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12883-021-02521-0