Abstract
Background
The objective of the study was to describe the epidemiology, management and cost of non-tuberculous mycobacteria pulmonary disease (NTM-PD) in France.
Methods
A retrospective analysis was performed using the SNDS (“Système national des données de santé”) database over 2010–2017. Patients with NTM-PD were identified based on the ICD10 codes during hospitalizations and/or specific antibiotics treatment regimens. The study population was matched (age, sex and region) to a control group (1:3) without NTM-PD.
Results
5628 patients with NTM-PD (men: 52.9%, mean age = 60.9 years) were identified over the study period and 1433 (25.5%) were treated with antibiotics. The proportion of patients still receiving treatment at 6 and 12 months was 40% and 22%, respectively. The prevalence of NTM-PD was estimated at 5.92 per 100,000 inhabitants and the incidence rate of NTM-PD remained stable over time between 1.025/100,000 in 2010 and 1.096/100,000 in 2017. Patients with NTM-PD had more co-morbidities compared to controls: corticoids (57.3% vs. 33.8%), chronic lower respiratory disease (34.4% vs. 2.7%), other infectious pneumonia (24.4% vs. 1.4%), malnutrition (based on hospitalization with the ICD-10 code reported during a hospital stay as a main or secondary diagnosis) (22.0% vs. 2.0%), history of tuberculosis (14.1% vs. 0.1%), HIV (8.7% vs. 0.2%), lung cancer and lung graft (5.7% vs. 0.4%), cystic fibrosis (3.2% vs. 0.0%), gastro-esophageal reflux disease (2.9% vs. 0.9%) and bone marrow transplant (1.3% vs. 0.0%) (p < 0.0001). The mean Charlson comorbidity index score was 1.6 (vs. 0.2 for controls; p < 0.0001). NTM-PD was independently associated with an increased mortality rate with a hazard ratio of 2.8 (95% CI: 2.53; 3.11). Mortality was lower for patients treated with antibiotics compared to untreated patients (HR = 0.772 (95% CI [0.628; 0.949]). Annual total expenses the year following the infection in a societal perspective were € 24,083 (SD: 29,358) in NTM-PD subjects vs. € 3402 (SD: 8575) in controls (p < 0.0001). Main driver of the total expense for NTM-PD patients was hospital expense (> 50% of the total expense).
Conclusion
Patients with NTM-PD in France were shown to have many comorbidities, their mortality risk is high and mainly driven by NTM-PD, and their management costly. Only a minority of patients got treated with antibiotics and of those patients treated, many stopped their therapy prematurely. These results underline the high burden associated with NTM-PD and the need for improvement of NTM-PD management in France.
Similar content being viewed by others
Background
More than 190 species and subspecies of non-tuberculous mycobacteria (NTM) have been described. Among these, some like Mycobacterium avium complex (MAC), may cause pulmonary disease (NTM-PD) with increasing frequency in non-acquired immunodeficiency syndrome (AIDS) patients [1, 2]. The incidence and prevalence of NTM-PD has even surpassed that of tuberculosis in some settings [3, 4]. This increase has been observed in older individuals and those with underlying bronchiectasis and may be associated with multifactorial factors. In Europe, it is estimated that the annual prevalence rate of diagnosed NTM-PD cases is in the range of 3–6/100,000 [4,5,6]. In France studies on NTM-PD have been conducted either based on cystic fibrosis registries or from laboratory databases from university hospitals [7] but not nationally generalizeable estimates.
The economic burden of NTM-PD in France is expected to be substantial due to its challenging management with frequent hospitalizations and the need for long-term antibiotic treatment. In Germany, NTM-PD mean direct expenditure was estimated to be nearly fourfold that of matched controls [8] and hospitalizations accounted for 63% of the total costs. Similar costs for NTM-PD have been observed in other western countries [9]. However, estimates of treatment costs for patients with newly diagnosed NTM-PD are currently not available in France. Therefore, we aimed to describe the epidemiology, comorbidities, management, mortality, and costs of NTM-PD in a real world setting in France.
Methods
Study design
We conducted a retrospective study using the French nationwide claims and hospitalisation database, the Système National des Données de Santé (SNDS) which covers more than 99% of the French population (nearly 66 million people) and represents one of the largest medico-administrative databases in the world. The SNDS database contains anonymous data on healthcare encounters (public and private), diagnostic-related groups, drugs, medical devices, procedures, laboratory tests (without results), date of death excluding cause, hospitalizations with ICD10 codes and discharge summaries with the main and secondary diagnoses.
Patients with NTM-PD were identified in the database based on hospitalizations with ICD-10 codes specific for NTM-PD infection and/or antibiotics combination for the treatment of the disease using outpatient drug consumptions. The detailed algorithm is reported in the additional files (Additional file 1: Tables S1–S3).
Newly diagnosed patients with NTM-PD (adults and children) not previously treated or hospitalized for NTM-PD in the last 3 years were included in the study from January 01/2010 to December 31/2017.
Socio-demographic characteristics included age, sex, residence, supplementary universal health care coverage (CMUc), status and registration for long-term disease with ICD-10 codes, and date of death, if any. Patients with NTM-PD were matched on age, sex, and region of residence with controls who were not selected by the algorithm to identify NTM-PD patients, with a ratio of 1:3. Risk factors for NTM-PD (including malnutrition) were identified through specific ICD-10 codes for long-term diseases, hospitalizations, and treatment in the past 3 years prior to NTM-PD diagnosis. Healthcare consumptions and costs related to hospitalizations and outpatient visits were also assessed.
An economic analysis was conducted and involved patients who were newly diagnosed with NTM-PD between January 01/2012 and December 31/2016; healthcare resource use and costs over one-year period following the diagnosis of NTM-PD were analyzed. The economic evaluation was assessed from the French Public Health Insurance perspective, with a separate analysis that also included patient out of pocket costs for services or drugs that are only partially reimbursed.
Outpatient costs that were analyzed relating to medical honoraria, dental fees, pharmacy and other products, laboratory tests, paramedical interventions, medical devices, transportation, and other cares. Indirect costs due to daily allowance for sick leave are reported separately.
Statistical analysis
Mean, standard deviation, median, minimum, and maximum were used to describe quantitative variables and percentages for qualitative variables. Bivariate analyses were performed and the Chi2 test was used to assess qualitative variables, whereas the Yates continuity correction or Fisher’s exact test evaluated sample sizes less than 5. For quantitative variables, a Student’s t-test or analysis of variance was performed when distribution was close to normal; otherwise, non-parametric tests including Wilcoxon and Kruskal–Wallis were used. Survival was analyzed with Kaplan–Meier curves and log rank test with December 31/2017 as the time point for censored observation. Cox multivariate model adjusted for age, sex, residence and comorbidities was used to compare: (1) NTM-PD patients to the controls and (2) NTM-PD patients treated with antibiotics compared to untreated NTM-PD patients.
Results
A total of 5628 patients with NTM-PD were identified in the SNDS database between 2010 and 2017, 4898 patients from hospitalizations and 730 from outpatient drug consumptions. Among patients identified through hospitalizations, 703 had a specific treatment for NTM-PD vs. 4195 with no treatment. The mean age was 60.9 years (SD ± 19.5) and 52.9% were males. The Universal Health Coverage for low-income individuals was observed in 8.8% of patients (Table 1).
A total of 3954 patients were diagnosed with NTM-PD between January 01/2010 and December 31/2017; thus, the prevalence of NTM-PD was estimated at 5.92 per 100,000 inhabitants over 8 years. The incidence rate of NTM-PD (Fig. 1) remained stable over time, with a min of 1.025/100,000 in 2010 (N = 662) and a max of 1.096/100,000 (N = 732) in 2017 with slight variations in-between years.
Matched controls could not be identified for all patients in the study; therefore, comparisons included 4447 NTM-PD cases matched to 13,341 controls for age, sex, and region.
Analysis of comorbidities (Table 2) found a significantly higher Charlson [10] Comorbidity Index (CCI) mean score in patients with NTM-PD (1.6) compared to controls (0.2) (p < 0.0001). However, there was no difference between treated and untreated groups on the CCI score (p = 0.3). Overall, patients with NTM-PD had a higher proportion of risk factors compared to controls including respectively corticoid treatment in the last 3 years (57.3% vs. 33.8%, p < 0.0001), chronic lower respiratory disease (34.4% vs. 2.7%, p < 0.0001), other infectious pneumonia (24.4% vs. 1.4%, p < 0.0001), malnutrition (22.0% vs. 2.0%, p < 0.0001), and history of tuberculosis (14.1% vs. 0.1%, p < 0.0001).
Even if statistically significant, no notable differences between the treated and untreated group were found, except higher rates of HIV infection in treated vs. untreated patients (14.8% vs. 5%).
Mortality
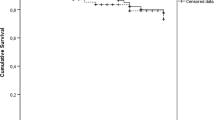
A first analysis showed that the mortality of 4447 NTM-PD cases was significantly higher than that of 13,341 controls (p < 0.0001) (Fig. 2).
In multivariate analysis (Table 3), after adjustment for age, residence, sex,, and risk factors, the risk of mortality was more than 2 times higher in NTM-PD patients compared to controls (HR = 2.804 (95% CI [2.532; 3.104]). The risk of mortality was more likely to increase in older patients with NTM-PD including those aged 70–79 years (HR = 16.081, 95% CI: 6.646–38.908), 80–89 years (HR = 33.654, 95% CI: 13.910–81.423), and more than 90 years (HR = 56.860, 95 CI: 23.209–139.303). Mortality was also higher in patients with Universal Health Coverage, HIV infection, lung cancer and lung graft, other infectious pneumonia, malnutrition, and other chronic obstructive pulmonary disease. Mortality was lower in female patients vs. males and in patients who had mucopurulent chronic bronchitis and bronchiectasis.
In a second multivariate analysis performed on the NTM-PD cohort and adjusted for age, residence, sex, and comorbidities, the risk of mortality was lower for treated patients with antibiotics compared to untreated patients (HR = 0.776 (95% CI [0.628; 0.949]).
Treatments
Out of the 5628 patients, 25.5% (1433) had received antibiotics to treat NTM-PD vs. 74.5% (4195) with no treatment (Table 4).
Among the 4,898 hospitalized patients, 703 (14.4%) had a specific treatment for NTM and 4195 (85.6%) did not receive any treatment. The most frequently used treatment first line regimens were: Clarithromycin + Ethambutol for 34.4% of patients, Clarithromycin + Rifampin + Ethambutol (22.1%), Clarithromycin + Rifampin (10.3%), and Clarithromycin monotherapy (9.3%). Other combinations represented less than 5% of patients for each of them (Table 4).
Figure 3 depicts the proportion of patients maintaining treatments at 3, 6, 9, and 12 months. Half of the patients (56%) were still treated at 3 months, 40% at 6 months, 30% at 9 months, and only 22% at 12 months. The majority of patients discontinued before 12 months of treatment.
Healthcare expenditures
This analysis included 3950 cases and 11,850 controls. A total of 3,641 NTM-PD patients (92.2%) had been hospitalized at least once since diagnosis compared to 2484 controls (21.0%). For those hospitalized at least once, the length of hospital stay was 40.3 days for cases vs. 16.5 days for controls; mean hospital stay per patient was 5.0 for cases and 2.7 for controls.
Economic analysis
A total of 2,683 cases and 8,049 controls were included in the economic analysis.
The mean total cost reimbursed in the year following NTM-PD diagnosis was significantly higher for cases compared to controls, €22,966 vs. €2709, respectively (p < 0.0001). Total expenses (societal perspective) followed the same trend with a greater amount for cases than controls, €24,083 vs. €3402, respectively (p < 0.0001).
From the French Public Health Insurance perspective (Fig. 4) the mean cost for hospitalizations in the year following NTM-PD diagnosis was €12,524 for cases vs. €1156 for controls (p < 0.0001). The total cost for outpatient care in the year following NTM-PD diagnosis was €10,442 for NTM-PD patients and €1553 for controls. Costs of drugs were estimated at €5493 for cases (52.6% of the total amount) vs. €517 for controls (33% of the total amount).
From a perspective incl. out-of-pocket costs (Fig. 4), the mean cost for hospitalizations in the year following NTM-PD diagnosis was €12,499 for cases vs. €1303 for controls (p < 0.0001). The total cost of outpatient care in the year following NTM-PD diagnosis was €11,584 for NTM-PD patients and €2099 for controls. Costs of drugs were estimated at €5872 for cases (50.6% of the total amount) vs. € 629 for controls (30% of the total amount).
Discussion
This retrospective study using the SNDS, a French nationwide claims and hospitalisation database, described the epidemiology, comorbidities, management, mortality, and costs of NTM-PD in a real world setting in France. Thanks to its comprehensive coverage of the whole population (99%), the SNDS database allowed this study to include all NTM-PD patients identified with the ICD-10 codes in France. Our study not only has demonstrated the higher mortality rate in NTM-PD patients and the economic burden related to this condition but also suggested that guidelines are not always respected.
Prevalence (6/100,000 inhabitants over 8 years) and incidence (1/100,000) were close to those reported in previous studies conducted in Europe with same or different methods including Delphi method, registry analysis, retrospective study of laboratory data [5, 8, 11,12,13]. These converging data suggest that the true incidence and prevalence must be close to those we found. Whether incidence of NTM-PD is increasing or is better diagnosed or reported is debated in the literature. The present study over 8 years did not show any increase of incidence which was close to the one reported in France 15 years ago [13].
Surprisingly, the proportion of men and women was comparable in our study in contrast to the higher proportion of women found in previous studies. As we did not include NTM-PD cases that were both not treated and not hospitalized, we may have missed women who outlived men and were followed by watchful waiting. This could explain the lower ratio of women we observed compared to previous studies. It should be noted that the same proportion of men and women, as compared to our study, was also found in Germany [11] and Denmark [12].
Among the different factors associated with NTM-PD that can be prevented, malnutrition was 10 times more frequent in NTM-PD patients than in controls. Weight loss has been reported as being a negative prognostic factor for NTM-PD [14]. Therefore, patients’ nutritional status should be assessed by a dietician particularly in those with NTM-PD cumulative risk factors.
We found that NTM-PD patients had a twice higher risk of mortality than controls in line with previous studies conducted in Germany and in the US [8, 18]. After 8 years there was a 30% difference in mortality between NTM-PD patients and controls. The 10 years survival rate predicted by Charlson index [10] for NTM-PD cases was between 90 and 95% whereas we found 61% after 8 years of follow up, underlying the impact of NTM-PD on mortality.
We observed that advanced age was associated with a greater risk of mortality. Interestingly, elderly patients were less likely to be treated in our study. This shows the reluctance of healthcare providers to initiate long-term and complex regimens in older patients who may be already treated for other comorbid conditions with multitude treatments. However, given this increasing mortality our data rather support treatment initiation in line with recent recommendations [15]. This is further supported by the survival analysis that showed that treated patients had 23% reduced risk of dying compared to untreated after controlling for confounding factors.
In a study conducted on a Danish registry [12], predictors of death were age ≥ 65 years (HR: 9.17) and male sex (female sex HR, 0.73). This is consistent with our study which shows a decreased risk of mortality in female NTM-PD patients. NTM-PD in women tend to manifest with bronchiectasis and has better prognosis than patients, usually men, who have the cavitary form of the disease [16]. Indeed, our study suggests a lower risk of mortality in NTM-PD patients with bronchiectasis compared to NTM-PD patients without bronchiectasis (HR: 0.713, 95% CI [0.591–0.861]). We did not find a significant association between emphysema and the risk of mortality in NTM-PD patients; however, those with other COPD were more likely to die compared to those without other COPD. A higher rate of mortality in NTM-PD patients with COPD has been consistently reported and accounted for 41.5% over the period of 39 months compared to 22.4% for the overall NTM-PD group, including those with and without COPD [8].
In our study, 85.6% of patients identified through hospitalizations did not receive any antibiotics to treat NTM-PD. However, in those treated for NTM-PD, clarithromycin monotherapy was observed in 19.5% of patients. This is particularly alarming since clarithromycin resistance has been reported in 16% of NTM-PD patients treated with clarithromycin monotherapy and this resistance that is prevented by multidrug therapy is associated with a poor prognosis [17, 18]. Also worrying was the rapid decline of the proportion of patients on treatment reaching 30% at 9 months whereas all recommended regimens last at least on year [16]. Together with increased long-term mortality, these data on poor treatment management underline the need for implementation of educational measures in France.
In our study, total expenses for cases were sevenfold higher than that of controls, €24,083 and €3402, respectively (p < 0.0001). Total costs were driven by hospitalizations followed by drugs, and finally outpatient care. Our results are consistent with previous studies reporting a higher cost in NTM-PD patients compared to matched-controls. In Germany, Diel et al. [8] estimated the mean direct expenditure per NTM-PD patient to be €39,559.60 over a period of 39 months which was almost fourfold that of matched control (€10, 006.71). Furthermore, NTM-PD patients were three times more likely to be hospitalized than controls, and hospitalizations represented 63% of the total costs. Goring et al. [9] conducted a study in a cohort of MAC patients refractory to treatment and estimated annual NTM-PD-related costs at $16,209 in Canada, €11,626 in Germany, €17,881 in France, and £9727 in the UK. Marras et al. [19] reported higher healthcare expenditures in newly diagnosed NTM-PD patients than in controls the first year ($72,475 vs. $28,405) and second year ($48,114 vs. $28,990), respectively. Interestingly, cost for an NTM-PD patient clearly surpasses the annual cost per patient for chronic respiratory diseases including COPD (€1013) or asthma (€1950) among others [20].
The limitations of the SNDS database are inherent to those of claims databases. The paucity of clinical information and laboratory results did not allow the use of the ATS/IDSA guidelines which include clinical, radiologic, and bacteriologic criteria to pose a diagnosis of NTM-PD [2]. We also did not have information on specific NTM species to be able to have species-specific results. Identification of NTM-PD cases using the ICD-10 codes along with the prescription of antimycobacterial treatments may have covered the majority of patients with NTM-PD; however, we may have missed patients who may have been misclassified with the ICD-10 codes. A better specificity and positive predictive value in detecting active TB with the ICD-10 codes combined with antibiotic prescriptions have been reported [21]. We also missed patients managed in outpatient care setting who were diagnosed with NTM-PD but did not receive any treatments due to their advanced age or due to milder stage of disease among other reasons. In addition, patients who died before the diagnosis could be established may have also led to an underestimation of our results. However, the incidence and prevalence of NTM-PD in our study are in line with other European studies and thus suggest that we did not miss many cases.
Conclusion
Despite the incremental risk of mortality in NTM-PD patients and financial burden associated with this condition, there is still a critical unmet need for the diagnosis and management of NTM-PD. Only a minority of patients get treated with antibiotics, but many discontinue treatment before the infection can be successfully eradicated. These results underline the high burden associated with NTM-PD and emphasize the need for effective management.
Availability of data and materials
All data generated or analyzed during this study are included in this published article [and its additional information files]. The data that support the findings of this study are available from [the French Health Insurance (CNAM)] but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available.
Abbreviations
- CCI:
-
Charlson Comorbidity Index
- CMUc:
-
Supplementary universal health care coverage
- COPD:
-
Chronic obstructive pulmonary disease
- HR:
-
Hazard ratio
- ICD:
-
International Classification of Diseases
- MAC:
-
Mycobacterium avium Complex
- NTM-PD:
-
Non-tuberculous mycobacteria pulmonary disease
- SNDS:
-
Système National des Données de Santé
- UK:
-
United Kingdom
References
Daley CL, et al. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline: executive summary. Clin Infect Dis. 2020;14:e1–36.
Griffith DE, et al. ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Disease Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–416.
Ringshausen FC, et al. Burden and trends of hospitalisations associated with pulmonary non-tuberculous mycobacterial infections in Germany, 2005–2011. BMC Infect Dis. 2013;13:231.
Shah NM, et al. Pulmonary Mycobacterium avium-intracellulare is the main driver of the rise in non-tuberculous mycobacteria incidence in England, Wales and Northern Ireland, 2007–2012. BMC Infect Dis. 2016;6:16–195.
Schildkraut JA, et al. Epidemiology of nontuberculous mycobacterial pulmonary disease in Europe and Japan by Delphi estimation. Respir Med. 2020;173:106164.
Prevots DR, Marras TK. Epidemiology of human pulmonary infection with nontuberculous mycobacteria: a review. Clin Chest Med. 2015;36:13–34.
Roux AL, et al. Multicenter study of prevalence of nontuberculous mycobacteria in patients with cystic fibrosis in France. J Clin Microbiol. 2009;47:4124–8.
Diel R, et al. Burden of non-tuberculous mycobacterial pulmonary disease in Germany. Eur Respir J. 2017;49:1602109.
Goring SM, et al. The cost of Mycobacterium avium complex lung disease in Canada, France, Germany, and the United Kingdom: a nationally representative observational study. BMC Health Serv Res. 2018;10:700.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83.
Ringshausen FC, et al. Prevalence of nontuberculous mycobacterial pulmonary disease, Germany. Emerg Infect Dis. 2016;22:1102–5.
Andrejak C, et al. Nontuberculous pulmonary mycobacteriosis in Denmark: incidence and prognostic factors. Am J Respir Crit, Care Med. 2010;181:514–21.
Dailloux M, et al. French Mycobacteria Study Group. Respiratory infections associated with nontuberculous mycobacteria in non-HIV patients. Eur Respir J. 2006;28:1211–5.
Berthon BS, Wood LG. Nutrition and respiratory health–feature review. Nutrients. 2015;7:1618–43.
Daley CL, et al. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Clin Infect Dis. 2020;71:e1–36.
Diel R, Lipman M, Hoefsloot W. High mortality in patients with Mycobacterium avium complex lung disease: a systematic review. BMC Infect Dis. 2018;18:206.
Griffith DE, et al. Clinical and molecular analysis of macrolide resistance in Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2006;174:928–34.
Wallace RJ Jr, et al. Initial clarithromycin monotherapy for Mycobacterium avium-intracellulare complex lung disease. Am J Respir Crit Care Med. 1994;149:1335–41.
Marras TK, et al. Health care utilization and expenditures following diagnosis of Nontuberculous mycobacterial lung disease in the United States. J Manag Care Spec Pharm. 2018;24:964–74.
Gibson GJ, Loddenkemper R, Lundbäck B, Sibille Y. The European Lung White Book. 2nd ed. Sheffield: European Respiratory Society; 2013.
Calderwood MS, et al. Real-time surveillance for tuberculosis using electronic health record data from an ambulatory practice in eastern Massachusetts. Public Health Rep. 2010;125:843–50.
Acknowledgements
Not applicable.
Funding
The study was sponsored by INSMED. INSMED validated the design of the study, the analysis, the interpretation of data and the writing of the manuscript which was first written by the Scientific experts and CEMKA.
Author information
Authors and Affiliations
Contributions
NV have participated to the study design and the writing of the article. CA have participated to the study design and the writing of the article. SB have participated to the study design, the statistical analysis, and the writing of the article. CE have participated to the study design, the statistical analysis, and the writing of the article. MO have participated to the study design and the writing of the article. RC have participated to the study design and the writing of the article. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Committee of Expertise for Research, Studies and Evaluations in the field of Healthcare (Comité d’Expertise pour les Recherches, les Etudes et les Evaluations dans le domaine de la Santé) and by the National Commission on Informatics and Liberty (Commission nationale de l’informatique et des libertés). As it is a SNDS database analysis, the informed consent is exempted by the Committee of Expertise for Research, Studies and Evaluations in the field of Healthcare (Comité d’Expertise pour les Recherches, les Etudes et les Evaluations dans le domaine de la Santé). All methods were carried out in accordance with relevant guidelines and regulations (e.g., Helsinki declaration.
Consent for publication
Not applicable.
Competing interests
NV was an expert in the Scientific Committee of this study and received grants from Insmed. CA was an expert in the Scientific Committee of this study and received grants from Insmed. SB is an employee of CEMKA that was supported by Insmed to perform this research. CE is an employee of CEMKA that was supported by Insmed to perform this research. MO is an employee of Insmed that supported this research. RC was an expert in the Scientific Committee of this study and received Grants from Insmed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Algorithm used to identify NTM patients (ICD-10 codes and antibiotics combination).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Veziris, N., Andréjak, C., Bouée, S. et al. Non-tuberculous mycobacterial pulmonary diseases in France: an 8 years nationwide study. BMC Infect Dis 21, 1165 (2021). https://doi.org/10.1186/s12879-021-06825-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-021-06825-x