Abstract
Background
The goal of this study was to investigate the effects of treatment with Saccharomyces boulardii and Lactobacillus reuteri on the eradication of Helicobacter pylori and Adverse effects (AEs) of the treatment.
Results
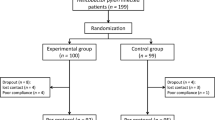
This study was a double-blind, randomized, placebo-controlled trial. And, eradication of H. pylori was reported comparing quadruple therapy include of PPI (proton pomp inhibitor), bismuth subcitrate, clarithromycin, and amoxicillin versus quadruple therapy supplemented with S. boulardii and L. reuteri DSMZ 17648. For this aim, a total of 156 patients were included in the current study; and patients positive for H. pylori infection (n = 156) were randomly assigned to 3 groups: 52 patients (Group P) received conventional quadruple therapy plus L. reuteri, 52 patients (Group S) received conventional quadruple therapy plus S. boulardii daily, for 2 weeks, and 52 patients were in the control group (Group C). At the end of the treatment period, all the subjects continued to take proton pump inhibitor (PPI) alone for 14 days, and then, no medication was given for 2 weeks again. During follow-up, gastrointestinal symptoms were assessed using an evaluation scale (Glasgow dyspepsia questionnaire [GDQ]), and AEs were assessed at 7, 14, 21, and 28 days. As a result, all patients completed the treatment protocol in all groups by the end of the study. Additionally, eradication therapy was effective for 94.2% of subjects in Group S, 92.3% of subjects in Group P, and 86.5% of subjects in the control group, with no differences between treatment arms. In Group S, the chance of develo** symptoms of nausea (OR = 2.74), diarrhea (OR = 3.01), headache (OR = 10.51), abdominal pain (OR = 3.21), and anxiety (OR = 3.58) was significantly lower than in the control group (p < 0.05).
Conclusion
S. boulardii could significantly reduce some AEs of H. pylori eradication therapy, but effectiveness of Lactobacillus reuteri on these cases was not significant. It is recommended to conduct the future research with larger sample size in order to investigate the effect.
Trial registration: IRCT20200106046021N1, this trial was registered on Jan 14, 2020.
Similar content being viewed by others
Introduction
Helicobacter pylori is a gram-negative infective bacterium affecting more than half of the world's population [1]. H. pylori infection contributes to etiology of a variety of diseases, such as gastric ulcer disease, dyspepsia, lymphoma, and gastric cancer [2,3,4]. Treatment of H. pylori infection is still being debated around the world, owing to emergence of multidrug-resistant strains of H. pylori. At the present, the most effective treatment options for H. pylori infection are complete pathogen eradication using multiple combinations of proton pump inhibitors (PPI) and two or three antibiotics [5,6,7]. This complicated approach carries a high risk of adverse effects (AEs), antibiotic resistance, and incompatibility [8, 9]. Reduced H. pylori load in the stomach by selective bacterial-bacterial surface interaction indicates a novel treatment approach to mitigating the danger posed by this human infection. Bacterial accumulation has previously been discussed in terms of infection elimination via specific binding to pathogens and formation of co-aggregates [10,11,12]. Many observations support the use of probiotics with bioactive components in people infected with H. pylori, recommending the use of multiplex probiotic microorganisms in control and treatment of infections caused by H. pylori [13, 14]. It has been proposed that Lactobacillus supplementation may be effective in accelerating removal of H. pylori in first-time patients, as well as having a positive effect on some AEs associated with H. pylori treatment [15]. To date, saccharomyces boulardii and several Lactobacillus reuteri strains have been used alone or in combination with various H. pylori treatment regimens in clinical studies [16,17,18,19,20,21,22,23,24,25,Statistical analysis After entering the SPSS 24 software, the data were described using central and dispersion indices for quantitative variables and frequency and agreement tables for qualitative variables. Homogeneity of groups was assessed using analysis of variance (ANOVA) and Chi-Square tests. Marginal logistic model with generalized estimating equation (GEE) approach was used for modeling the changes in complications during the study. In the tests, significance level was set at 0.05.
Results
Patients
A total of 156 subjects with confirmed H. pylori infection completed the treatment protocol in all groups by the end of the study. The study included 60.9 percent female participants and 39.1% male participants, with a mean age of 47.76 ± 13.92 years old (age range of 16–74 years old). They were randomly assigned into three study arms: 52 patients were assigned into the L. Reuteri group (Group P), 52 patients were assigned into the S. boulardii group (Group S), and 52 patients were assigned into the placebo group (Group C). The majority of our patients (93.6%) were from rural areas, housewife (46.8%), and non-smokers (76.9%). Dyspepsia was the most common reason for undergoing an endoscopy procedure (64.7%). At the time of the first endoscopy, the majority of patients had antral gastritis. Antral and corpus predominant gastritis were found in 88.5and 27.6% of patients, respectively. The three treatment groups were similar in terms of their demographic, clinical, and endoscopic characteristics at baseline (Table 1) (p > 0.05).
Eradication rate
The highest rate of H. pylori eradication occurred in all groups in the second week after treatment. Totally, 86.5, 94.2, and 92.3% of eradication was observed in the control, S, and P groups, respectively. In general, eradication rate in the studied groups was not statistically significant in the second and sixth weeks, (with P = 0.46 in the second week and P = 0.53 in the sixth week, respectively) (Fig. 3).
Patients compliance and adverse effects
Headache, abdominal pain, and anxiety were significantly reduced in the Group S (who received saccharomyces boulardii probiotic-DAILYEAST®). But in Group P, headache and abdominal pain were significantly reduced and anxiety was significantly increased (p < 0.05). However, none of the study groups experienced significant changes in vomiting, insomnia, bitter taste in the mouth, or epigastric discomfort (p > 0.05) (Table 2). According to Table 2, after adjusting the effect of time, in the Group S, chance of develo** symptoms of headache (OR = 10.51), abdominal pain (OR = 3.21), and anxiety (OR = 3.58) was significantly lower than control group (p < 0.05). Also, except for headache (OR = 3.75), the Group P did not differ significantly from the control group in incidence of complications (p > 0.05) (Fig. 4). For more details, see Additional file 1.
Discussion
Treatment of H. pylori infection is becoming increasingly important, particularly in develo** countries. Despite availability of various therapeutic regimens, treatment failure has remained a growing problem in daily clinical practices. Several factors could contribute to failure of eradication, but the most important factors are antibiotic resistance and clinical efficacy [31]. According to findings of the current study, there was no statistically significant difference between the study results at the second and sixth weeks after treatment. These findings were in agreement with the Zojaji et al. study that evaluated the efficacy and safety of adding S. boulardii to standard triple therapy in 160 adult patients with biopsy confirmed H. pylori infection. They randomized patients into two treatment regimens: patients in group A (n = 80) were given amoxicillin, clarithromycin, omeprazole, and a probiotic of Saccharomyces boulardii for 14 days. Moreover, patients in group B (n = 80) were given amoxicillin, clarithromycin, and omeprazole for 14 days. After the second week, the success rate for H. pylori eradication in group A was higher at 75 (87.5%) than in group B at 65 (81.2%), but the difference between the two groups was not significant [27]. Furthermore, Cindoruk et al., in a study on assessing the effect of S. boulardii on eradication of H. pylori infection and reduction of AEs reported no significant difference in H. pylori eradication between the study groups (71% in the S. boulardii-treated group and 60% in the placebo group), which was consistent with our findings [32]. Also, Shavakhi et al., found that using a combination of probiotics containing Lactobacillus, Bifidobacterium, and Streptococcus thermophilus species along with a standard quadruple therapy had no beneficial effect in treating H. pylori infection. This could be due to the probiotic diet's low dose or high frequency of upper gastrointestinal AEs, which can reduce H. pylori eradication [33]. On the other hand, Poonyam et al., indicated that eradication of H. pylori was significantly increased compared to the control group when Lactobacillus reuteri was used in combination with a standard quadruple therapy [34]. Moreover, Yu et al. demonstrated that Lactobacillus can significantly eradicate H. pylori in a meta-analysis with a sample size of 724 patients to investigate the probiotic effect of Lactobacillus in combination with a triple eradication regimen [35]. In addition, Zhou et al., in a meta-analysis showed that S. boulardii can significantly increase eradication of H. pylori [36], all these studies were not in agreement with our findings. Among the reasons for this mismatch, one can mention a difference in the type of probiotic used in the studies in terms of the strains used or the large sample size and multi-center nature of these studies are reasons for the inconsistency between the findings.
With the widespread use of probiotics in clinical practice in recent years, the concept of "treating bacteria with probiotics" has been proposed as a new strategy to eradicate H. pylori. However, the mechanism underlying L. reuteri and S. Bouvardia’s eradication of H. pylori has not been fully elucidated. Several potential mechanisms have been proposed, including the following:
-
1.
The volume of S. boulardii is 10 times larger than that of common bacterial strains of probiotics, which increases the surface area and can better adhere to pathogenic bacteria, affecting the colonization of H. pylori in the gastric mucosa [37]. S. boulardii comprises neuraminidase activity selective for alpha (2–3)‐linked sialic acid, and acts as a ligand binding to H. pylori adhesin, which in turn inhibits the adhesion of H. Pylori in the duodenum [38].
-
2.
Increasing antimicrobial substances, such as short‐chain fatty acids, inhibiting the growth and proliferation of H. pylori [39].
-
3.
S. boulardii may stimulate an increase in secretory IgA and immunoglobulin levels [40], strengthening the mucosal immune barrier. H. pylori infection can stimulate gastric epithelial cells to produce inflammatory mediators such as interleukin (IL) and tumor necrosis factor (TNF) [41, 42].
-
4.
Reducing the incidence of adverse effects and, subsequently, compliance may improve, which may indirectly improve the H. pylori eradication rates.
In the case of L. reuteri, In vitro and in artificial gastric juice, H. pylori specifically co-aggregates with H. pylori without interfering with other commensal intestinal flora bacteria [43]. This specific binding may obscure H. pylori surface structures and impair Helicobacter motility. Pathogens that have aggregated are presumably no longer adhered to the gastric mucosa and are cleared from the stomach. Competition for specific binding proteins could be another mode of action [44].
According to findings of our study, distribution frequency of some AEs in the subjects receiving S. boulardii probiotic was significantly reduced after the intervention but, except for headache, no significant change was observed in the Lactobacillus reuteri-treated group. According to the findings, the most common AEs in the first week were vomiting, bitter taste in the mouth, epigastric discomfort, and insomnia, which were decreased after that time. However, no statistically significant difference was found between the studied groups at different times. Our findings were consistent with those of the studies by Pourmasoumi et al., and Lv et al., who showed that probiotic administration can reduce the AEs of anti H. pylori treatment [ In general, our findings revealed that the use of probiotic supplements containing S. boulardii could significantly reduce some of the AEs of H. pylori eradication therapy. But, the effectiveness of Lactobacillus reuteri (DSMZ 17648 strain) on these cases was not significant, and only the headache was remarkably reduced, which was in accordance with the previous evidence in the literature. Therefore, it is recommended to conduct future research with a larger sample size in order to investigate the effect of S. boulardii supplementation on eradicating H. pylori infection and reducing treatment AEs.Conclusion
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Hooi JK, Lai WY, Ng WK, Suen MM, Underwood FE, Tanyingoh D, Malfertheiner P, Graham DY, Wong VW, Wu JC. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology. 2017;153(2):420–9.
Sjomina O, Heluwaert F, Moussata D, Leja M. Helicobacter pylori infection and nonmalignant diseases. Helicobacter. 2017;22:e12408.
Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345(11):784–9.
Wotherspoon AC, Ortiz-Hidalgo C, Falzon MR, Isaacson PG. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. The Lancet. 1991;338(8776):1175–6.
Nishizawa T, Suzuki H. Mechanisms of Helicobacter pylori antibiotic resistance and molecular testing. Front Mol Biosci. 2014;1:19.
Fiorini G, Zullo A, Vakil N, Saracino IM, Ricci C, Castelli V, Gatta L, Vaira D. Rifabutin triple therapy is effective in patients with multidrug-resistant strains of Helicobacter pylori. J Clin Gastroenterol. 2018;52(2):137–40.
Thung I, Aramin H, Vavinskaya V, Gupta S, Park J, Crowe S, Valasek M. the global emergence of Helicobacter pylori antibiotic resistance. Aliment Pharmacol Ther. 2016;43(4):514–33.
Zhang M. High antibiotic resistance rate: a difficult issue for Helicobacter pylori eradication treatment. World J Gastroenterol. 2015;21(48):13432.
Malfertheiner P, Megraud F, O’morain C, Gisbert J, Kuipers E, Axon A, Bazzoli F, Gasbarrini A, Atherton J, Graham DY. Management of Helicobacter pylori infection—the Maastricht V/Florence consensus report. Gut. 2017;66(1):6–30.
Younes JA, van der Mei HC, van den Heuvel E, Busscher HJ, Reid G. Adhesion forces and coaggregation between vaginal staphylococci and lactobacilli. PLoS ONE. 2012;7(5):e36917.
Lang C, Böttner M, Holz C, Veen M, Ryser M, Reindl A, Pompejus M, Tanzer J. Specific lactobacillus/mutans streptococcus co-aggregation. J Dent Res. 2010;89(2):175–9.
McMillan A, Dell M, Zellar MP, Cribby S, Martz S, Hong E, Fu J, Abbas A, Dang T, Miller W. Disruption of urogenital biofilms by lactobacilli. Colloids Surf, B. 2011;86(1):58–64.
Gotteland M, Brunser O, Cruchet S. Systematic review: are probiotics useful in controlling gastric colonization by Helicobacter pylori? Aliment Pharmacol Ther. 2006;23(8):1077–86.
Vítor JM, Vale FF. Alternative therapies for Helicobacter pylori: probiotics and phytomedicine. FEMS Immunol Med Microbiol. 2011;63(2):153–64.
Zou J, Dong J, Yu X. Meta-analysis: lactobacillus containing quadruple therapy versus standard triple first-line therapy for Helicobacter pylori eradication. Helicobacter. 2009;14(5):449–59.
Francavilla R, Lionetti E, Castellaneta SP, Magistà AM, Maurogiovanni G, Bucci N, De Canio A, Indrio F, Cavallo L, Ierardi E. Inhibition of Helicobacter pylori infection in humans by Lactobacillus reuteri ATCC 55730 and effect on eradication therapy: a pilot study. Helicobacter. 2008;13(2):127–34.
Dore MP, Cuccu M, Pes GM, Manca A, Graham DY. Lactobacillus reuteri in the treatment of Helicobacter pylori infection. Intern Emerg Med. 2014;9(6):649–54.
Emara MH, Mohamed SY, Abdel-Aziz HR. Lactobacillus reuteri in management of Helicobacter pylori infection in dyspeptic patients: a double-blind placebo-controlled randomized clinical trial. Ther Adv Gastroenterol. 2014;7(1):4–13.
Holz C, Busjahn A, Mehling H, Arya S, Boettner M, Habibi H, Lang C. Significant reduction in helicobacter pylori load in humans with non-viable lactobacillus reuteri DSM17648: a pilot study. Probiotics Antimicrob Proteins. 2015;7(2):91–100.
Mehling H, Busjahn A. Non-viable Lactobacillus reuteri DSMZ 17648 (Pylopass™) as a new approach to Helicobacter pylori control in humans. Nutrients. 2013;5(8):3062–73.
Holz C, Busjahn A, Mehling H, Arya S, Boettner M, Habibi H, Lang C. Significant reduction in Helicobacter pylori load in humans with non-viable Lactobacillus reuteri DSM17648: a pilot study. Probiotics Antimicrob Proteins. 2015;7(2):91–100.
Saulnier DM, Santos F, Roos S, Mistretta T-A, Spinler JK, Molenaar D, Teusink B, Versalovic J. Exploring metabolic pathway reconstruction and genome-wide expression profiling in Lactobacillus reuteri to define functional probiotic features. PLoS ONE. 2011;6(4):e18783.
Yadegar A, Mobarez AM, Alebouyeh M, Mirzaei T, Kwok T, Zali MR. Clinical relevance of cagL gene and virulence genotypes with disease outcomes in a Helicobacter pylori infected population from Iran. World J Microbiol Biotechnol. 2014;30(9):2481–90.
Yadegar A, Alebouyeh M, Zali MR. Analysis of the intactness of Helicobacter pylori cag pathogenicity island in Iranian strains by a new PCR-based strategy and its relationship with virulence genotypes and EPIYA motifs. Infect Genet Evol. 2015;35:19–26.
Pourmasoumi M, Najafgholizadeh A, Hadi A, Mansour-Ghanaei F, Joukar F. The effect of synbiotics in improving Helicobacter pylori eradication: a systematic review and meta-analysis. Complement Ther Med. 2019;43:36–43.
Lv Z, Wang B, Zhou X, Wang F, **e Y, Zheng H, Lv N. Efficacy and safety of probiotics as adjuvant agents for Helicobacter pylori infection: a meta-analysis. Exp Ther Med. 2015;9(3):707–16.
Zojaji H, Ghobakhlou M, Rajabalinia H, Ataei E, Sherafat SJ, Moghimi-Dehkordi B, Bahreiny R. The efficacy and safety of adding the probiotic Saccharomyces boulardii to standard triple therapy for eradication of Helicobacter pylori; a randomized controlled trial. Gastroenterol Hepatol Bed Bench. 2013;2016(Suppl. 2011):S2099–104.
Dore MP, Bibbo S, Pes GM, Francavilla R, Graham DY. Role of probiotics in Helicobacter pylori eradication: lessons from a study of Lactobacillus reuteri strains DSM 17938 and ATCC PTA 6475 (Gastrus®) and a proton-pump inhibitor. Can J Infect Dis Med Microbiol. 2019. https://doi.org/10.1155/2019/3409820.
Buckley M, Lacey S, Doolan A, Goodbody E, Seamans K. The effect of Lactobacillus reuteri supplementation in Helicobacter pylori infection: a placebo-controlled, single-blind study. BMC Nutr. 2018;4(1):1–8.
Schulz KF, Altman DG, Moher D, The CG. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8(1):18.
Ibrahim NH, Gomaa AA, Abu‐Sief MA, Hifnawy TM, Tohamy MAE. The use of different laboratory methods in diagnosis of Helicobacter pylori infection; a comparative study. Life Sci J‐Acta Zhengzhou Univ Overseas Ed. 2012;9(4):249–59.
Cindoruk M, Erkan G, Karakan T, Dursun A, Unal S. Efficacy and safety of Saccharomyces boulardii in the 14-day triple anti-Helicobacter pylori therapy: a prospective randomized placebo-controlled double-blind study. Helicobacter. 2007;12(4):309–16.
Shavakhi A, Tabesh E, Yaghoutkar A, Hashemi H, Tabesh F, Khodadoostan M, Minakari M, Shavakhi S, Gholamrezaei A. The effects of multistrain probiotic compound on bismuth-containing quadruple therapy for Helicobacter pylori infection: a randomized placebo-controlled triple-blind study. Helicobacter. 2013;18(4):280–4.
Poonyam P, Chotivitayatarakorn P, Vilaichone RK. High effective of 14-day high-dose PPI-bismuth-containing quadruple therapy with probiotics supplement for Helicobacter pylori eradication: a double blinded-randomized placebo-controlled study. Asian Pac J Cancer Prev. 2019;20(9):2859–64.
Yu M, Zhang R, Ni P, Chen S, Duan G. Efficacy of Lactobacillus-supplemented triple therapy for H. pylori eradication: a meta-analysis of randomized controlled trials. PLoS ONE. 2019;14(10):e0223309.
Zhou B-G, Chen L-X, Li B, Wan L-Y, Ai Y-W. Saccharomyces boulardii as an adjuvant therapy for Helicobacter pylori eradication: a systematic review and meta-analysis with trial sequential analysis. Helicobacter. 2019;24(5):e12651.
Czerucka D, Piche T, Rampal P. Yeast as probiotics–Saccharomyces boulardii. Aliment Pharmacol Ther. 2007;26(6):767–78.
Sakarya S, Gunay N. S accharomyces boulardii expresses neuraminidase activity selective for α2, 3-linked sialic acid that decreases Helicobacter pylori adhesion to host cells. APMIS. 2014;122(10):941–50.
McFarland LV. Systematic review and meta-analysis of Saccharomyces boulardii in adult patients. World J Gastroenterol. 2010;16(18):2202.
Buts J-P, Bernasconi P, Vaerman J-P, Dive C. Stimulation of secretory IgA and secretory component of immunoglobulins in small intestine of rats treated with Saccharomyces boulardii. Dig Dis Sci. 1990;35(2):251–6.
Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347(15):1175–86.
Lee J, Choi S, Park J, Shin J, Joo Y. The effect of Saccharomyces boulardii as an adjuvant to the 14-day triple therapy for eradication of Helicobacter pylori. J Gastroenterol Hepatol. 2011;26:257.
Lang C. DSMZ17648 specifically co-aggregates H. pylori under in vitro conditions. 2011. unpublished work.
Mukai T, Asasaka T, Sato E, Mori K, Matsumoto M, Ohori H. Inhibition of binding of Helicobacter pylori to the glycolipid receptors by probiotic Lactobacillus reuteri. FEMS Immunol Med Microbiol. 2002;32(2):105–10.
Acknowledgements
Not applicable.
Funding
This study was funded by ZistTakhmir pharmaceutical company, Tehran, Iran.
Author information
Authors and Affiliations
Contributions
NN coordinated the experiments, FS performed the Statistical analysis and Interpretated the data, SN prepared the manuscript, TT revised the important intellectual content and Supervision, all authors reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All methods were carried out in accordance with relevant guidelines and regulations. This study was approved by the Research Council and Ethics Committee of the Birjand University of Medical Sciences with the ethics code of IR.BUMS.REC.1398.305. It was also registered on the website of the Iranian registry of clinical trials (IRCT) with the following Number: IRCT20200106046021N1 on 14/01/2020 (https://en.irct.ir/trial/44865). Informed written consent was obtained from all the patients. No costs were imposed on patients. The drugs used in the study, as well as the dose administered to patients, had no AEs or toxicity. The final report and analysis were performed without the names of the participants in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Supplementary figures: The frequency distribution of side effects over time in the study groups.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Naghibzadeh, N., Salmani, F., Nomiri, S. et al. Investigating the effect of quadruple therapy with Saccharomyces boulardii or Lactobacillus reuteri strain (DSMZ 17648) supplements on eradication of Helicobacter pylori and treatments adverse effects: a double-blind placebo-controlled randomized clinical trial. BMC Gastroenterol 22, 107 (2022). https://doi.org/10.1186/s12876-022-02187-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12876-022-02187-z