Abstract
Background
Pleural effusion (PE) is a common clinical feature that presents a diagnostic challenge for clinicians. In this retrospective study, we aimed to assess the biomarkers, ratios, and multiple indicators in serum and Pleural effusion for the differential diagnosis of tuberculous pleural effusion (TPE) from non-tuberculosis effusion (non-TPE).
Methods
The participants, who were divided into two groups: TPE and non-TPE (MPE and PPE), from Ningbo First Hospital, were incorporated in this study. The clinical and laboratory features were collected and analyzed using logistic regression analysis. Twelve biomarkers and their ratios in serum and PE were investigated for TPE versus non-TPE. Additionally, the value of multiple indicators for joint diagnosis was estimated.
Results
Biomarkers and ratios showed good diagnostic performance. The five variables including Serum ADA, IGRA, Effusion ADA, Effusion ADA/Serum ADA and Effusion LDH/Effusion ADA were identified as valuable parameters for differential diagnosis of TPE from non-TPE. The combined diagnosis of the five indexes yielded the highest diagnostic accuracy for TPE with an AUC (0.919), sensitivity (90.30%), and specificity (94.50%).
Conclusions
The biomarkers and ratios demonstrated strong diagnostic performance, and the utilization of multiple indicators for joint diagnosis can improve the diagnostic efficacy of tuberculous pleurisy.
Similar content being viewed by others
Background
Tuberculosis (TB) is the second deadliest infectious disease behind the COVID-19. The World Health Organization (WHO) estimates that 10.6 million people worldwide suffered from TB in 2021, an increase of 4.5% from 10.1 million in 2020. The TB incidence rate (new cases per 100 000 population per year) rose by 3.6% between 2020 and 2021, reversing declines of about 2% per year for most of the previous 2 decades [1]. Tuberculous pleural effusion (TPE) is the second most common form of extrapulm- onary tuberculosis [2, 3], with presentations ranging from benign effusions that are absorbed spontaneously to complicated effusions with pleural thickening, empyema, and even Pleural fibrosis, all of which may result in lasting lung function impairment [4]. So, early and accurate diagnosis of TPE is extremely critical for the management of the disease.
Confirmation of TPE requires the isolation and/or culture of Mycobacterium tuberculosis (Mtb) from Pleural effusions and Pleural biopsy specimens or the demonstration of granulomas by pleural biopsy [5, 6]. In addition, the invasiveness and technical difficulty of medical thoracoscopic surgery appear to offer greater sensitivity (93–100%) and accuracy for diagnosing TPE.
However, it is an invasive and expensive diagnostic procedure with a complication rate of 2–6% [7, 8]. The common complications include bleeding, fever, empyema, pneumonia, and long-term air leakage air leak and so on [9]. Besides, some patients with advanced underlying disease progression and elderly patients could not tolerate the test.
In order to diagnose TPE, Pleural effusion and peripheral blood tests have been proposed as an alternative method [4]. These specimens are commonly used in clinical practice and are minimally invasive and easy to obtain. IGRA, CRP, ESR, serum TP, ALB, ADA, LDH, Pleural effusion TP, ALB, ADA, and LDH are the primary examinations for hospitalized patients. However, it is crucial to further investigate the application value. Therefore, we conducted a retrospective analysis.
Materials and methods
Study population
This retrospective study focused on patients newly diagnosed PE between January 2015 and March 2022 from Ningbo First Hospital. Patients under the age of 18 and those who were unwilling to provide informed consent were excluded from the study. The patient enrollment process was shown in Fig. 1.The whole patients included in the study were hospitalized for the first time owing to pleural effusion. All PE samples and followed peripheral blood samples were collected and tested. The study analyzed data from the first sample of PE and blood collected from each patient. The correlated statistics, laboratory, and clinical characteristics for all patients were obtained from the clinical electronic record system. A total of 362 patients with PE were included in this study. Of the 362 patients, 185 cases with Tuberculous pleural effusion (TPE) were diagnosed with tuberculous pleurisy effusion, 177 cases with non-TPE,104 cases were caused by parapulmonary effusion (PPE), and 73 cases with malignant Pleural effusion (MPE) were caused by primary lung cancer. All the following guidelines were included for all subjects: (i) Diagnoses of PE was experienced either ultra-sonography, chest CT, or X-ray (ii) All participants were diagnosed by cytology, thoracentesis or Pleural biopsy and follow-up (no less than 6 months). The exclusion criteria were as follows: (i) age below 18 years old; (ii) participants with incomplete clinical data;(iii)pregnant women; (iv) uncertain of the clinical diagnosis.
Standardized diagnostic criteria for TPE, PPE, and MPE
Patients with TPE diagnosed and treated in our hospital for the first time were registered in our study, and the diagnostic criteria were: (a) The culture of pleural effusion or pleural tissue was positive for Mycobacterium tuberculosis. (b) Mycobacterium tuberculosis has been isolated from the granulomatous inflammation, which was found in pleural biopsy histology. (c)granulomatous inflamed tissue in the pleural biopsy coexisting with clinical response to antituberculosis therapy [10,11,12].
The diagnosis of PPE is based on: (1) Bacterial pneumonia, with no MTB in the PF obtained by continuous thoracentesis procedures and no evidence of MTB in the pathological manifestations of inflammatory pleuritis, pleural fibrosis, plaques, or chronic empyema [13]; (2) parapneumonic PE, which disappeared after anti-inflamm- atory treatment [14].
MPE was diagnosed based on: (i) The combination of cytology, thoracoscopy, and imaging studies with a minimum follow-up of 6 months. (ii) MPE was diagnosed when Pleural effusion cytology or Pleural biopsy was positive for malignant cells [15, 16].
Data capture
All the data of clinical and laboratory, including age, gender, smoking history, effusion biochemical indexes [TP (total protein), ALB(albumin), ADA (adenosine deaminase), LDH (lactatedehy drogenase), peripheral blood indexes [CRP(C-reactive protein), ESR(erythrocyte sedimentation rate), IGRA(interferon-gamma release assay), serum indexes [TP, ALB, ADA, and LDH](Table 1), they were obtained from the clinical electronic record system.
PE and blood indexes analysis
The subjects of the TB-IGRA experiment used dehyrogenated vacuum tubes to collect heparinized anticoagulated whole blood, and culture filter protein 10(CFP-10) and early secretory antigen 6 (ESAT-6) containing Mycobacterium tuberculosis (MTB) specific antigen were added to the test tubes. CFP-10 and ESAT-6 stimulated MTB-specific T lymphocytes to proliferate and release IFN-γ, which was detected in plasma by enzyme-linked immunoassay (Elisa). The linear range of the method is 2-400pg/ml, the value of ≤ 2 is counted as 2. The kit was provided by Wantai Biopharmaceutical Co., Ltd (Bei**g, China).CRP was assayed by Immunoturbidimetry with ARISTO from Guosai Technology Co., Ltd (Shenzhen, China). ESR was assayed with Test1 provided by Italian company ALIFAX.PE and serum TP were assayed by the biuret method, ALB by the bromocresol green end point assay method, and LDH by the modified IFCC method with Olympus AU5821 of Beckman Coulter (Suzhou, China). ADA was assayed by the enzyme colorimetric method of Saike Biotechnology Co., Ltd. (Ningbo, China) with Olympus AU5821 of Beckman Colter.
Statistical analysis
Statistical analyses were performed using SPSS 26.0 (SPSS Inc., Chicago, IL USA), and P < 0.05 was considered to be significantly different. The categorical variables were expressed as number and percentage (n, %). The continuous variables were expressed as median and interquartile range (IQR, 25–75), and analyzed by the Mann-Whitney U test. Use univariate logistic regression analysis to select the independent indicators, and the Akaike information criterion (AIC) of the multivariable logistic regression models was used to choose statistically significant variables. Expressed as estimated odds ratios (OR) and 95% confidence intervals (CI). The receiver operating characteristic (ROC) curve and the corresponding AUCs were used to evaluate the value of biomarkers to distinguish TPE from non-TPE. We also calculated sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive predictive value (PLR), and negative predictive value (NLR) to measure the diagnostic accuracy.
Results
Participants
From January 2015 to March 2022, a total of 435 patients from Ningbo First Hospital were investigated in this study, Among them, 73 were excluded according to the exclusion criteria, including (1) age below 18 years old(n = 3); (2) pregnant women(n = 2); (3) incomplete clinical data(n = 37); (4) unknown etiology of PE (n = 31); Finally, 362 patients were included in final analysis (Fig. 1). Demographic, clinical and laboratory characteristics of the study population are summarized in Table 1.
The results of univariate and multivariate logistic regression analysis for distinguishing TPE from non-TPE
The cutoff values of those variables were determined by Youden’s indices. Supplementary file 1: Tables S1-3 showed that all of the variables were analysed by the Mann-Whitney U test between TPE and non-TPE. Additionally, the results of the univariate logistic analysis were presented in supplementary file 1: Tables S1 including 16 variables. To further investigate the diagnostic value of biomarkers, 13 variables with an AUC > 0.65 were selected for multiple regression analysis, respectively. Using the AIC method to stepwise select the regression model, resulting in the identification of the 5 most valuable variables for distinguishing TPE from non-TPE (Table 2). The results of the multivariate logistic regression analysis were summarized in Table 2, as follows: serum ADA (OR (95%CI), 0.252(0.106–0.600)), IGRA (OR (95%CI), 0.099(0.047–0.212)), effusion ADA(OR(95%CI), 0.236(0.092–0.606)), Effusion ADA/ADA(OR (95%CI), 0.186(0.066–0.524)), Effusion LDH/ ADA, (OR(95%CI), 0.242(0.113–0.520)) (Table 2).
The diagnostic performance of indicators for TPE
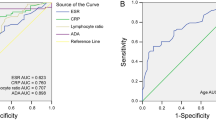
To distinguish TPE from non-TPE, the diagnostic performance of all indicators was based on ROC. We have defined an AUC greater than 0.65 as a valid marker. The detailed comparative diagnostic reference indicators and their corresponding performance were listed in (Table 3; Fig. 2). The AUCs of effective indexes for differentiating TPE from non-TPE were as follows: serum ADA (0.680, 95% CI 0.624–0.735), IGRA (0.833, 95% CI 0.788–0.878), effusion ADA (0.867, 95% CI 0.825–0.908), effusion ADA/Serum ADA (0.810, 95% CI 0.0.754–0.853), effusion LDH/effusion ADA (0.857, 95% CI 0.0.754–0.853), and 0.919 (0.888–0.951) for combined diagnosis of the five indexes (Table 3; Fig. 2 ).
Compared to the Serum ADA, IGRA, Effusion ADA, Effusion ADA/Serum ADA and Effusion LDH/Effusion ADA demonstrated a good diagnostic accuracy for TPE in terms of sensitivity (85.41%, 80.50, 82.20, 75.14), and specificity (75.14%, 88.10%, 76.80%, 85.90%), respectively. However, the combined diagnosis of five indexes yielded the highest diagnostic accuracy for TPE with sensitivity of 90.30%, and specificity of 94.50% (Table 3; Fig. 2 ).
The level of Serum ADA, IGRA, Effusion ADA, Effusion ADA/Serum ADA and Effusion LDH/Effusion ADA and the combined diagnosis of the five indexes are used to discriminate TPE from non-TPE. ROC curve of Serum ADA, IGRA, Effusion ADA, Effusion ADA/Serum ADA and Effusion LDH/Effusion ADA and the combined diagnosis of the five indexes prediction probability discrimination TPE from non-TPE
Discussion
For patients with TPE, early diagnosis and timely treatment are essential to keep away from severe complications such as Pleural thickening, empyema, and calcification. However, the early differentiation of TPE from non-TPE (such as PPE and MPE) is still a clinical challenge, despite the availability of various diagnostic methods. In addition, factors such as the low number of bacteria causing the disease, insufficient and unsuitable laboratory samples, and the ineffectiveness of traditional microbiological methods make it difficult for diagnosing TPE.
In this study, we selected 13 variables to differentiate TPE from non-TPE, respectively. These variables comprised of primary clinical and laboratory variables as well as calculated ratios. Finally, we identified the 5 most significant variables for distinguishing TPE from PPE non-TPE, which included Serum ADA, IGRA, Effusion ADA, Effusion ADA/Serum ADA and Effusion LDH/Effusion ADA. These findings demonstrate a strong diagnostic performance. The integration of five commonly used indexes proved to be cost-effective, convenient, and easily accessible in most hospitals.
ADA is a widely studied and recommended biomarker that has shown good performance in diagnosing TPE [17, 18]. A meta-analysis of 2162 citations evaluated the value of Pleural ADA activity in identifying TPE and non-TPE, demonstrating its high sensitivity and specificity (92% and 90%, respectively), including 65 studies with an ADA threshold of 40 ± 4 IU/L [19]. However, a recent study from China showed that the best cutoff value of effusion ADA for TBE was 27U/L with a sensitivity of 81% and a specificity of 78% [20]. Our study also found a similar cutoff value for effusion ADA (25.20 U/L) in differentiating TPE from non-TPE. Therefore, the optimal cutoff values for ADA are still a matter of debate, which may be attributed to variations in disease prevalence rates, sample sizes, different test methods, or the presence of HIV co-infection [17].
The effusion LDH/ADA ratio was also evaluated in differentiating TPE from non-TPE. Blakiston et al. discovered a cutoff value of 15.0 for the effusion LDH/ADA ratio with a high sensitivity and specificity in distinguishing TPE from non-TBE [21]. Another study indicated that the effusion LDH/ADA ratio with other indexes showed a sensitivity and specificity of 80.0% and 87.40% for MPE diagnosis [ Combined detection of Pleural effusion of Serum ADA, IGRA, Effusion ADA, Effusion ADA/Serum ADA and Effusion LDH/Effusion ADA can improve the diagnostic efficacy of tuberculous pleurisy.Conclusion
Data Availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ADA:
-
Adenosine deaminase
- AIC:
-
Akaike information criterion
- ALB:
-
Albumin
- AUC:
-
Area under the curve
- CI:
-
Confidence intervals
- CRP:
-
C-reactive protein
- CXCL9:
-
C-X-C motif chemokine ligand 9
- ESR:
-
Erythrocyte sedimentation rate
- IGRA:
-
Interferon-gamma release assay
- IL-27:
-
Interleukin 27
- LDH:
-
Lactatedehy drogenas
- MPE:
-
Malignant Pleural effusion
- NLR:
-
Negative likelihood ratio
- NPV:
-
Negative predictive value
- ORs:
-
Odds ratios
- PE:
-
Pleural effusion
- PLR:
-
Positive likelihood ratio
- PPE:
-
Parapneumonic Pleural effusion
- PPV:
-
Positive predictive
- ROC:
-
Receiver operating characteristic
- TB:
-
Tuberculosis
- TBP:
-
Tuberculous pleurisy
- TNF-α:
-
Tumor necrosis factor-α. TP:total protein
- TPE:
-
Tuberculous Pleural effusion
References
Bagcchi S. WHO’s global Tuberculosis report 2022. The Lancet Microbe. 2023;4(1):e20.
Light RW. Update on tuberculous pleural effusion. Respirology. 2010;15(3):451–8.
Shaw JA, Diacon AH, Koegelenberg CFN. Tuberculous pleural effusion. Respirology. 2019;24(10):962–71.
Shaw JA, Koegelenberg CFN. Pleural Tuberculosis. Clin Chest Med. 2021;42(4):649–66.
Sahn SA, Huggins JT, San Jose ME, Alvarez-Dobano JM, Valdes L. Can tuberculous pleural effusions be diagnosed by pleural fluid analysis alone? Int J Tuberc Lung Dis. 2013;17(6):787–93.
Herrera Lara S, Fernandez-Fabrellas E, Juan Samper G, Marco Buades J, Andreu Lapiedra R, Pinilla Moreno A, Morales Suarez-Varela M. Predicting Malignant and Paramalignant Pleural effusions by combining Clinical, Radiological and Pleural Fluid Analytical parameters. Lung. 2017;195(5):653–60.
Shaikh F, Lentz RJ, Feller-Kopman D, Maldonado F. Medical thoracoscopy in the diagnosis of pleural Disease: a guide for the clinician. Expert Rev Respir Med. 2020;14(10):987–1000.
Wang Z, Xu LL, Wu YB, Wang XJ, Yang Y, Zhang J, Tong ZH, Shi HZ. Diagnostic value and safety of medical thoracoscopy in tuberculous pleural effusion. Respir Med. 2015;109(9):1188–92.
MacDuff A, Arnold A, Harvey J, Group BTSPDG. Management of spontaneous pneumothorax: British thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65(Suppl 2):ii18–31.
Lin MT, Wang JY, Yu CJ, Lee LN, Yang PC, Group T. Mycobacterium tuberculosis and polymorphonuclear pleural effusion: incidence and clinical pointers. Respir Med. 2009;103(6):820–6.
Bielsa S, Palma R, Pardina M, Esquerda A, Light RW, Porcel JM. Comparison of polymorphonuclear- and lymphocyte-rich tuberculous pleural effusions. Int J Tuberc Lung Dis. 2013;17(1):85–9.
Villena Garrido V, Cases Viedma E, Fernandez Villar A, de Pablo Gafas A, Perez Rodriguez E, Porcel Perez JM, Rodriguez Panadero F, Ruiz Martinez C, Salvatierra Velazquez A, Valdes Cuadrado L. Recommendations of diagnosis and treatment of pleural effusion. Update. Arch Bronconeumol. 2014;50(6):235–49.
Light RW. Clinical practice. Pleural effusion. N Engl J Med. 2002;346(25):1971–7.
Wang J, Liu J, **e X, Shen P, He J, Zeng Y. The pleural fluid lactate dehydrogenase/adenosine deaminase ratio differentiates between tuberculous and parapneumonic pleural effusions. BMC Pulm Med. 2017;17(1):168.
Chen KY, Feng PH, Chang CC, Chen TT, Chuang HC, Lee CN, Su CL, Lin LY, Lee KY. Novel biomarker analysis of Pleural effusion enhances differentiation of tuberculous from malignant pleural effusion. Int J Gen Med. 2016;9:183–9.
Samanta S, Sharma A, Das B, Mallick AK, Kumar A. Significance of total protein, albumin, globulin, serum effusion albumin gradient and LDH in the Differential diagnosis of Pleural Effusion secondary to Tuberculosis and Cancer. J Clin Diagn Res. 2016;10(8):BC14–18.
Gopi A, Madhavan SM, Sharma SK, Sahn SA. Diagnosis and treatment of tuberculous pleural effusion in 2006. Chest. 2007;131(3):880–9.
Keng LT, Shu CC, Chen JY, Liang SK, Lin CK, Chang LY, Chang CH, Wang JY, Yu CJ, Lee LN. Evaluating pleural ADA, ADA2, IFN-gamma and IGRA for diagnosing tuberculous Pleurisy. J Infect. 2013;67(4):294–302.
Aggarwal AN, Agarwal R, Sehgal IS, Dhooria S. Adenosine deaminase for diagnosis of tuberculous pleural effusion: a systematic review and meta-analysis. PLoS ONE. 2019;14(3):e0213728.
Lei X, Wang J, Yang Z, Zhou S, Xu Z. Diagnostic value of pleural effusion mononuclear cells count and Adenosine Deaminase for Tuberculous Pleurisy patients in China: a case-control study. Front Med (Lausanne). 2019;6:301.
Blakiston M, Chiu W, Wong C, Morpeth S, Taylor S. Diagnostic performance of Pleural Fluid Adenosine Deaminase for Tuberculous Pleural Effusion in a low-incidence setting. J Clin Microbiol 2018, 56(8).
Lin L, Li S, **ong Q, Wang H. A retrospective study on the combined biomarkers and ratios in serum and pleural fluid to distinguish the multiple types of pleural effusion. BMC Pulm Med. 2021;21(1):95.
Gao L, Wang W, Zhang Y, Hu X, An J, Li Y, Chen M, Shen Y. Adenosine deaminase-based measurement in the differential diagnosis of pleural effusion: a multicenter retrospective study. Ther Adv Respir Dis. 2023;17:17534666231155747.
Zhang Q, Zhou C. Comparison of laboratory testing methods for the diagnosis of tuberculous Pleurisy in China. Sci Rep. 2017;7(1):4549.
Krenke R, Safianowska A, Paplinska M, Nasilowski J, Dmowska-Sobstyl B, Bogacka-Zatorska E, Jaworski A, Chazan R. Pleural fluid adenosine deaminase and interferon gamma as diagnostic tools in Tuberculosis Pleurisy. J Physiol Pharmacol. 2008;59(Suppl 6):349–60.
Liao M, Yang Q, Zhang J, Zhang M, Deng Q, Liu H, Graner MW, Kornfeld H, Zhou B, Chen X. Gamma interferon immunospot assay of pleural effusion mononuclear cells for diagnosis of tuberculous Pleurisy. Clin Vaccine Immunol. 2014;21(3):347–53.
Aggarwal AN, Agarwal R, Gupta D, Dhooria S, Behera D. Interferon Gamma release assays for diagnosis of Pleural Tuberculosis: a systematic review and Meta-analysis. J Clin Microbiol. 2015;53(8):2451–9.
Fenhua J, Daohui W, Hui L, **aodong X, Wen H. Diagnostic value of combined pleural interleukin-33, adenosine deaminase and peripheral blood Tuberculosis T cell spot detection TB for tuberculous Pleurisy. BMC Infect Dis. 2021;21(1):861.
Acknowledgements
Thanks to the doctors and nurses in the department of Tuberculosis for recruiting and following up the donors of this study.
Funding
No funding.
Author information
Authors and Affiliations
Contributions
Mo Yijun and JIN Weijiang researched literature and conceived the study. Chen Huimin and Liu Fang were involved in protocol development, gaining ethical approval, patient recruitment, data collection, and analysis. All authors reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study involving human participants were reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Ningbo University. The written informed consent for patients was exempted by the Ethics Committee of the First Affiliated Hospital of Ningbo University.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Fei, G., Yijun, M., Weijiang, J. et al. Biomarkers for distinguishing tuberculous pleural effusion from non-tuberculosis effusion: a retrospective study. BMC Infect Dis 23, 771 (2023). https://doi.org/10.1186/s12879-023-08781-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-023-08781-0