Abstract
Background
Sarcoptes scabiei is one of the most impactful mammalian parasites. There has been much research on immunological and clinical pathological changes associated with S. scabiei parasitism across a range of host species. This rich body of literature is complex, and we seek to bring that complexity together in this study. We first (1) synthesise narrative reviews of immunopathological relationships to S. scabiei infection to construct overarching hypotheses; then (2) undertake a systematic meta-analysis of primary literature on immunological and clinical pathological changes; and lastly (3) contrast our findings from the meta-analysis to our synthesis from narrative reviews.
Methods
We synthesised 55 narrative reviews into two overarching hypotheses representing type I and type IV immune responses to S. scabiei infection. We then systematically extracted all literature reporting immunological variables, acute phase proteins, oxidant/antioxidant status, and erythrocytic, hepatological and nephrological changes, calculating 565 effect sizes between controls and sarcoptic mange affected grou**s, refining (simplifying) hypotheses from narrative reviews.
Results
Immunological and clinical pathological parameters were most often studied in dogs (n = 12) and humans (n = 14). Combining immunological and clinical pathological information across mammalian species (n = 19) helped yield general insights into observed disease responses. This is evidenced by interspecific consensus in 27 immunological and clinical pathology variables (6/26 type I hypersensitivity, 3/20 type IV hypersensitivity, 6/10 oxidant/antioxidant status, 3/6 acute phase protein, 4/7 erythrocytic, and 5/10 hepatological/nephrological).
Conclusions
Elevated IgE, eosinophils and mast cells in type I hypersensitivity response corresponded to what was described in narrative reviews. Results from type IV hypersensitivity response suggested typical antibody response, however cell-mediated response was less evident. Some consensus of acute phase protein response and shifted oxidant/antioxidant balance and slight evidence of anemia. We highlight the need for mange/scabies studies to more routinely compare immunological and clinical pathological changes against controls, and include collection of a more standardised suite of variables among studies.
Similar content being viewed by others
Introduction
Sarcoptes scabiei is one of the most impactful of mammalian parasites [1]. It is documented to infest nearly 150 species, and is a Neglected Tropical Disease of humans with prevalence of approximately 100 million cases [2,3,4]. Infection with this parasitic mite causes the disease ‘scabies’ in humans, which is termed ‘sarcoptic mange’ in non-human animals (hereafter, ‘mange’) [5]. Mange has been recognised since antiquity [6], and knowledge of the mechanisms that dictate disease severity has grown substantially over the last two decades, creating a complex body of literature on the associated immunological and clinical pathology variables.
Clinical pathological signs of mange are driven by host immune responses to the mite. Physical signs range from mild erythema to the development of severe dermatitis, hyperkeratotic crusts, alopecia and progressive systemic disease characterised by weight loss, secondary bacterial infections [7,8,9] and death [10]. A fascinating aspect of mange is that pathological outcomes broadly fall into two categories, depending on the type of hypersensitivity reaction mounted by the host [5]: a Type I immediate antibody-mediated immune response [4, 11,12,13]; or Type IV delayed cell-mediated immune response [14,15,16,17]. Type I reactions are typified by the Immunoglobulin(Ig) E-mediated activation of mast cells and eosinophils, whereas Type IV is associated with sensitised T cells that either cause damage directly or activate other leukocytes [18] (see Additional file 1: S1 and Additional file 3: S3 Appendicies). Such responses are often referred to as immunopathological because of the innapropriate immune response to the infection that can cause harm to the host. Intra- and interspecific variation is observed in the type and magnitude of the hypersensitivity reaction that develops. In some hosts, such as humans and domestic dogs, Type I hypersensitivity is the most frequently observed, with disease termed ‘ordinary’ mange with pathological and clinical signs of mild to intense pruritus and alopecia. However, other hosts, such as wombats, canids (e.g., red foxes, raccoon dogs and San Joaquin kit foxes), and immunocompromised humans, appear predisposed to develo** a Type IV hypersensitivity reaction, resulting in a more severe form of disease known as ‘crusted’ mange/scabies [15] with signs of severe hyperkeratosis and crusts.
The immunological and clinical pathological changes associated with hypersensitivity reactions to S. scabiei infection have been subject to much research across host species [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46]. Several narrative reviews have brought together the complex array of empirical studies into excellent syntheses, albeit mostly focused on pathology observed in humans [7, 13, 17, 47, 48] or a single species [4, 49]. However, a synthesis of mange associated changes in immunology and clinical pathology, that is both empirical and brings together data from across a compilation of host species, is yet to be undertaken. Meta-analysis provides a useful way to synthesise S. scabiei studies, comparing the effect of infected groups with uninfected individuals, as well as across observed disease severity from the same experiment. By bringing together empirical studies on S. scabiei from across host species in a formal analysis framework, a deeper understanding of the interspecies (dis)similarities in the pathology of mange may be developed to the benefit of all hosts, as well as highlight potential knowledge gaps in disease pathology.
In this research, we undertake a comprehensive empirical examination of reported immune, erythrocytic and biochemical parameters observed in host species with clinical signs of mange to give as holistic a view as possible. We synthesise both narrative reviews and empirical studies to describe the immunological and clinical pathological changes occurring in hosts affected by S. scabiei. We include information on all reported variables in the literature, comprising of immunological variables, acute phase proteins (APPs), oxidant/antioxidant status, and erythrocytic, hepatological and nephrological changes. We have three overarching objectives: (1) synthesise existing narrative reviews of immunopathological relationships to S. scabiei infection and construct overarching hypotheses of immunopathological relationships based on these reviews; (2) undertake a systematic meta-analysis of primary literature on immunological and pathological changes observed in association with scabies/mange; and (3) utilise the formal meta-analysis to describe immunological and clinical pathological changes with comparison to the literature from Objective 1.
Methods
Synthesising narrative reviews of sarcoptic mange pathology
In October 2020, we sought to capture all narrative literature reviews available on S. scabiei infection in online databases Web of Science, PubMed, and Scopus. We used broad search criteria by including variations on the disease name, such as sarcoptic mange, scabies and Sarcoptes scabiei, and solely selected to view reviews. Following literature collection, we screened all reviews based on their abstracts and titles to eliminate any review articles not focused on immunology or pathology associated with sarcoptic mange. With the remaining reviews, we synthesised all immunopathological relationships reported and used this to create a conceptual diagram detailing proposed immunopathological relationships associated with infection. The conceptual diagram was created based on the most frequently described immunopathological parameters and relationships reported, with some less commonly mentioned parameters used to draw ‘likely’ connections in a small number of instances (e.g., B cells and some cytokines). Hypersensitivity type was usually listed in the reviews explaining the response to S. scabiei infection. In reviews where this was not the case, we categorised the response according to previous knowledge or observations regarding the host’s response, either from other reviews focusing on the same hosts or by screening the observations from the referenced articles.
Meta-analysis of immunopathological responses associated with S. scabiei infection
Between April and October 2020, we undertook a systematic search of the existing literature in the same online databases as mentioned above, to obtain relevant studies reporting the empirical effects of sarcoptic mange on animals and humans. We used the following keywords in two separate searches, which we found best captured both human and non-human animal studies:
(“Sarcoptes scabiei” OR “sarcoptic mange” OR “scabies”) AND (“patho*” OR “physio*” OR “disease” OR “immun*” OR “biochem*” OR “skin” OR “lesion” OR “metabol*” OR “heat” OR “therm*”) AND (“wild*” OR “domestic*”) NOT (“human*” OR “child*” OR “m?n” OR “wom?n” OR “patient*”) NOT (“potato scab” OR “streptomyces”).
(“Sarcoptes scabiei” OR “sarcoptic mange” OR “scabies”) AND (“patho*” OR “physio*” OR “disease” OR “immun*” OR “biochem*” OR “skin” OR “lesion” OR “metabol*” OR “heat” OR “therm*”) AND (“human” OR “child*” OR “m?n” OR “wom?n” OR “patient*” AND “control”) NOT (“potato scab” OR “streptomyces”).
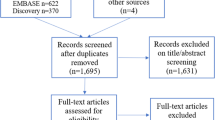
Any variation of the name of the disease was accommodated (e.g., sarcoptic mange or scabies) as well as the name of the parasite (Sarcoptes scabiei), and irrelevant diseases (e.g., potato scab or streptomyces) were excluded. Any studies reporting empirical information linking host immunology or pathology with mange/scabies were retained. This resulted in 1394 research articles that were downloaded to an EndNote Library and duplicates were removed. All studies were screened by evaluating the results section, excluding those studies: (i) not mentioning or comparing to a relevant control group; (ii) not informing of sample sizes; (iii) lacking mean values; or (iv) lacking standard deviation (SD) or standard error (SE) values. 63 research articles were included in the final meta-analysis (see Additional file 4: Fig. S1).
For each study, we noted the author, year, parameter(s) examined, key findings, sample sizes across control and treatment groups, hypersensitivity type, host species and country of origin for the study. The mean, SD or SE and sample size for each parameter were recorded to calculate the effect size and confidence intervals (CI). In cases where a control group wasn’t specified, but a time point of sample collecting was, we chose the values the studies used as a ‘base line’ (e.g., healthy, pre-exposure or day 0) as a control group. When multiple means were reported (e.g., separate means for each control subject or males and females separately), the values were pooled using the Cochrane method [50]; the same method was applied for SE and SD.
Mange affected and healthy control groups were compared by the calculation of effect sizes (g) and variance (Vg) using the following equations [51];
where x1 and x2 are the mean values of the control and treatment groups, n1 and n2 are sample sizes, Swithin is the within-groups standard deviation, and J is the correction factor for small sample sizes, \(J=1-\frac{3}{4df-1}\). Upper and lower CI was calculated lastly for each effect size using the following equation, \(CI=g\pm 1.96*SEg\), where SEg is the square root of the variance (Vg).
Due to the large number of immunopathological parameters and differences in focus groups with some studies dividing their data based on disease severity (mild/moderate/severe clinical sign presentation) or simply affected/not affected, the data were divided into four different categories against the control group: (a) mange affected; (b) mild cases of mange; (c) moderate cases of mange; and (d) severe cases of mange. By separating our data into these categories, we somewhat reduced the variability observed in study methods. From now on we will refer to category a as ‘mange affected’ or ‘diseased relative to control comparisons; and category b–d as ‘mange severity’. The classification of disease severity was based on the author’s determination or was otherwise categorised based on the percentage of lesions present according to Additional file 4: Table S1. The effect sizes were compared and computed together in R (ver. 1.3.1093) using the metafor package [52]. We investigated whether publication bias existed in the literature for studies reporting immunological parameters for Type I and Type IV hypersentivity, as well as parameters falling into common responses (i.e. oxidant/antioxidant status, acute phase protein response, and erythrocytic, hepatological and nephrological changes). To test for publication bias we plotted standard error against effect sizes for the previous mentioned categories (Additional file 5: Figs. S1–S3). We additionally conducted a regression test to test for data distribution asymmetry using regtest in the metafor package [52]. Finally, a conceptual diagram based on the meta-analysis was created, partially informed by the proposed processes from the narrative reviews in Objective 1, but with only significant relationships (95% Cis not overlap** zero) computed from the meta-analysis added (Fig. 1B).
Diagrams of the immunopathological cascades arising from Sarcoptes scabiei infection depending on host hypersensitivity response (Type I or IV). Diagram A represents the immunopathological processes as currently proposed in narrative literature reviews of S. scabiei, and diagram B represents the Immunopathological relationships supported by the meta-analysis undertaken in this study. Solid arrow ( →) indicates a stimulation or influence from one parameter to the other, whereas a dashed arrow (– →) indicates a hypothesised link; small up or down triangle next to parameter indicates an increase or decrease; red text indicates missing immunological links considered likely to connect parameters; Parameters in non-bold indicates secreted cytokines or immunoglobulins; *in panel B IV indicates no direct measure of macrophages instead measured by MCP-1; **in panel A IV indicates epidermal cells to include keratinocytes, Langerhans cells and fibroblasts; *** in panel B indicates no direct measure of T cells or B cells however could be included in the measurement of lymphocytes. IL interleukin; IFN-γ Interferon gamma; TNF-α tumour necrosis factor alpha; TGF-ß transforming growth factor beta; CD4+= T helper cells; CD8+ cytotoxic T cells; Ig immunoglobulin; C3 complement 3; MCV mean corpuscular volume; TEC total erythrocyte concentration; PCV packed cell volume; AGP Acid(1)-alpha glycoprotein; SAA serum amyloid A; A:G ratio albumin:globulin ratio; ALT alanine aminotransferase; BUN blood urea nitrogen; MCHC mean corpuscular haemoglobin concentration; MCH mean corpuscular haemoglobin; LPO lipid peroxidation; CAT catalase; GSH:GSSH free glutathione:oxidized glutathione ratio; GGT Gamma-glutamyl transferase. Created in Inkscape
Results
Synthesising narrative reviews of sarcoptic mange-associated clinical pathology
From the initial literature search, 244 narrative review articles were identified. These reviews were diverse, covering topics from socioeconomic impacts of sarcoptic mange to the pathology associated with S. scabiei. A conceptual diagram (Fig. 1A) was created based on 55 reviews that primarily described immunological and clinical pathology variables associated with sarcoptic mange (see Additional file 1: S1).
There was an almost equal amount of information regarding Type I and IV hypersensitivity responses (Type I = 32; Type IV = 30). The most common parameters described across studies were eosinophils (Type I, 19 times; Type IV, 20 times), mast cells (Type I, 9 times; Type IV, 5 times) and immunoglobulin E (IgE) (Type I, 16 times; Type IV, 16 times) for both hypersensitivity types. In addition, a Type 1 T helper cell (Th1) response was described 9 times for Type I and 1 time for Type IV, and a Type 2 T helper cell (Th2) response was described 3 times for Type I and 13 times for Type IV hypersensitivity type. Observations from reviews provided evidence of Type I hypersensitivity being associated with IgE activation of basophils and mast cells, and the subsequent release of histamine and other proinflammatory cytokines. Processes documented as part of Type IV hypersensitivity responses included the proliferation of macrophages, neutrophils, and B cells. Antibody responses appeared to be present during both Type I and Type IV hypersensitivity responses, depending on whether there was a Th1 focused response or a Th2 focused response to S. scabiei infection. Almost all hosts infested with S. scabiei reportedly had altered antioxidant defence, mild to severe anaemia, and hepatological and nephrological abnormalities.
Publication bias
We chose to look at whether a bias existed in the literature to determine whether the effect sizes we obtained from our analysis were potentially influenced by publication bias. Plotting effect sizes against the standard errors for all immunological parameters for either Type I or Type IV hypersentivity or common responses extracted from the literature resulted in some of the funnel plots with the appearance of largely scattered data points, and plots that appeared asymmetrically distributed (see Additional file 5: Figures). To investigate this further, we conducted an Egger’s regression for funnel plot asymmetry for each category across Type I hypersentivity, Type IV hypersensitivity and common responses. Regressions for Ctrl vs. affected (Type I hypersensitivity: z = 12.60, p < 0.0001; Type IV hypersensitivity: z = 5.79, p < 0.0001; Common responses: z = 4.03, p < 0.0001), mild mange (Type IV hypersensitivity: z = − 4.67, p < 0.0001; Common responses: z = − 6.70, p < 0.0001), moderate mange (Type I hypersensitivity: z = 5.17, p < 0.0001; Type IV hypersensitivity: z = 6.11, p < 0.0001; Common responses: z = − 5.21, p < 0.0001), and severe mange (Type IV hypersensitivity: z = 5.14, p < 0.0001) suggests that a bias does exists (see Additional file 5: Table S1 for full overview).
Meta-analysis of immunological and clincal pathology changes associated with S. scabiei infection
The full dataset included 565 effect sizes from 63 research articles that reported immune and pathological changes in association with S. scabiei infection. 244 effect sizes were categorised as being linked to type I hypersensitivity and 321 were categorised as being linked to type IV hypersensitivity. Neutrophil counts were the most frequently reported parameters (25 times), globulin was the second most reported (23 times) and eosinophils, haematocrit and haemoglobin concentration were the third most reported parameters (21 times each). Data were available for a total of five different orders (Artiodactyla, 39%; Carnivora, 26%; Primates, 24%; Diprotodontia, 8%; Lagomorpha, 3%) and 19 species (see Figs. 1 and 2 and Additional file 1: S1 and Additional file 2: Appendicies for the full dataset), consisting mostly of domestic dogs (12 studies, 138 effect sizes), humans (14 studies, 76 effect sizes), bare-nosed wombats (4 studies, 67 effect sizes) and Iberian ibex (8 studies, 63 effect sizes).
Heatmap illustrating the four host species for which effect sizes were most commonly calculated (dog = 138, human = 76, bare-nosed wombat = 67 and Iberian ibex = 63). The heat reflects the percentage of studies for each category (immunological process) with each category amounting to a total of 100%. Parameters not falling directly into a definitive category, such as ‘Erythrocytic changes or ‘Acute phase proteins’, were included in the category ‘Other’
Type I hypersensitivity immune variable changes
Of the parameters examined for their association with host disease status, all leukocyte types exhibited a significant change (increase or decrease with CIs not overlap** zero) in one or more of the mange status and severity categories, except monocytes and basophils (Table 1). These changes included 3 species and 10 individual studies. Overall leukocyte and lymphocyte numbers were only significantly elevated in the moderate and severe mange categories, respectively. However, significant effect size changes in the numbers of several other cell types were observed across the spectrum of disease severity. For variables where increases were observed from mild to severe disease (mast cells, neutrophils and macrophages), effect sizes also increased sequentially, except from eosinophils, where the effect size peaked at moderate mange severity. T helper CD4+ were the only leukocyte type that decreased in effect size. The only antibody or cytokine with measurements reported in studies with a mange severity scale was IgE, which showed an increase in effect sizes with severity. Antibodies IgG and IgM, and cytokines IL-2, IL-4, IL-5, IL-10, IL-13, and transforming growth factor (TGF)-ß were elevated in diseased relative to control comparisons. Effect sizes for monocytes, basophils, monocyte chemoattractant protein (MCP)-1, macrophage inflammatory protein (MIP)-1ɑ, IgA, IL-8, IL-17, tumor necrosis factor (TNF)-α, interferon (IFN)-γ and complement (C) 3 were not significantly changed in relation to disease status.
Type IV hypersensitivity immune variable responses
Fewer immune variable measurements were investigated in association with Type IV hypersensitivity responses to S. scabiei infection (Table 2, 20 for Type IV vs. 26 for Type I). Although severity data was available for total leukocytes, lymphocytes, neutrophils, eosinophils, monocytes and mast cells, significant increases in effect sizes were only observed in monocytes in mild and severe cases, in neutrophils and eosinophils in mild cases of mange, as well as in total leukocytes and monocytes in affected cases relative to controls. Although no significant changes were observed in lymphocyte number, T cells, CD4+, and CD8+ cells were all significantly elevated in diseased relative to control comparisons. Macrophage numbers were not measured directly in hosts exhibiting Type IV hypersensitivity response, however, MCP-1, a key chemokine for regulation of macrophage migration and infiltration, increased significantly in diseased relative to control comparisons. Of the antibodies, only IgG have been examined in relation to mange severity categories, which showed an increase in severe cases, as well as in diseased relative to control comparisons. Antibody IgM increased significantly, whereas the effect size of IgA decreased significantly (both in diseased relative to control comparisions). The only significant change in cytokines was observed in IL-17 that increased in diseased relative to control comparisons. Changes in lymphocytes, basophils, mast cells, IgE, TNF-a, IL-1, IL-6, and IL-8 did not significantly differ relative to infection status or disease severity.
Oxidant/antioxidant status
All but one of the parameters associated with oxidative stress, mentioned in the literature, exhibited a significant change (increase or decrease with CIs not overlap** zero) (Table 3). Lipid peroxidation (LPO), free gluthathione (GSH), total oxidative stress (TOS) and malondialdehyde (MDA) had elevated effect sizes relative to control and/or increased with disease severity. Catalase (CAT), vitamin C, zinc, and copper were reduced, relative to the controls and/or with disease severity. Free glutathione:oxidised glutathione ratio (GSH:GSSH) decreased significantly in severe mange cases, but no significant change was observed in diseased relative to control comparisons nor in mild or moderate mange severity. Superoxide dismutase (SOD) did not significantly differ relative to infection status or disease severity.
Acute phase protein response
Three out of the six acute phase proteins (APPs) mentioned in the literature showed increased effect sizes in diseased relative to control comparisons [e.g. ceruloplasmin, transferrin, acid(1)-alpha glycoprotein (AGP)]. Serum amyloid A (SAA) increased in severe cases, but not in mild and moderate disease categories and in diseased relative to control comparisons. Only albumin of the APPs decreased in effect size in both moderate and severe cases of infection and in diseased relative to control comparisons. Haptoglobin was the only acute phase protein in the analysis without evidence of any significant changes in either disease categories.
Erythrocytic changes
Six out of seven erythrocytic parameters, mentioned in literature, significantly decreased in one or more of the mange severity categories (Table 3). Total red blood cells (RBC) decreased significantly in all categories. Haemoglobin and iron decreased in severe cases of mange and in diseased relative to controls. Haematocrit values decreased in mild and moderate cases of mange and in diseased relative to controls. Mean corpuscular haemoglobin concentration (MCHC)’s effect size decreased in severe cases of mange. In contrast, the mean corpuscular volume (MCV) had an elevated effect size in severe cases of mange, compared to controls. Mean corpuscular haemoglobin (MCH) was the only parameter not showing any significant changes in effect sizes.
Hepatological and nephrological changes
Only five out of ten parameters, mentioned in the literature, had significant effect size changes. Alanine aminotransferase (ALT), increased in diseased relative to control comparisons. Gamma-glutamyl transferase (GGT) significantly increased in severe cases. Blood urea nitrogen (BUN) was increased in moderate and severe cases of sarcoptic mange compared to controls (no data for severe cases of mange). The BUN:creatinine ratio had a significant increased effect size in moderate/severe cases, but not in mild. There was no data in diseased relative to control comparisons for the BUN:creatinine ratio. Urea:Nitrogen ratio increased significantly in severe cases as well, but not in moderate. No data was available for mild cases of disease nor diseased relative to control comparisons for Urea:N ratio. The remaining parameters (total protein, globulin, bilirubin, creatinine and urea) did not significantly change with S. scabiei infection status or severity.
Discussion
Sarcoptic mange affects a large range of mammal species globally, with clinical manifestations of disease generally depending on the type of hypersensitiy response experienced by the host (Type I and IV). Thus, combining studies across host species with a meta-analysis provides a fuller understanding of the clinical pathology of sarcoptic mange. We brought together both narrative reviews on the topic, which help develop proposed immunological processes/hypotheses, and empirical studies, formally examined with the use of a meta-analysis. We showed which immunological parameters changed significantly during Type I and Type IV hypersensitivity responses, as well as oxidant/antioxidant balance, APPs, erythrocytic, hepatological and nephrological changes compared to controls. Overall, our results reveal the complex nature of immune and clinical pathological changes associated with scabies/mange. As anticipated, our empirical findings broadly align with narrative reviews (see Fig. 1), although they also simplify proposed processes. Our results provide evidence of interspecific consensus in 27 immunological and clinical pathology variables across Type I (6/26 parameters) and Type IV (3/20 parameters) hypersensitivity, and in oxidant/antioxidant balance (6/10 parameters), APPs (3/6 parameters), erythrocytic (4/7 parameters) and hepatological/nephrological (5/10 parameters) changes. The only parameter showing interspecific consensus in both Type I and Type IV hypersensitivity responses was neutrophils, which showed a similar increase in effect sizes across severity and in affected compared to controls.
Type I hypersensitivity-associated changes
The immediate immune response associated with Type I hypersensitivity reactions is considered to be primarily driven by a combination of IgE, mast cells and eosinophils [53]. Activation of these immunological variables are confirmed in our meta-analysis. Eosinophils, which primarily help promote inflammation in response to a parasitic infection, increased in scabies/mange affected hosts relative to controls and across disease severity. IgE stimulates mast cells and basophils to secrete proinflammatory reagents in response to infection [54,55,56]. Our results indicated increased levels of IgE and mast cells with mange severity, which corresponds to what is described in the literature [57, 58]. There was evidence of interspecific consensus in the response of eosinophils and IgE, including humans, domestic dogs and pigs, and humans, bulls, rabbits and domestic dogs, respectively.
Looking at the effect sizes of changes in lymphocyte numbers, an increase was observed in moderate cases compared to controls, but not in other severity categories. Importantly, results in this moderate severity category only stem from a single study involving domestic dogs. Increased lymphocytes numbers could be due to the increased pruritus and the subsequent abrasion of the skin, which enhances microbial invasion leading to the increase of white blood cells [29]. However, both intra- and inter-specific variation was evident in mild and severe cases of mange with both non-significant decreases and increases in effect sizes being documented. This suggests variation when it comes to lymphocyte numbers in response to S. scabiei infection. A potential explaination for this variation could be linked to the shift or overlap between Th1 and Th2 responses [59], discussed further below.
According to narrative reviews (illustrated in Fig. 1A) of Type I hypersensitivity response to S. scabiei infection affected hosts most often exhibit a predominant Th1 response. With regards to specific lymphocytes lineages, our meta-analysis provides some evidence of significant changes in response to disease. For example, a single study showed significant decreases in CD4+ T cells in diseased domestic dogs relative to controls indicating cell differentiation from naïve CD4+ T cells to specific linages such as Th1 and/or Th2 T cells [44] in transferrin and ceruloplasmin. The increases in effect sizes of ceruloplasmin, AGP and SAA corresponds to what is expected in the host response to infection as illustrated in dogs, pigs and cattle [79]. SAA is secreted during the acute phase of inflammation with a key role of recruiting white blood cell types to the site of inflammation [79]. Surprisingly, we only observed a significant effect size in severe cases of mange in Iberian ibex relative to controls. This is particularly surprising as we would expect APPs to be highest in early stages of infection (mild and moderate cases) and slowly wane with chronic disease (e.g. severe cases) [80], however chronic expression of SAA has been observed in rats causing systemic amyloidosis [81] indicating that it is likely species and/or condition specific APP responses. Another unexpected result is the lack of significance in haptoglobin. Haptoglobin is known to increase several fold during an inflammatory event and the levels (e.g. high or low of haptoglobin subtypes) of haptoglobin has an important role in the disease development for other parasitic diseases in regards to exacterbated oxidative stress [82]. Looking at the individual studies’ effect sizes the two studies with Type I hypersensitivity response (pig and alpine ibex) have significantly increased effect sizes, whereas the Iberian ibex study (involving both severity range and diseased relative to control) exhibiting Type IV hypersensitivity had non-significantly increased effect sizes. To understand if this difference is due to Type I or Type IV hypersensitivity responses or due to species variation or lastly a function of the assays used in the analysis, more research is needed.
Albumin is the most commonly studied APP in our analysis incompassing 12 different species in total. Despite its antioxidant properties that could influence oxidative status, as a negative acute phase protein its decreased levels are most-likely explained by reduced hepatic synthesis [30] and catabolic processes during sarcoptic mange [35, 83]. Unexpectedly, the effect size of transferrin, in diseased relative to control comparisons, showed an increase. This contradicts other studies into immune-modulated inflammation, as these found that transferrin, which is a negative APP associated with the innate immune response [79], typically decreases during inflammation [84, 1]. Here, we undertook a detailed assessment of S. scabiei. We hope the information brought together help create and generalise therapeutic strategies, as well as help illustrate disease aspects with little to no information.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files.
Abbreviations
- Cis:
-
95% Confidence intervals
- IL:
-
Interleukin
- IFN-γ:
-
Interferon gamma
- TNF-α:
-
Tumour necrosis factor alpha
- TGF-β:
-
Transforming Growth Factor beta
- CD4+:
-
T helper cells
- CD8+:
-
Cytotoxic T cells
- Ig:
-
Immunoglobulin
- C3:
-
Complement 3
- MCV:
-
Mean corpuscular volume
- TEC:
-
Total erythrocyte concentration
- PCV:
-
Packed cell volume
- AGP:
-
Acid(1)-alpha glycoprotein
- SAA:
-
Serum amyloid A
- A:G ratio:
-
Albumin:globulin ratio
- ALT:
-
Alanine aminotransferase
- BUN:
-
Blood urea nitrogen
- MCHC:
-
Mean corpuscular haemoglobin concentration
- MCH:
-
Mean corpuscular haemoglobin
- LPO:
-
Lipid peroxidation
- CAT:
-
Catalase
- GSH:GSSH:
-
Free glutathione:oxidized glutathione ratio
- GGT:
-
Gamma-glutamyl transferase
References
Escobar LE, Carver S, Cross PC, Rossi L, Almberg ES, Yabsley MJ, et al. Sarcoptic mange: an emerging panzootic in wildlife. Transbound Emerg Dis. 2021;69:927–42.
Old JM, Sengupta C, Narayan E, Wolfenden J. Sarcoptic mange in wombats—a review and future research directions. Transbound Emerg Dis. 2018;65(2):399–407.
Pence DB, Ueckermann E. Sarcoptic mange in wildlife. Rev Sci Tech Off Int Epizoot. 2002;21(2):385–98.
Kraabøl M, Gundersen V, Fangel K, Olstad K. The taxonomy, life cycle and pathology of sarcoptes scabiei and notoedres cati (Acarina, sarcoptidae): a review in a fennoscandian wildlife perspective. Fauna Norvegica. 2015;35:21–33.
Bornstein S, Mörner T, Samuel W. Sarcoptes scabiei and Sarcoptic Mange. In: Samuel WM, Pybus MJ, Kocan AA, editors. Parasitic diseases of wild mammals. Ames: Iowa State University Press; 2001. p. 107–19.
Currier RW, Walton SF, Currie BJ. Scabies in animals and humans: history, evolutionary perspectives, and modern clinical management. In: Nahmias A, Danielsson D, Nahmias SB, editors. Evolution of infectious agents in relation to sex. Annals of the New York Academy of Sciences. Oxford: Blackwell Science Publ; 2011. p. 50–60.
Chandler DJ, Fuller LC. A review of scabies: an infestation more than skin deep. Dermatology. 2019;235(2):79–90.
Pérez JM, Granados JE, Espinosa J, Ráez-Bravo A, López-Olvera JR, Rossi L, et al. Biology and management of sarcoptic mange in wild Caprinae populations. Mamm Rev. 2020;51:82.
Thomas C, Coates SJ, Engelman D, Chosidow O, Chang AY. Ectoparasites: scabies. J Am Acad Dermatol. 2020;82(3):533–48.
Turchetto S, Obber F, Rossi L, D’Amelio S, Cavallero S, Poli A, et al. Sarcoptic mange in wild caprinae of the Alps: could pathology help in filling the gaps in knowledge? Front Vet Sci. 2020. https://doi.org/10.3389/fvets.2020.00193.
Laha R. Sarcoptic mange infestation in pigs: an overview. J Parasit Dis. 2015;39(4):596–603.
Arlian LG, Morgan MS. A review of Sarcoptes scabiei: past, present and future. Parasit Vectors. 2017;10(1):297.
Arora P, Rudnicka L, Sar-Pomian M, Wollina U, Jafferany M, Lotti T, et al. Scabies: A comprehensive review and current perspectives. Dermatol Ther. 2020;33(4):12.
McCarthy JS, Kemp DJ, Walton SF, Currie BJ. Scabies: More than just an irritation. Postgrad Med J. 2004;80(945):382–7.
Walton SF, Oprescu FI. Immunology of scabies and translational outcomes: identifying the missing links. Curr Opin Infect Dis. 2013;26(2):116–22.
Shimose L, Munoz-Price LS. Diagnosis, prevention, and treatment of scabies. Curr Opin Infect Dis. 2013;15(5):426–31.
Mounsey EK, McCarthy SJ, Walton FS. Scratching the itch: new tools to advance understanding of scabies. Trends Parasitol. 2013;29(1):35–42.
Dispenza MC. Classification of hypersensitivity reactions. Allergy Asthma Proc. 2019;40(6):470–3.
Dalapati MR, Bhowmik MK, Sarkar S. Clinico-haematological, biochemical and pathomorphological changes of scabies in goats. Indian J Anim Sci. 1996;66(4):351–4.
Rathod A, Singh AP, Dixit SK, Dadhich H, Tanwar RK, Gahlot AK. Haemato-biochemical profile of camels (Camelus dromedarius) suffering from sarcopticosis. Vet Pract. 2008;9(2):104–9.
Allaam MA, Allam TS, Elkhatam AO. Biochemical and circulating oxidative stress biomarkers in Egyptian buffaloes (Bubalus bubalis) infested by sarcoptic mange. Global Veterinaria. 2014;13(4):656–61.
Rahman MM, Lecchi C, Fraquelli C, Sartorelli P, Ceciliani F. Acute phase protein response in Alpine ibex with sarcoptic mange. Vet Parasitol. 2010;168(3–4):293–8.
Lastras ME, Pastor J, Marco I, Ruiz M, Viñas L, Lavin S. Effects of sarcoptic mange on serum proteins and immunoglobulin G levels in chamois (Rupicapra pyrenaica) and Spanish ibex (Capra pyrenaica). Vet Parasitol. 2000;88(3–4):313–9.
Raez-Bravo A, Granados JE, Serrano E, Dellamaria D, Casais R, Rossi L, et al. Evaluation of three enzyme-linked immunosorbent assays for sarcoptic mange diagnosis and assessment in the Iberian ibex Capra pyrenaica. Parasites Vectors. 2016;9:8.
Skerratt LF. Clinical response of captive common wombats (Vombatus ursinus) infected with Sarcoptes scabiei var. wombati. J Wildl Dis. 2003;39(1):179–92.
Hartley M, English A. Sarcoptes scabei var wombati infection in the common wombat (Vombatus ursinus). Eur J Wildl Res. 2005;51(2):117–21.
Skerratt LF, Beveridge I. Human scabies of wombat origin. Aust Vet J. 1999;77(9):607.
Ruykys L, Breed B, Schultz D, Taggart D. Effects and treatment of sarcoptic mange in southern hairy-nosed wombats. J Wildl Dis. 2013;49(2):312–20.
Arlian LG, Morgan MS, Rapp CM, Vyszenski-Moher DL. Some effects of sarcoptic mange on dogs. J Parasitol. 1995;81(5):698–702.
Beigh SA, Soodan JS, Bhat AM. Sarcoptic mange in dogs: Its effect on liver, oxidative stress, trace minerals and vitamins. Vet Parasitol. 2016;227:30–4.
Behera SK, Dimri U, Singh SK, Mohanta RK. The curative and antioxidative efficiency of ivermectin and ivermectin plus vitamin E-selenium treatment on canine Sarcoptes scabiei infestation. Vet Res Commun. 2011;35(4):237–44.
Chandy J, Nambi AP, Jeyaraja K, Gowri B. Clinicopathological and biochemical studies in scabies in dogs. Indian Vet J. 2000;77(9):755–7.
Aujla RS, Singla LD, Juyal PD, Gupta PP. Prevalence and pathology of mange-mite infestations in dogs. J Vet Parasitol. 2000;14(1):45–9.
Nwufoh OC, Sadiq NA, Adediran OA, Jarikre TA, Emikpe BO. Sequential histopathological changes and cytokine expressions in dogs naturally infested with sarcoptes scabiei mites. Acta Parasitol. 2020;65:452–61.
Kido N, Kamegaya C, Omiya T, Wada Y, Takahashi M, Yamamoto Y. Hematology and serum biochemistry in debilitated, free-ranging raccoon dogs (Nyctereutes procyonoides) infested with sarcoptic mange. Parasitol Int. 2011;60(4):425–8.
Noviana D, Otsuka Y, Horii Y. Proliferation of protease-enriched mast cells in sarcoptic skin lesions of raccoon dogs. J Comp Pathol. 2004;131(1):28–37.
Newman TJ, Baker PJ, Harris S. Nutritional condition and survival of red foxes with sarcoptic mange. Can J Zool. 2002;80(1):154–61.
Rudd J, Clifford D, Richardson D, Cypher B, Westall T, Kelly E, et al. Hematologic and serum chemistry values of endangered san joaquin kit foxes (Vulpes macrotis mutica) with sarcoptic mange. J Wildl Dis. 2019;55(2):410–5.
Al-Hadraawy SK, Hessen HB. Hematological and epidemiological study for patients infected with scabies. J Pharm Sci Res. 2017;9(6):897–900.
Mohammed ZAA, Hadi NA, Kawen AA. Immunological aspects of patients infested with sacbies in Thi-Qar Province Southern Iraq. Medico-Legal Update. 2020;20(3):1–8.
Al-Musawi ND, Al-Bayati NY, Hussain MJ. Evaluation of some interleukins and immunomodulatory factors in Iraqi scabies patients. J Garmian Univ. 2018;5:30–40.
Sarma K, Roychoudhury P, Das G, Borthaku SK, Kalita G, Prasad H, et al. Seroprevalence of Sarcoptes scabiei var suis infestation in swine population and its effect on haemato-biochemical and oxidative stress indices and its management with special reference to herbal ointmen. Indian J Anim Res. 2019;53(11):1489–96.
Dimri U, Bandyopadhyay S, Singh SK, Ranjan R, Mukherjee R, Yatoo MI, et al. Assay of alterations in oxidative stress markers in pigs naturally infested with Sarcoptes scabiei var. suis. Vet Parasitol. 2014;205(1–2):295–9.
Zalunardo M, Cargill CF, Sandeman RM. Identification of auto-antigens in skin scra**s from scabies-infected pigs. Int J Parasitol. 2006;36(10–11):1133–41.
Seddiek SA, Khater HF, El-Shorbagy MM, Ali AM. The acaricidal efficacy of aqueous neem extract and ivermectin against Sarcoptes scabiei var cuniculi in experimentally infested rabbits. Parasitol Res. 2013;112(6):2319–30.
Casais R, Millán J, Rosell JM, Dalton KP, Prieto JM. Evaluation of an ELISA using recombinant Ssλ20δB3 antigen for the serological diagnosis of Sarcoptes scabiei infestation in domestic and wild rabbits. Vet Parasitol. 2015;214(3–4):315–21.
Bhat SA, Mounsey KE, Liu X, Walton SF. Host immune responses to the itch mite, Sarcoptes scabiei, in humans. Parasit Vectors. 2017;10(1):385.
Fischer K, Walton S. Parasitic mites of medical and veterinary importance—is there a common research agenda? Int J Parasitol. 2014;44(12):955–67.
Perez JM, Granados JE, Espinosa J, Raez-Bravo A, Lopez-Olvera JR, Rossi L, et al. Biology and management of sarcoptic mange in wild Caprinae populations. Mam Rev. 2021;51(1):82–94.
Higgins J, Thomas J, Chandler J, Cumpston M, Li Y, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions: Cochrane; 2021 [updated February 2021. Version 6.2. www.training.cochrane.org/handbook.
Borenstein M, Hedges LV, Higgins JP, Rothstein HR. Introduction to meta-analysis. Chichester: Wiley; 2009.
Viechtbauer W. Conducting meta analyses in R with the metafor package. J Stat Softw. 2010;36:1–48.
Abbas AK, Lichtman AH. Basic immunology: functions and disorders of the immune system. Philadelphia: Elsevier Saunders; 2006.
Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med. 2012;18(5):693–704.
Galli SJ, Tsai M. Mast cells in allergy and infection: versatile effector and regulatory cells in innate and adaptive immunity. Eur J Immunol. 2010;40(7):1843–51.
Mukai K, Tsai M, Starkl P, Marichal T, Galli SJ. IgE and mast cells in host defense against parasites and venoms. Semin Immunopathol. 2016;38(5):581–603.
Morgan MS, Arlian LG. Serum antibody profiles of Sarcoptes scabiei infested or immunized rabbits. Folia Parasitol (Praha). 1994;41(3):223–7.
Arlian LG, Morgan MS, Rapp CM, Vyszenski-Moher DL. The development of protective immunity in canine scabies. Vet Parasitol. 1996;62(1–2):133–42.
Spellberg B, Edwards JE Jr. Type 1/Type 2 immunity in infectious diseases. Clin Infect Dis. 2001;32(1):76–102.
Luckheeram RV, Zhou R, Verma AD, **a B. CD4+T cells: differentiation and functions. Clin Dev Immunol. 2012;2012:1–12.
Berger A. Th1 and Th2 responses: what are they? BMJ. 2000;321(7258):424.
Shinkai K, Mohrs M, Locksley RM. Helper T cells regulate type-2 innate immunity in vivo. Nature. 2002;420(6917):825–9.
Draijer C, Robbe P, Boorsma CE, Hylkema MN, Melgert BN. Characterization of macrophage phenotypes in three murine models of house-dust-mite-induced asthma. Mediat Inflamm. 2013;2013:1–10.
Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20(6):355–62.
Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164(12):6166–73.
Prud’homme GJ. Pathobiology of transforming growth factor β in cancer, fibrosis and immunologic disease, and therapeutic considerations. Lab Invest. 2007;87(11):1077–91.
Brake DK, PérezDeLeón AA. Immunoregulation of bovine macrophages by factors in the salivary glands of Rhipicephalus microplus. Parasites Vectors. 2012;5(1):38.
Grönwall C, Vas J, Silverman G. Protective roles of natural IgM antibodies. Front Immunol. 2012. https://doi.org/10.3389/fimmu.2012.00066.
Boes M, Prodeus AP, Schmidt T, Carroll MC, Chen J. A critical role of natural immunoglobulin M in immediate defense against systemic bacterial infection. J Exp Med. 1998;188(12):2381–6.
Marwa K, Kondamudi N. Type IV Hypersensitivity Reaction StatPearls [Internet]: Treasure Island (FL): StatPearls Publishing; 2021. https://www.ncbi.nlm.nih.gov/books/NBK562228/.
Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29(6):313–26.
Liu X, Walton S, Mounsey K. Vaccine against scabies: necessity and possibility. Parasitology. 2014;141(6):725–32.
Bhat SA, Walton SF, Ventura T, Liu X, McCarthy JS, Burgess STG, et al. Early immune suppression leads to uncontrolled mite proliferation and potent host inflammatory responses in a porcine model of crusted versus ordinary scabies. PLoS Negl Trop Dis. 2020;14(9): e0008601.
Forman HJ, Zhang H. Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat Rev Drug Discov. 2021;20:689–709.
Kurutas EB. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutr J. 2016;15(1):71.
Evans P, Halliwell B. Micronutrients: oxidant/antioxidant status. Br J Nutr. 2001;85(S2):S67.
Schorah CJ, Downing C, Piripitsi A, Gallivan L, Al-Hazaa AH, Sanderson MJ, et al. Total vitamin C, ascorbic acid, and dehydroascorbic acid concentrations in plasma of critically ill patients. Am J Clin Nutr. 1996;63(5):760–5.
Raez-Bravo A, Granados JE, Ceron JJ, Cano MFJ, Fandos P, Perez JM, et al. Acute phase proteins increase with sarcoptic mange status and severity in Iberian ibex (Capra pyrenaica, Schinz 1838). Parasitol Res. 2015;114(11):4005–10.
Jain S, Gautam V, Naseem S. Acute-phase proteins: As diagnostic tool. J Pharm Bioallied Sci. 2011;3(1):118–27.
Hooijberg EH, Cray C, Steenkamp G, Buss P, Goddard A, Miller M. Assessment of the Acute Phase Response in Healthy and Injured Southern White Rhinoceros (Ceratotherium simum simum). Frontiers in Veterinary Science. 2020;6(475):1–9.
Cray C. Acute phase proteins in animals. Amsterdam: Elsevier; 2012. p. 113–50.
Quaye IK. Haptoglobin, inflammation and disease. Trans R Soc Trop Med Hyg. 2008;102(8):735–42.
Laboratories I. Albumin Interpretive Summary 2013 [updated 11/1/2013. https://www.idexx.no/files/8088-us-albumin-interpretive-summary.pdf.
Matusiewicz M, Neubauer K, Lewandowska P, Gamian A, Krzystek-Korpacka M. Reduced transferrin levels in active inflammatory bowel disease. Biomed Res Int. 2017;2017:1–8.
Vanarsa K, Ye Y, Han J, **e C, Mohan C, Wu T. Inflammation associated anemia and ferritin as disease markers in systemic lupus erythematosus. Arthritis Res Ther. 2012;14(4):R182.
Bermejo F, García-López S. A guide to diagnosis of iron deficiency and iron deficiency anemia in digestive diseases. World J Gastroenterol. 2009;15(37):4638.
Laboratories I. Hematocrit Interpretive Summary 2013 https://www.idexx.dk/files/8947-us-hct-interpretive-summary.pdf.
Laboratories I. Hemoglobin Intepretive Summary 2013. https://www.idexx.no/files/8948-us-hemoglobin-interpretive-summary.pdf.
Laboratories I. Mean Corpuscular Hemoglobin Concentration (MCHC) 2013 [updated 11/1/2013.
Pérez JM, Granados JE, González FJ, Ruiz-Martinez I, Soriguer RC. Hematologic parameters of the Spanish ibex (Capra pyrenaica). J Zoo Wildl Med. 1999;30(4):550–4.
Laboratories I. Blood Urea Nitrogen (BUN) 2013.
Chung SD, Wang KH, Huang CC, Lin HC. Scabies increased the risk of chronic kidney disease: a 5-year follow-up study. J Eur Acad Dermatol Venereol. 2014;28(3):286–92.
Hoy WE, White AV, Dowling A, Sharma SK, Bloomfield H, Tipiloura BT, et al. Post-streptococcal glomerulonephritis is a strong risk factor for chronic kidney disease in later life. Kidney Int. 2012;81(10):1026–32.
Acknowledgements
The authors acknowledge the aboriginal custodians on whose traditional lands this research was conducted. The authors would like to thank Dr. Christopher Burridge, University of Tasmania; and Dr. David Lizárraga, University of Tasmania. This research was supported by the Australian Research Council Linkage Programme (Grant No. LP180101251), The authors declare that they have no competing interests.
Funding
This research was supported by funds from the Australian Research Council Linkage Programme (Grant No. LP180101251).
Author information
Authors and Affiliations
Contributions
Conceived and designed the study: CNN, SC. Analysed the data: CNN, SC, NMP. Wrote the manuscript: CNN, VW, SC. Reviewed the manuscript: CNN, VW, NMP, SC. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This article does not contain any studies with participants or animals performed by any of the authors.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Narrative reviews.
Additional file 2.
Full meta-data.
Additional file 3.
Reference table.
Additional file 4.
Figure 1 Flowdiagram & Table 1 Severity grou**s.
Additional file 5.
Funnel plots and Egger’s regression results
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Næsborg-Nielsen, C., Wilkinson, V., Mejia-Pacheco, N. et al. Evidence underscoring immunological and clinical pathological changes associated with Sarcoptes scabiei infection: synthesis and meta-analysis. BMC Infect Dis 22, 658 (2022). https://doi.org/10.1186/s12879-022-07635-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-022-07635-5