Abstract
Background
Chronic kidney disease (CKD) has become an increasingly important public health disease with a high incidence rate and mortality. Although several studies have explored the effectiveness of resistance exercise in improving the prognosis of CKD patients, the number of studies is still limited and the results are still controversial.
Objectives
We conducted this meta-analysis of randomized controlled trials (RCT) studies to evaluate the effectiveness of resistance exercise on CKD patients.
Methods
The PubMed, Embase, and Cochrane Library databases were searched from the inception date to October 2023. The meta-analysis was conducted to evaluate 12 main indicators, including glomerular filtration rate (GFR)(ml/(min•1.73m2)), C-reactive protein (CRP) (mg/L), serum creatinine (mg/dL), hemoglobin (g/dL), Glycosylated Hemoglobin, Type A1C (HBA1c) (%), high Density Lipoprotein (HDL) (mg/dL), low Density Lipoprotein (LDL) (mg/dL), 6-min walk(m), body mass index (BMI) (kg/m2), fat-free mass (kg), fat mass (kg), grip strength (kgf).
Results
Sixteen RCT studies were included in this meta-analysis from 875 records. GFR exhibited no significant change in CKD patients treated with resistance exercise (WMD 1.82; 95%CI -0.59 to 4.23; P = 0.139). However, 6-min walk (WMD 89.93; 95%CI 50.12 to 129.74; P = 0.000), fat-free mass (WMD 6.53; 95%CI 1.14 to 11.93; P = 0.018) and grip strength (WMD 3.97; 95%CI 1.89 to 6.05; P = 0.000) were significantly improved with resistance exercise. The level of CRP (WMD − 2.46; 95%CI -4.21 to -0.72; P = 0.006) and HBA1c (WMD − 0.46; 95%CI -0.63 to -0.29; P = 0.000) dropped significantly after resistance exercise treatment.
Conclusions
Resistance exercise can improve physical function, metabolic condition, inflammatory response and cardiopulmonary function in CKD patients, specifically reflected in the increase of indicators fat-free mass, grip strength, 6-min walk, as well as the decrease of indicators HBA1c and CRP.
Similar content being viewed by others
Introduction
Chronic kidney disease (CKD) has become an increasingly important public health priority [1, 2]. Since 1990, the global all-age prevalence of CKD has increased by 29.3% [3]. Moreover, it is reported that 1.2 million people died from CKD globally in 2017 [3]. Chronic kidney disease is a progressive disease, which cannot be cured. It has a high incidence rate and mortality, and is common in the general adult population, especially in patients with diabetes and hypertension [4].
Long term chronic kidney disease can lead to many complications. Its main complication is cardiovascular disease (CVD), and it was reported that CKD is a strong independent risk factor of poor cardiovascular outcome [5,6,7]. It was reported that among patients aged 65 and above in the United States with CKD, the prevalence of CVD is 64.5%, while among patients without CKD, this proportion was only 32.4% [8]. Most patients with CKD will die due to cardiovascular disease before the CKD reaches its final stage [9]. In addition, CKD can also lead to complications such as metabolic syndrome and sarcopenia [10]. This process involves some complex molecular mechanisms, including reactive oxygen species (ROS) and NF-E2-related factor 2 (NRF2) [11]. According to previous studies, the incidence of muscle atrophy in CKD patients who started dialysis was 30%. There was also a significant correlation between the reduction of lean mass and the severity of kidney disease and physical function [12].
The impact of resistance exercise on improving the prognosis of CKD patients has also received increasing attention. For CKD patients, resistance exercise may be an ideal choice for improving prognosis. On the one hand, resistance exercise is not necessarily a very intense exercise that can cause harm to the body, including various types and forms (such as resistance bands, dumbbells) [13, 14]. On the other hand, resistance exercise can improve metabolic parameters with less energy consumption [15]. Therefore, resistance exercise may be more feasible for CKD patients, especially those with poor cardiopulmonary function. Moreover, resistance exercise can effectively alleviate the complications of CKD, including but not limited to metabolic syndrome, sarcopenic obesity, and reduce related biomarkers [16, 17].
The current evidence for resistance exercise treatment in CKD is encouraging. However, its practical application in clinical settings is still relatively limited [18]. We conducted this meta-analysis of existing literature on resistance exercise for the treatment of CKD, aiming to explore the effectiveness of resistance exercise in improving the prognosis of CKD patients.
Methods
Search strategy
According to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) and AMSTAR (Assessing the Methodological Quality of Systematic Reviews) guidelines, the authors predetermined the eligibility criteria for the meta-analysis. We searched the PubMed, Embase, and Cochrane Library databases from the inception date to October 2023 using the keywords “resistance exercise”, “chronic kidney disease”, and “CKD” to identify published studies comparing patients with and without resistance exercise. We searched only English-language studies, and links within the search results and references were also examined to find additional literature. Grey literature was also reviewed. The primary outcome was assessed by glomerular filtration rate (GFR)(ml/(min•1.73m2)). The secondary outcomes were assessed by C-reactive protein (CRP) (mg/L), serum creatinine (mg/dL), hemoglobin (g/dL), Glycosylated Hemoglobin, Type A1C (HBA1c) (%), high Density Lipoprotein (HDL) (mg/dL), low Density Lipoprotein (LDL) (mg/dL), 6-min walk(m), body mass index (BMI) (kg/m2), fat-free mass (kg), fat mass (kg), grip strength (kgf).
Eligibility criteria
In this study, two investigators independently reviewed all the literature and browsed the titles and abstracts of all papers. All the selected articles had to meet the following criteria: (1) Patients diagnosed with CKD; (2) Compared studies with and without resistance exercise; (3) studies that had sufficient data for statistical analysis. (4) The studies should be randomized controlled studies.
Articles including any of the following were excluded: (1) noncomparative studies such as meta-analyses, reviews, and case reports; (2) studies that were not focused on CKD patients underwent resistance exercise; (3) patients diagnosed with cancer, inflammatory disease, autoimmune disease; (4) duplicate studies.
Data extraction and quality assessment
A thorough and independent review of the publications was conducted by two reviewers. Data extracted included the following items: author, year, study design, stage, age, number of female and male, dialysis duration, pathogen, GFR, CRP (mg/L), serum creatinine (mg/dL), hemoglobin (g/dL), HBA1c (%), HDL (mg/dL), LDL (mg/dL), 6-min walk(m), body mass index (BMI) (kg/m2), fat-free mass (kg), fat mass (kg), grip strength (kgf). The tool published by the Cochrane Collaboration in the Cochrane Handbook (version 5.3) was used to evaluate the risk of bias of one RCT and contained seven items: random sequence generation, blinding of participants and personnel, allocation concealment, blinding of outcome assessors, selective reporting, incomplete outcome data and other biases. An independent risk of bias assessment was carried out by two reviewers, and a third reviewer arbitrated unresolved differences. Potential biases were evaluated by 2 independent authors and were classified into three categories: “high risk”, “low risk”, and “unclear risk” according to the Cochrane bias risk assessment tool.
Statistical analysis
Continuous variables were assessed using the weighted mean difference (WMD) and heterogeneity was assessed using the Q test with the corresponding P value and I2 test. It indicated that there was no heterogeneity if I2 was less than 25%; if 25% < I2 ≤ 50%, it implied moderate heterogeneity; if 50% < I2 ≤ 75%, it indicated substantial heterogeneity. If I2 ≥ 75%, it indicated considerable heterogeneity. An analysis of pooled data was conducted with a random-effects model if P > 0.05; otherwise, a fixed-effects model was applied. A funnel plot was used to assess publication bias. Egger’s test and Begg’s test were applied to determine publication bias. A sensitivity analysis was performed using the removal method. When studies provided only medians, we calculated the means, standard deviations and ranges. A statistically significant level of P < 0.05 was considered when analyzing the data using STATA version 15.0 software.
Results
Studies selected and characteristics
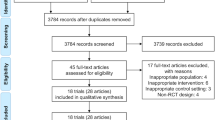
A total of 875 potential articles published from the inception date to October 2023 were found. A total of 630 records remained after duplicates were removed. Thirty-six records were selected for further retrieval and evaluation after reviewing title and abstract. Two records were not retrieved. In addition, 10 reviews, 4 letters, 1 nonrandomized controlled trial study and 3 editorials were excluded during the assessment for eligibility. Eventually, 16 randomized controlled trial (RCT) studies met the criterion for our study and were included in the meta-analysis [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34]. A corresponding flowchart is shown in Fig. 1.
A total of 787 CKD patients were included in this meta-analysis, of which the clearly reported causes were diabetes (n = 128), renovascular (n = 27), glomerulonephritis (n = 57), Polycystic kidney disease (n = 8). Seven articles clearly indicated the CKD stage of included patients. Fourteen studies described the gender ratio of the resistance exercise group and the control group. In general, the baseline features of the included studies are illustrated in Table 1.
Quality assessment
The evaluation of quality according to the Cochrane Bias Risk Assessment Tool is shown in Table 2; Fig. 2. Six studies were evaluated as high-risk on randomization. Six studies were identified as high-risk on blinding of participants and investigators. One study was considered high-risk on blinding of outcome assessment. In addition, 1 study exhibited high-risk on selective report of outcomes.
Outcomes
GFR
The GFR was reported in 7 studies. The pooled outcome exhibited that there was no significant difference in GFR between CKD patients with and without resistance exercise (WMD 1.82; 95%CI -0.59 to 4.23; P = 0.139). The forest plot is illustrated in Fig. 3A.
CRP
The CRP was examined in 9 records. The pooled effect revealed that the level of CRP in CKD patients with resistance exercise was significantly lower than that without resistance exercise (WMD − 2.46; 95%CI -4.21 to -0.72; P = 0.006). The forest plot is shown in Fig. 3B.
Serum creatinine
The serum creatinine was detected in 5 trials. The level of serum creatinine in CKD patients with resistance exercise did not change significantly compared to that without resistance exercise (WMD − 0.01; 95%CI -0.17 to 0.14; P = 0.849). The corresponding forest plot could be seen in Fig. 3C.
Hemoglobin
Five studies described the flow of the level of hemoglobin. No significant difference in the level of hemoglobin was found between CKD patients with and without resistance exercise (WMD − 0.07; 95%CI -0.62 to 0.47; P = 0.787). The forest plot is exhibited in Fig. 3D.
HBA1c
HBA1c was mentioned in 3 studies. The pooled analysis suggested that the level of HBA1c in CKD patients met significant decrease when treated with resistance exercise (WMD − 0.46; 95%CI -0.63 to -0.29; P = 0.000). The forest plot is listed in Fig. 3E.
HDL and LDL
HDL was presented in 6 studies. The level of HDL did not exhibit significant change when CKD patients were treated with resistance exercise (WMD 2.24; 95%CI -2.54 to 7.02; P = 0.359). In contrast, 6 studies reported the level of LDL. The pooled analysis illustrated the level of LDL with resistance exercise was significantly higher than that without resistance exercise in CKD patients (WMD 1.72; 95%CI 0.07 to 3.36; P = 0.512). The forest plot is shown in Fig. 3F and G.
BMI, fat-free mass and fat mass
BMI was examined in 8 studies. The pooled outcome indicated that resistance exercise did not significantly reduce BMI in CKD patients (WMD 0.09; 95%CI -0.52 to 0.70; P = 0.776). The forest plot is shown in Fig. 3H.Fat-free mass was reported in 2 studies. The pooled analysis showed that the level of fat-free mass with resistance exercise was higher than that without resistance exercise (WMD 6.53; 95%CI 1.14 to 11.93; P = 0.018) (shown in Fig. 3I). 2 studies described the level of fat mass. The pooled effect showed no significant changes in fat mass when CKD patients were treated with resistance exercise (WMD − 5.38; 95%CI -13.29 to 2.53; P = 0.183) (shown in Fig. 3J).
6-min walk
Two studies presented the data of 6-min walk. The pooled analysis revealed that CKD patients with resistance exercise had a longer 6-min walk compared to the control group and the difference was statistically significant (WMD 89.93; 95%CI 50.12 to 129.74; P = 0.000). The forest plot is presented in Fig. 3K.
Grip strength
Grip strength was evaluated in 7 studies. The grip strength of CKD patients in the resistance exercise group was significantly better than that of the control group according to the pooled outcome (WMD 3.97; 95%CI 1.89 to 6.05; P = 0.000). The forest plot is shown in Fig. 3L.
The pooled WMDs of all indicators included in this study are summarized in Table 3.
Sensitivity analysis and publication bias
Sensitivity analysis revealed that the pooled WMDs were not materially influenced by any single study in all outcomes, indicating that the outcomes were statistically robust (shown in Fig. 4A).
Publication bias was evaluated by using a funnel plot visually (shown in Fig. 4B), which indicated no significant publication bias. Statistically, Egger’s test also suggested no significant publication bias though outcomes with less than 10 included studies (P = 0.625).
Discussion
This meta-analysis demonstrated the comparisons of 12 outcomes between CKD patients with resistance exercise treatment and those without resistance exercise. The primary outcome, the level of GFR, was not improved significantly after resistance exercise in CKD patients (WMD 1.82; 95%CI -0.59 to 4.23; P = 0.139). The secondary outcomes, including the level of serum creatinine, hemoglobin, HDL, BMI and fat mass, did not change significantly when CKD patients were treated with resistance exercise. Moreover, the level of LDL, 6-min walk, fat-free mass and grip strength increased significantly with resistance exercise, while the level of CRP and HBA1c dropped significantly with resistance exercise.
This meta-analysis did not demonstrate the beneficial effects of resistance exercise on renal function, which was consistent with the results of some other RCT studies [28, 33]. The changes in GFR and serum creatinine of patients receiving resistance exercise treatment were not significant compared to that of the control group. The impact of resistance exercise on GFR in CKD patients is still controversial. The research data of Corrêa, H. L. et al. [23] confirms that resistance exercise could alleviate the decrease in GFR in CKD patients, while the research data of Erin et al. [28] could not support this outcome. A study on resistance exercise for the elderly showed that resistance exercise programs can improve GFR [35]. However, the study by Amorim and Machado et al. showed that at 24, 48, and 72 h after resistance exercise, serum creatine kinase (CK) activity was elevated while eGFR dropped, showing a negative correlation between the two [36, 37]. Only 7 studies included in the meta-analysis recorded the GFR indicator. RCT studies on the impact of resistance exercise on renal function are still limited and need further research.
However, it is encouraging to note that this meta-analysis found that resistance exercise can improve physical function, metabolic condition, inflammatory response and cardiopulmonary function in CKD patients. Resistance exercise could increase the 6-min walk, fat-free mass and grip strength of the CKD patients significantly according to our meta-analysis. The increase in distance of 6-min walk indicated an improvement in cardiopulmonary function in CKD patients after receiving resistance exercise. Half a SD (standard deviation) was reported to be the estimated MCiD (minimal clinically important difference) for chronic diseases [38]. Therefore, we used half a SD to calculate the estimated MCiD. The WMD of 6-min walk (89.93) was greater than the estimated MCiD (20.31), indicating that this difference is not only statistically significant but also clinically significant. Moreover, the increase in fat-free mass and grip strength suggested an enhancement in muscle mass and muscle strength, respectively. The estimated MCiD of fat-free mass and grip strength were 2.75 and 1.06, respectively, suggesting that the increase in fat-free mass and grip strength were both clinically significant. The above three outcomes suggested that resistance exercise could improve the physical function of CKD patients. Moreover, resistance exercise can significantly reduce the levels of CRP and HBA1c in CKD patients according to our meta-analysis. CRP is not only an inflammatory marker, but also a predictor and risk factor for cardiovascular disease [39]. CRP, whose MCiD was − 0.89, showed a significant drop (WMD − 2.46; 95%CI -4.21 to -0.72; P = 0.006) indicating a relief of the level of inflammation and the risk of cardiovascular complications in CKD patients after resistance exercise, which revealed resistance exercise can alleviate the inflammatory response of CKD and improve the prognosis of CKD patients. In addition, the decrease in HBA1c ratio (WMD − 0.46; 95%CI -0.63 to -0.29; P = 0.000), which exceeded its corresponding estimated MCiD (-0.09), indicated that CKD patients can improve their metabolic condition and alleviate the progression of CKD by resistance exercise in controlling blood sugar and other aspects. However, it is worth noting that CKD patients showed a significant elevation in LDL after resistance exercise, which is one of the risk factors for cardiovascular disease. We subsequently conducted a sensitivity analysis on the included studies that recorded LDL, and the result showed that excluding the study by Barcellos et al., the pooled outcome changed significantly, indicating that the study by Barcellos et al. influenced the stability of the pooled outcome [20]. We excluded the research data from Barcellos et al. and conducted a meta-analysis again. The pooled effect showed that there was no significant change in LDL levels in CKD patients after resistance exercise (WMD − 1.83; 95%CI -9.29 to 5.62; P = 0.630). Overall, this meta-analysis suggested that resistance exercise could improve the physical function and metabolic condition of CKD patients.
Research and clinical implications
Resistance exercise has been reported to have beneficial effects on physical function, metabolic condition, inflammatory response and cardiopulmonary function in adults with CKD, which is consistent with our meta-analysis outcomes [17, 18, 40]. However, the mechanisms behind these outcomes have received little attention [40]. ROS and NRF2 might be key molecules in the progression of CKD [11, 19, 40]. The kidney is one of the organs with the highest oxygen consumption. The kidneys only account for 0.5% of total body weight, but they account for approximately 7% of the body’s total oxygen consumption [11]. Mitochondria is one of the important sources of endogenous ROS in the kidney. Mitochondria in kidney cells may leak some electrons during electron transport, leading to the production of superoxide. Usually, the generation and elimination of ROS reach a balance. But in CKD patients, this balance is disrupted, and excessive ROS ultimately leads to oxidative stress [10]. The oxidative stress leads to a decrease in the degradation of NRF2, which transfers from the cytoplasm to the nucleus, inducing the expression of glutathione synthase and HO1, which are crucial for protecting cells and organs from oxidative stress. However, in animal models of CKD, a decrease in NRF2 activity was observed in the kidneys [41]. The weakened antioxidant stress capacity of the kidneys ultimately leads to the progression of CKD. The progression of CKD leads to a decline in physical function, deterioration of metabolic condition [42], increased inflammatory response, and impaired cardiopulmonary function in CKD patients, while resistance exercise could alleviate these complications. For physical function, resistance exercise could decrease the expression of myostatin and elevate the expression of insulin-like growth factor-1 (IGF-1), which could improve the synthesis of protein in skeletal muscle and attenuate protein degradation [12, 40]. Moreover, resistance exercise is proved to increase the expression of peroxisome proliferator-activated receptor coactivator-γ-1α4 (PGC-1α4, an isoform of the transcriptional co-activator PGC-1α), which not only enhances muscle hypertrophy but also improves glycolysis [43]. For metabolic condition, resistance exercise is found to improve the bind between PGC-1α4 and a nuclear receptor named peroxisome proliferator-activated receptor β (PPARβ), enhancing anaerobic glycolysis, which could promote the glucose uptake and fat oxidation in skeletal muscle [43]. As a result, resistance exercise is beneficial for improving glucose and lipid metabolism. For inflammatory, NRF2 has been mentioned as a potential key molecule in the impact of resistance exercise on the progression of CKD. Resistance exercise could improve the expression of NRF2 thereby alleviate oxidative stress and inflammatory response [19]. For cardiopulmonary function, resistance exercise improves the muscular contractions, resting heart rate and blood pressure, resulting in shear stress-induced adaptations in nitric oxide metabolism. Therefore, resistance exercise might play a critical role in improving flow-mediated dilatation, enhancing cardiopulmonary function and reducing the risk of cardiovascular diseases [44]. In summary, resistance exercise improves the prognosis of CKD patients by affecting the expression and activity of multiple molecules, including ROS, NRF2, IGF-1 and PGC-1α4. Moreover, further research is needed to verify the changes in other indicators. Different stages and causes of CKD may affect the effectiveness of resistance exercise in improving CKD prognosis and alleviating CKD progression.
Conclusion
This meta-analysis shows that resistance exercise can improve physical function, metabolic condition, inflammatory response and cardiopulmonary function in CKD patients, specifically reflected in the increase of indicators fat-free mass, grip strength, 6-min walk, as well as the decrease of indicators HBA1c and CRP.
Data availability
All data analyzed in this study are included in this published article.
References
Zoccali C, Mark PB, Sarafidis P, et al. Diagnosis of cardiovascular disease in patients with chronic kidney disease. Nat rev Nephrol. 2023;19:733–46.
Matsushita K, Ballew SH, Wang AY, et al. Epidemiology and risk of cardiovascular disease in populations with chronic kidney disease. Nat rev Nephrol. 2022;18:696–707.
Global regional. National burden of chronic kidney disease, 1990–2017: a systematic analysis for the global burden of Disease Study 2017. Lancet. 2020;395:709–33.
Kalantar-Zadeh K, Jafar TH, Nitsch D, et al. Chronic kidney disease. Lancet. 2021;398:786–802.
Beldhuis IE, Lam CSP, Testani JM, et al. Evidence-based medical therapy in patients with heart failure with reduced ejection fraction and chronic kidney disease. Circulation. 2022;145:693–712.
Noels H, Jankowski J. Increased risk of Cardiovascular complications in chronic kidney disease: introduction to a Compendium. Circ res. 2023;132:899–901.
Shroff GR, Bangalore S, Bhave NM, et al. Evaluation and management of aortic stenosis in chronic kidney Disease: A Scientific Statement from the American Heart Association. Circulation. 2021;143:e1088–114.
Lai AC, Bienstock SW, Sharma R, et al. A Personalized Approach to chronic kidney Disease and Cardiovascular Disease: JACC Review topic of the Week. J am coll Cardiol. 2021;77:1470–9.
Patel N, Yaqoob MM, Aksentijevic D. Cardiac metabolic remodelling in chronic kidney disease. Nat rev Nephrol. 2022;18:524–37.
Wang XH, Mitch WE, Price SR. Pathophysiological mechanisms leading to muscle loss in chronic kidney disease. Nat rev Nephrol. 2022;18:138–52.
Kishi S, Nagasu H, Kidokoro K et al. Oxidative stress and the role of redox signalling in chronic kidney disease. Nat rev nephrol 2023;null:null.
Gollie JM, Harris-Love MO, Patel SS, et al. Chronic kidney disease: considerations for monitoring skeletal muscle health and prescribing resistance exercise. Clin Kidney j. 2018;11:822–31.
Chekroud SR, Chekroud AM. Efficacy of Resistance Exercise Training with depressive symptoms. Jama Psychiat. 2018;75:1091–2.
Courneya KS, Segal RJ, Mackey JR, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. J clin Oncol. 2007;25:4396–404.
Hashida R, Kawaguchi T, Bekki M, et al. Aerobic vs. resistance exercise in non-alcoholic fatty liver disease: a systematic review. J Hepatol. 2017;66:142–52.
Dieli-Conwright CM, Courneya KS, Demark-Wahnefried W, et al. Effects of Aerobic and Resistance Exercise on metabolic syndrome, Sarcopenic Obesity, and circulating biomarkers in overweight or obese survivors of breast Cancer: a Randomized Controlled Trial. J clin Oncol. 2018;36:875–83.
Colleluori G, Aguirre L, Phadnis U, et al. Aerobic Plus Resistance Exercise in obese older adults improves muscle protein synthesis and preserves myocellular quality despite weight loss. Cell Metab. 2019;30:261–e2736.
Noor H, Reid J, Slee A. Resistance exercise and nutritional interventions for augmenting Sarcopenia outcomes in chronic kidney disease: a narrative review. J cachexia Sarcopeni. 2021;12:1621–40.
Abreu CC, Cardozo L, Stockler-Pinto MB, et al. Does resistance exercise performed during dialysis modulate Nrf2 and NF-κB in patients with chronic kidney disease? Life Sci. 2017;188:192–7.
Barcellos FC, Del Vecchio FB, Reges A, et al. Exercise in patients with hypertension and chronic kidney disease: a randomized controlled trial. J Hum Hypertens. 2018;32:397–407.
Castaneda C, Gordon PL, Parker RC, et al. Resistance training to reduce the malnutrition-inflammation complex syndrome of chronic kidney disease. Am J Kidney Dis. 2004;43:607–16.
Cheema B, Abas H, Smith B, et al. Progressive exercise for anabolism in kidney disease (PEAK): a randomized, controlled trial of resistance training during hemodialysis. J Am Soc Nephrol. 2007;18:1594–601.
Corrêa HL, Neves RVP, Deus LA, et al. Low-load resistance training with blood flow restriction prevent renal function decline: the role of the redox balance, angiotensin 1–7 and vasopressin(). Physiol Behav. 2021;230:113295.
de Araújo TB, de Corrêa L, de Deus H. The effects of home-based progressive resistance training in chronic kidney disease patients. Exp Gerontol. 2023;171:112030.
Dong J, Sundell MB, Pupim LB, et al. The effect of resistance exercise to augment long-term benefits of intradialytic oral nutritional supplementation in chronic hemodialysis patients. J Ren Nutr. 2011;21:149–59.
Headley S, Germain M, Wood R, et al. Short-term aerobic exercise and vascular function in CKD stage 3: a randomized controlled trial. Am J Kidney Dis. 2014;64:222–9.
Hiraki K, Shibagaki Y, Izawa KP, et al. Effects of home-based exercise on pre-dialysis chronic kidney disease patients: a randomized pilot and feasibility trial. BMC Nephrol. 2017;18:198.
Howden EJ, Coombes JS, Strand H, et al. Exercise training in CKD: efficacy, adherence, and safety. Am J Kidney Dis. 2015;65:583–91.
Leehey DJ, Collins E, Kramer HJ, et al. Structured Exercise in obese Diabetic patients with chronic kidney disease: a Randomized Controlled Trial. Am J Nephrol. 2016;44:54–62.
Marinho SM, Mafra D, Pelletier S, et al. In Hemodialysis patients, Intradialytic Resistance Exercise improves osteoblast function: a pilot study. J Ren Nutr. 2016;26:341–5.
Olvera-Soto MG, Valdez-Ortiz R, López Alvarenga JC, et al. Effect of resistance exercises on the indicators of muscle reserves and handgrip strength in adult patients on Hemodialysis. J Ren Nutr. 2016;26:53–60.
Song WJ, Sohng KY. Effects of progressive resistance training on body composition, physical fitness and quality of life of patients on hemodialysis. J Korean Acad Nurs. 2012;42:947–56.
Uchiyama K, Adachi K, Muraoka K, et al. Home-based aerobic exercise and resistance training for severe chronic kidney disease: a randomized controlled trial. J Cachexia Sarcopenia Muscle. 2021;12:1789–802.
Watanabe K, Kamijo Y, Yanagi M, et al. Home-based exercise and bone mineral density in peritoneal dialysis patients: a randomized pilot study. BMC Nephrol. 2021;22:98.
Ramel A, Arnarson A, Geirsdottir OG, et al. Glomerular filtration rate after a 12-wk resistance exercise program with post-exercise protein ingestion in community dwelling elderly. Nutrition. 2013;29:719–23.
Machado M, Zini EN, Valadão SD, et al. Relationship of glomerular filtration rate and serum CK activity after resistance exercise in women. Int Urol Nephrol. 2012;44:515–21.
Amorim MZ, Machado M, Hackney AC, et al. Sex differences in serum CK activity but not in glomerular filtration rate after resistance exercise: is there a sex dependent renal adaptative response? J Physiol Sci. 2014;64:31–6.
Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41:582–92.
Gedebjerg A, Bjerre M, Kjaergaard AD, et al. CRP, C-Peptide, and risk of First-Time Cardiovascular events and mortality in early type 2 diabetes: a Danish cohort study. Diabetes Care. 2023;46:1037–45.
Bishop NC, Burton JO, Graham-Brown MPM, et al. Exercise and chronic kidney disease: potential mechanisms underlying the physiological benefits. Nat rev Nephrol. 2023;19:244–56.
Ruiz S, Pergola PE, Zager RA, et al. Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int. 2013;83:1029–41.
Mitrofanova A, Merscher S, Fornoni A. Kidney lipid dysmetabolism and lipid droplet accumulation in chronic kidney disease. Nat rev Nephrol. 2023;19:629–45.
Koh JH, Pataky MW, Dasari S, et al. Enhancement of anaerobic glycolysis - a role of PGC-1α4 in resistance exercise. Nat Commun. 2022;13:2324.
Ashton RE, Tew GA, Aning JJ, et al. Effects of short-term, medium-term and long-term resistance exercise training on cardiometabolic health outcomes in adults: systematic review with meta-analysis. Brit j Sport med. 2020;54:341–8.
Funding
No.
Author information
Authors and Affiliations
Contributions
Study conception and design: Chong-Cheng Chen, Zheng-Wen Chen, Tao **; Acquisition of data: Chong-Cheng Chen, Yue-Yang Huang, Hua-Zhang, **a-Liu, Xue-Qin Li, Yan-Qiong Long; Analysis and interpretation of data: Chong-Cheng Chen, Yue-Yang Huang, Hua-Zhang; Drafting of manuscript: Chong-Cheng Chen; Critical revision: Zheng-Wen Chen, Tao **; Approval of final manuscript: all authors. Tao ** and Zheng-Wen Chen contributed equally to this study.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was based on previously published studies, which didn’t need ethics. For this meta-analysis, informed consent was not needed.
Consent for publication
All authors consent to publish the article.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, CC., Huang, YY., Hua-Zhang et al. Impact of resistance exercise on patients with chronic kidney disease. BMC Nephrol 25, 115 (2024). https://doi.org/10.1186/s12882-024-03547-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12882-024-03547-5