Abstract
Background
There have been previous studies and earlier systematic review on the relationship between inflammatory bowel disease (IBD) and radiation exposure. With the diversification of current test methods, this study intended to conduct a meta-analysis to evaluate the IBD radiation exposure in recent years.
Methods
Three databases (PUBMED, EMBASE, and MEDICINE) for relevant literature up to May 1, 2023 were searched. The statistical data meeting requirements were collated and extracted.
Results
20 papers were enrolled. The overall high radiation exposure rate was 15% (95% CI = [12%, 19%]) for CD and 5% (95% CI = [3%, 7%]) for UC. The pooled result found that high radiation exposure rate was 3.44 times higher in CD than in UC (OR = 3.44, 95% CI = [2.35, 5.02]). Moreover, the average radiation exposure level in CD was 12.77 mSv higher than that in UC (WMD = 12.77, 95% CI = [9.93, 15.62] mSv). Furthermore, radiation exposure level of CD after 2012 was higher than those before 2012 (26.42 ± 39.61vs. 23.76 ± 38.46 mSv, P = 0.016), while UC did not show similar result (11.99 ± 27.66 vs. 10.01 ± 30.76 mSv, P = 0.1). Through subgroup analysis, it was found that disease duration (WMD = 2.75, 95% CI = [0.10, 5.40] mSv), complications (OR = 5.09, 95% CI = [1.50, 17.29]), and surgical history (OR = 5.46, 95% CI = [1.51, 19.69]) significantly increased the proportion of high radiation exposure.
Conclusion
This study found that radiation exposure level of IBD patients was high, which revealed the radiation risk in the process of diagnosis and treatment of IBD patients. In the future, longer follow-up and prospective studies are needed to reveal the relationship between high radiation exposure and solid tumorigenesis.
Similar content being viewed by others
Background
Inflammatory bowel disease (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC), refers to a group of lifelong idiopathic disorders characterized by gastrointestinal inflammation and extra-intestinal manifestations [1]. The incidence and prevalence of IBD is increasing worldwide especially in Asia, while it is still highest among developed countries in Europe and America [2]. Due to the unclear pathogenesis and the complexity of treatment, IBD has a significant disease and economic burden [3]. The diagnosis of IBD is a difficult and complicated process. In addition to gastrointestinal endoscopy, repeated imaging tests are also required, especially for the diagnosis of CD, which needs to assess the extent and severity of the disease, and the presence of complications [4]. Therefore, the assessment of radiation exposure is very important.
IBD itself increases the risk of intestinal tumors [5, 6], and the use of drugs such as azathioprine, other immunosuppressive agents and biological agents will increase the risk of malignant tumors such as lymphoma [7]. In addition, exposure to ionizing radiation may potentially increase the risk of malignancy [8]. Radiation exposure as low as 50 millisieverts (mSv) has been associated with the development of certain solid tumors such as colon, bladder cancer [9]. Globally, up to 2% of malignancies can be attributed to diagnostic medical radiation (DMR) [10]. Although some clinicians believe that DMR exposure is indeed a potential risk, the actual exposure of IBD patients in clinical practice still lacks sufficient multicenter large sample data to support, that leads to many concerns for patients, such as whether they are exposed to excessive DMR. Previous meta-analysis study have shown that IBD patients do have higher DMR [11]. With the continuous development of medical technology, such as the application of MRI and intestinal ultrasound, it is not clear whether DMR has changed from before.
Therefore, it is important to conduct this study to update our current knowledge by meta-analysis to analyze relevant studies found to date, especially recent studies, to determine the pooled prevalence of increased exposure in IBD patients and risk factors associated with exposure to potentially harmful ionizing levels.
Methods
Data selection
We searched three databases (PUBMED, EMBASE, and MEDICINE) for relevant literature up to May 1, 2023. Literature search limited to human studies and English version, including prospective and retrospective studies. The following search terms were used to retrieve potential articles: ((Inflammatory Bowel Disease) OR (IBD) OR (Crohn’s disease) OR (CD) OR (ulcerative colitis) OR (UC)) AND ((radiation exposure) OR (radiation injuries) OR (medical radiation)).
The search was independently performed by 2 authors according to title and abstract, and full text was retrieved if it met the requirement. In addition, disagreement would be evaluated by a third author independently.
Inclusion criteria and quality assessment
The diagnosis of IBD was based on symptoms, imaging, and histopathology [12]. High diagnostic medical radiation exposure was defined as ≥ 50 mSv. In addition, sufficient data for calculation were needed for inclusion in the study. STROBE checklist was used to assess Quality assessment and risk of bias for the studies included [13]. Moreover, the work was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [14].
Data extraction
Relevant data from every included study according to the unified standard were extracted by two independent authors and then they proceeded to cross-check the results. The extracted data contained author, country or region, published year, number of subjects, radiation exposure dose, number of high diagnostic medical radiation exposure and factors affecting radiation exposure. Agreement between the investigators was greater than 95%, and differences between the datasets were resolved by discussion.
Statistical analysis
Continuous variables were expressed as mean and standard deviation, and dichotomous variables were described by odds ratio (OR) and 95% confidence interval (CI). Heterogeneity of the data was quantified with the I2 statistic and assessed by Cochran’s Q statistic. In this study, when heterogeneity was less than 50%, the pooled estimates were obtained using the fixed-model (Mantel and Haenszel) method. On the contrary, the random-model (M-H heterology) method was chosen if heterogeneity was more than 50% [15]. This study compared the following: difference in radiation exposure between CD and UC; difference in high diagnostic medical radiation exposure between CD and UC; difference in radiation exposure of CD and UC patients before and after 2012 (According to the articles, it can be basically determined that articles after 2012 did not overlap the count of CT before), and the difference in radiation exposure under different influencing factors including disease duration, gender, complications, surgical history and medication. In addition, sensitivity analysis was used to evaluate whether the results were reliable. Begg’s test was conducted to estimate publication bias with a value of P > 0.05 suggesting no publication bias. All data analysis methods involved in this study were implemented through STATA 15 (StataCorp., College Station, Tex, USA).
Results
Basic characteristics
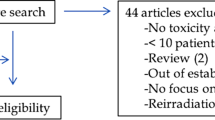
A total of 3894 relevant articles were screened, of which 20 papers were enrolled finally according to inclusion criteria. The flowchart has been schematically outlined in Fig. 1 which described the process of the study selection. 20 articles all referred to CD, and 15 of them referred to radiation exposure of UC. The included population of 17 articles came from Europe and the United States. Of the 20 articles reporting on CD, 17 mentioned average radiation exposure values, 16 mentioned numbers of high diagnostic medical radiation exposure, and 13 articles were published after 2012. Of the 15 articles reporting on UC, 13 mentioned average radiation exposure values, 12 mentioned numbers of high diagnostic medical radiation exposure, and 9 articles were published after 2012. UC did not perform subgroup analysis on influencing factors due to lack of literature support. About CD, 3 referred to disease duration, 3 referred to gender, 3 referred to complications, 4 referred to surgical history, and 3 referred to medication.
Radiation exposure in CD and UC patients
The total number and number of individuals with high radiation exposure of CD was 32,963 and 5181 respectively, and the average radiation exposure level was 26.31 mSv (Table 1). At the same time, The total number and number of individuals with high radiation exposure of UC was 34,854 and 2147 respectively, and the average radiation exposure level was 11.97 mSv (Table 2). Combining rates by meta-analysis found that the overall high radiation exposure rate was 15% (95% CI = [12%, 19%]) for CD (Fig. 2A) and 5% (95% CI = [3%, 7%]) for UC (Fig. 2B). The pooled result of meta-analysis found that high radiation exposure rate was 3.44 times higher in CD than in UC (OR = 3.44, 95% CI = [2.35, 5.02]) (Fig. 3A). Moreover, the pooled results of meta-analysis showed that the average radiation exposure level in CD was 12.77 mSv higher than that in UC (WMD = 12.77, 95% CI = [9.93, 15.62] mSv) (Fig. 3B).
Furthermore, we compared whether there was a difference in radiation exposure level before and after 2012 in order to judge whether the increase in imaging methods in recent years has affected radiation exposure. 11 articles on CD were published after 2012, while 6 articles were published before 2012 (Table 1). The pooled radiation exposure level was 26.42 ± 39.61 mSv after 2012 and 23.76 ± 38.46 mSv before 2012, and there was a statistical difference between two groups (P = 0.016). In addition, high radiation exposure rate was 16.10% after 2012 and 12.25% before 2012, and it also had statistical difference (P < 0.01). However, UC did not show similar results. 8 articles were published after 2012, while 5 articles were published before 2012 (Table 2). The pooled radiation exposure level was 11.99 ± 27.66 mSv after 2012 and 10.01 ± 30.76 mSv before 2012, and high radiation exposure rate was 6.64% after 2012 and 4.30% before 2012. Neither parameter had statistical difference (P = 0.1 and P = 0.62, respectively).
Finally, the study analyzed factors affecting radiation exposure. Due to lack of data, we only analyzed the influencing factors of CD. Disease duration, gender, complications, surgical history, and medication were the factors for our analysis. Through subgroup analysis, it was found that disease duration (WMD = 2.75, 95% CI = [0.10, 5.40] mSv), complications (OR = 5.09, 95% CI = [1.50, 17.29]), and surgical history (OR = 5.46, 95% CI = [1.51, 19.69]) significantly increased the proportion of high radiation exposure, while gender (OR = 1.16, 95% CI = [0.76, 1.77]) and medication (OR = 1.75, 95% CI = [0.99, 3.11]) had no effect. (Fig. 4)
Funnel plot analyses of studies assessing radiation exposure revealed no significant publication bias (P > 0.05). Sensitivity analysis showed that although some results were fluctuant, the overall results were stable and reliable.
Discussion
This updated meta-analysis showed that radiation exposure of IBD patients was significantly increased, and the proportion of patients with high radiation exposure was also significantly increased. In addition, radiation exposure level of CD patients was significantly higher than that of UC patients, and the high radiation exposure of CD was related to disease duration, complications and surgical history.
Radiation exposure in IBD patients was significantly higher, which was depended on the course of diagnosis and treatment of the disease. Especially for CD patients, because the entire digestive tract may be involved, doctors need to conduct a comprehensive evaluation, especially the evaluation of the small intestine, which requires the use of small intestine CT and abdominal CT. It reported that incidence and mortality of solid cancer were positively associated with higher radiation dose and younger age of exposure [16]. And it has been reported that ionising radiation levels as low as 50 mSv have been contributed to the development of solid tumors [9]. Based on the results of this study and the characteristics of IBD patients with young age of onset and high radiation exposure [17], we believed that IBD patients may be exposed to an environment with a higher tumor incidence. So what can be done to reduce the risk of solid tumors in patients with IBD? First, we could propose the creation of an IBD patient radiation diary to record total radiation exposure and increase physician awareness of patient exposure to ionizing radiation [18]. Second, in tertiary care institutions, the frequency of magnetic resonance enterography (MRE) examinations can be increased to replace CT enterography (CTE). MRE is used to obtain cross-sectional imaging of small bowel without exposure to DMR, which can show the inflammation and fibrotic bowel wall in detail [19]. In a prospective study, Fiorino et al. found that MRE and CTE were similar accuracy in localizing CD, bowel wall enhancement, enteroenteric fistula, and MRE was superior to CTE for assessing strictures and bowel wall thickening [20]. Therefore, European Crohn’s and Colitis Organisation advocate increased routine usage of MRI for the assessment of small bowel CD [21]. According to the results of this study, why do the articles published in recent years showed that radiation exposure dose of IBD was higher than before. The authors believed that there were many reasons for this. First, the popularity of MRE is still only available in large general hospitals. Therefore, CTE remains the primary usage of IBD examination. Second, with the tense medical environment, doctors are more careful to deal with complications that may occur at any time during the diagnosis and treatment of patients and pay more attention to the efficacy of patients, so the frequency of examinations may be increased. Finally, Although the article was published in recent years, the patients included in the article may go back several years.
The results of this study showed that disease duration, complications and surgical history were associated with high radiation exposure, which was clearly closely related to the diagnosis and follow-up of the disease. The earlier the onset, the earlier the initial exposure. In addition, complications and surgical history have also added additional imaging tests to assess the severity of the disease. CT imaging offers advantages of rapid acquisition of images, high sensitivity, widespread availability, and specificity for the detection of intestinal and extra-intestinal disease [22]. Combined with the improved visualization of the small bowel mucosa by CTE, the assessment of small bowel disease activity is more accurate [23]. However, previous studies have shown that the role of CT in assessing intestinal disease activity may be limited [24]. In turn, radiation-induced cancer occurs in 1/1000 patients who undergo at least10 mSv CT scan [25]. Therefore, the appropriate imaging examination methods and frequency in the process of IBD diagnosis and treatment still require doctors to pay close attention.
On the basis of previous studies, this study has carried out a more detailed and systematic study and obtained more convincing results, but there were still some shortcomings needed to be pointed out. First, this study included data from multiple centers, which can lead to patient heterogeneity. Although sensitivity analysis showed the overall results were stable and reliable, the existence of heterogeneity still made this study only select random effect model for data analysis. The inconsistency of equipment models in different centers, the inconsistency of doctors’ cognition of diseases, and the compliance of patients would all affect the total radiation exposure. Moreover, this study has conducted extensive screening of papers. But based on the data provided by the published papers, the data of some included papers was not complete. We also asked the authors about the data through email, but unfortunately there was no reply. Additionally, the estimated radiation dose may be greater or less than the actual exposure. It is also possible that tests performed at other centers may not have been captured, leading to underestimate the total radiation dose. Second, we lacked studies with large sample data. Some studies included limited patients, which affected the reliability of the results. In particular, in the subgroup analysis of high exposure risk factors, the number of articles and patients included was limited, so the reliability of the results was limited. Finally, we lacked longer-term follow-up and prospective studies to analyze the risk of solid tumor development in high radiation exposure patients. The emergence of such results will have important guiding significance for the selection of imaging examinations in the process of IBD diagnosis and treatment.
Conclusion
In conclusion, this study found that radiation exposure level of IBD patients was high, and exposure level of CD patients was higher than UC, which revealed the radiation risk in the process of diagnosis and treatment of IBD patients. In the future, longer follow-up and prospective studies are needed to reveal the relationship between high radiation exposure and solid tumorigenesis.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- IBD:
-
Inflammatory bowel disease
- CD:
-
Crohn’s disease
- UC:
-
Ulcerative colitis
- DMR:
-
Diagnostic medical radiation
- mSv:
-
Millisieverts
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- MRE:
-
Magnetic resonance enterography
- CTE:
-
CT enterography
References
Li L, Xu P, Zhang Z, Zhou X, Chen C, Lu C. Platelets can reflect the severity of Crohn’s disease without the effect of anemia. Clinics (Sao Paulo) (2020) 75:e1596. Epub 2020/07/16. https://doi.org/10.6061/clinics/2020/e1596. PubMed PMID: 32667493; PubMed Central PMCID: PMCPMC7337217.
Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142(1):46–54.e42. https://doi.org/10.1053/j.gastro.2011.10.001; quiz e30. Epub 2011/10/18.
Sairenji T, Collins KL, Evans DV. An update on inflammatory bowel disease. Prim Care. 2017;44(4):673–92. https://doi.org/10.1016/j.pop.2017.07.010. Epub 2017/11/15.
Flynn S, Eisenstein S. Inflammatory bowel disease presentation and diagnosis. Surg Clin North Am. 2019;99(6):1051–62. https://doi.org/10.1016/j.suc.2019.08.001. Epub 2019/11/05.
Jess T, Gamborg M, Matzen P, Munkholm P, Sørensen TI. Increased risk of intestinal cancer in Crohn’s disease: a meta-analysis of population-based cohort studies. Am J Gastroenterol (2005) 100(12):2724-9. Epub 2006/01/06. https://doi.org/10.1111/j.1572-0241.2005.00287.x. PubMed PMID: 16393226.
Loftus EV. Jr. Epidemiology and risk factors for colorectal dysplasia and cancer in ulcerative colitis. Gastroenterol Clin North Am. 2006;35(3):517–31. https://doi.org/10.1016/j.gtc.2006.07.005. Epub 2006/09/06.
Carvalho D, Russo P, Bernardes C, Saiote J, Ramos G, Mascarenhas L, et al. Hodgkin’s lymphoma in Crohn’s Disease treated with Infliximab. GE Port J Gastroenterol. 2017;24(6):279–84. https://doi.org/10.1159/000455180. Epub 2017/12/20.
Kroeker KI, Lam S, Birchall I, Fedorak RN. Patients with IBD are exposed to high levels of ionizing radiation through CT scan diagnostic imaging: a five-year study. J Clin Gastroenterol (2011) 45(1):34 – 9. Epub 2010/08/04. https://doi.org/10.1097/MCG.0b013e3181e5d1c5. PubMed PMID: 20679907.
Brenner DJ, Doll R, Goodhead DT, Hall EJ, Land CE, Little JB, et al. Cancer risks attributable to low doses of ionizing radiation: assessing what we really know. Proc Natl Acad Sci U S A. 2003;100(24):13761–6. https://doi.org/10.1073/pnas.2235592100. Epub 2003/11/12.
Tanooka H. Radiation cancer risk at different dose rates: new dose-rate effectiveness factors derived from revised A-bomb radiation dosimetry data and non-tumor doses. J Radiat Res. 2022;63(1):1–7. https://doi.org/10.1093/jrr/rrab109. Epub 2021/12/21.
Chatu S, Subramanian V, Pollok RC. Meta-analysis: diagnostic medical radiation exposure in inflammatory bowel disease. Aliment Pharmacol Ther. 2012;35(5):529–39. https://doi.org/10.1111/j.1365-2036.2011.04975. Epub 2012/01/14.
Lu C, Yang J, Yu W, Li D, **ang Z, Lin Y, et al. Association between 25(OH)D Level, Ultraviolet exposure, geographical location, and inflammatory bowel Disease Activity: a systematic review and Meta-analysis. PLoS ONE. 2015;10(7):e0132036. https://doi.org/10.1371/journal.pone.0132036. Epub 2015/07/15.
Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the reporting of Observational studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med. 2007;4(10):e297. https://doi.org/10.1371/journal.pmed.0040297. Epub 2007/10/19.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, the National Institute for Health Research in England. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med (2009) 6(7):e1000100. Epub 2009/07/22. https://doi.org/10.1371/journal.pmed.1000100. PubMed PMID: 19621070; PubMed Central PMCID: PMCPMC2707010 Oxford Radcliffe Hospitals Trust on behalf of the Department of Health and. This is a fixed term contract, the renewal of which is dependent upon the value placed upon his work, that of the UK Cochrane Centre, and of The Cochrane Collaboration more widely by the Department of Health. His work involves the conduct of systematic reviews and the support of the conduct and use of systematic reviews. Therefore, work–such as this manuscript–relating to systematic reviews might have an impact on his employment.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med (2002) 21(11):1539-58. Epub 2002/07/12. https://doi.org/10.1002/sim.1186. PubMed PMID: 12111919.
Ozasa K, Shimizu Y, Suyama A, Kasagi F, Soda M, Grant EJ, et al. Studies of the mortality of atomic bomb survivors, Report 14, 1950–2003: an overview of cancer and noncancer diseases. Radiat Res. 2012;177(3):229–43. https://doi.org/10.1667/rr2629.1. Epub 2011/12/17.
Seyedian SS, Nokhostin F, Malamir MD. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J Med Life. 2019;12(2):113–22. https://doi.org/10.25122/jml-2018-0075. Epub 2019/08/14.
Herfarth H, Palmer L. Risk of radiation and choice of imaging. Dig Dis. 2009;27(3):278–84. Epub 2009/09/30. doi: 10.1159/000228561. PubMed PMID: 19786752.
Amitai MM, Ben-Horin S, Eliakim R, Kopylov U. Magnetic resonance enterography in Crohn’s disease: a guide to common imaging manifestations for the IBD physician. J Crohns Colitis. 2013;7(8):603–15. https://doi.org/10.1016/j.crohns.2012.10.005. Epub 2012/11/06.
Fiorino G, Bonifacio C, Peyrin-Biroulet L, Minuti F, Repici A, Spinelli A, et al. Prospective comparison of computed tomography enterography and magnetic resonance enterography for assessment of disease activity and complications in ileocolonic Crohn’s disease. Inflamm Bowel Dis. 2011;17(5):1073–80. https://doi.org/10.1002/ibd.21533. Epub 2011/04/13.
Kucharzik T, Tielbeek J, Carter D, Taylor SA, Tolan D, Wilkens R et al. ECCO-ESGAR Topical Review on Optimizing Reporting for Cross-Sectional Imaging in Inflammatory Bowel Disease. J Crohns Colitis (2022) 16(4): 523–543. Epub 2022/05/10. https://doi.org/10.1093/ecco-jcc/jjab180. PubMed PMID: 34628504.
Brenner DJ, Hall EJ. Computed tomography–an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277–84. https://doi.org/10.1056/NEJMra072149. Epub 2007/11/30.
Nguyen GC, Low D, Chong RY, Diong C, Chawla T. Utilization of Diagnostic Imaging and Ionization Radiation exposure among an inflammatory bowel Disease Inception Cohort. Inflamm Bowel Dis. 2020;26(6):898–906. https://doi.org/10.1093/ibd/izz219. Epub 2019/09/29.
Patel B, Mottola J, Sahni VA, Cantisani V, Ertruk M, Friedman S et al. MDCT assessment of ulcerative colitis: radiologic analysis with clinicndoscopic, and pathologic correlation. Abdom Imaging (2012) 37(1):61 – 9. Epub 2011/05/24. https://doi.org/10.1007/s00261-011-9741-x. PubMed PMID: 21603899.
Zakeri N, Pollok RC. Diagnostic imaging and radiation exposure in inflammatory bowel disease. World J Gastroenterol. 2016;22(7):2165–78. https://doi.org/10.3748/wjg.v22.i7.2165. Epub 2016/02/24.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Chao Lu designed research; Chao Lu and **n Yao searched articles and collected data; Mosang Yu performed the statistical analysis; Chao Lu wrote the paper; **njue He revised the paper.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lu, C., Yao, X., Yu, M. et al. Medical radiation exposure in inflammatory bowel disease: an updated meta-analysis. BMC Gastroenterol 24, 173 (2024). https://doi.org/10.1186/s12876-024-03264-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12876-024-03264-1