Abstract
Background
Malnutrition increases the risk of poor prognosis in patients with cardiovascular disease, and our current research was designed to assess the predictive performance of the Geriatric Nutrition Risk Index (GNRI) for the occurrence of poor prognosis after percutaneous coronary intervention (PCI) in patients with stable coronary artery disease (SCAD) and to explore possible thresholds for nutritional intervention.
Methods
This study retrospectively enrolled newly diagnosed SCAD patients treated with elective PCI from 2014 to 2017 at Shinonoi General Hospital, with all-cause death as the main follow-up endpoint. Cox regression analysis and restricted cubic spline (RCS) regression analysis were used to explore the association of GNRI with all-cause death risk and its shape. Receiver operating characteristic curve (ROC) analysis and piecewise linear regression analysis were used to evaluate the predictive performance of GNRI level at admission on all-cause death in SCAD patients after PCI and to explore possible nutritional intervention threshold points.
Results
The incidence of all-cause death was 40.47/1000 person-years after a mean follow-up of 2.18 years for 204 subjects. Kaplan-Meier curves revealed that subjects at risk of malnutrition had a higher all-cause death risk. In multivariate Cox regression analysis, each unit increase in GNRI reduced the all-cause death risk by 14% (HR 0.86, 95% CI 0.77, 0.95), and subjects in the GNRI > 98 group had a significantly lower risk of death compared to those in the GNRI < 98 group (HR 0.04, 95% CI 0.00, 0.89). ROC analysis showed that the baseline GNRI had a very high predictive performance for all-cause death (AUC = 0.8844), and the predictive threshold was 98.62; additionally, in the RCS regression analysis and piecewise linear regression analysis we found that the threshold point for the GNRI-related all-cause death risk was 98.28 and the risk will be significantly reduced when the subjects' baseline GNRI was greater than 98.28.
Conclusions
GNRI level at admission was an independent predictor of all-cause death in SCAD patients after PCI, and GNRI equal to 98.28 may be a useful threshold for nutritional intervention in SCAD patients treated with PCI.
Similar content being viewed by others
Background
SCAD is a widespread cardiovascular disease, featured pathologically as the presence of atherosclerotic plaques within the walls of the coronary arteries [1, 2]. Atherosclerotic plaques can cause severe stenosis or even rupture of coronary vessels, leading to cardiovascular events like angina pectoris, ischemic heart failure, and acute myocardial infarction, which can lead to death in severe cases [3, 4]. Globally, CAD is a major cause of death, especially among the elderly population [5, 6]. PCI is, currently, one of the main invasive treatments for patients with CAD, which can effectively relieve myocardial ischemia and improve myocardial oxygenation, and reduce angina attacks and myocardial infarction [7, 8]. Previous studies have shown that the presence of malnutrition may increase the incidence of post-PCI complications and cardiovascular events in elderly SCAD patients, which has a significant impact on the long-term prognosis of patients [9, 10]; in addition, malnutrition can induce a chronic inflammatory response in the body, and long-term chronic inflammation can further aggravate malnutrition and oxidative stress, thereby exacerbating endothelial dysfunction and forming a malnutrition-inflammation-atherosclerosis syndrome [11, 12]. Therefore, evaluating the nutritional conditions of elderly SCAD patients undergoing PCI during hospitalization may have important prognostic implications.
GNRI is a nutrition-related index that can be applied to evaluate the nutritional conditions of hospitalized elderly patients and to predict morbidity and mortality from malnutrition-related diseases in elderly patients developed by Bouillanne O et al. [13]. Currently, several studies have reported the association of nutritional conditions assessed using GNRI with the occurrence and prognosis of CAD and the risk of CAD comorbidities [10, 11, 14,15,16,17]. Also, the association of GNRI with the long-term prognosis of CAD patients was explored in a study by Wada H et al., who concluded that lower GNRI was an independent risk factor for all-cause death in post-PCI CAD patients [18]. However, the predictive performance of GNRI at the time of admission for elective PCI in newly diagnosed SCAD patients for the future occurrence of all-cause death and the possible threshold points for nutritional interventions are unclear. Therefore, this study aimed to investigate the risk assessment/predictive value of GNRI levels at admission in elderly patients with newly diagnosed SCAD for the occurrence of all-cause death after PCI and to further search for possible thresholds of nutritional intervention.
Methods
Research design and ethics approval
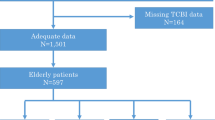
This study conducted a secondary analysis using inpatient examination data and post-discharge follow-up data of newly diagnosed SCAD patients treated with elective PCI at Shinonoi General Hospital from October 2014 to October 2017. All information regarding patient enrollment has been described in detail elsewhere [19]. In brief, the research team from Shinonoi General Hospital enrolled hospitalized patients who were newly diagnosed with SCAD and underwent selective PCI treatment at the hospital between 2014 and 2017. After excluding patients with a history of old myocardial infarction and those with malignant tumors, a total of 204 subjects were finally included; and the researcher collected data on various tests during the hospitalization of these patients and continued to follow the patients after their discharge. In a previous study, Suzuki S et al. used the data of this cohort of patients to investigate the association of serum albumin (ALB) with the occurrence of major adverse cardiac events, and uploaded the complete data of all subjects to the Daryd database [20]; additionally, Suzuki S et al. declared that all enrolled patients and their written informed consent were approved by Shinonoi General Hospital Ethics Committee, and the subjects’ identifying information was anonymized [19].
On this basis, the current study used data from this cohort to explore the risk assessment and predictive value of GNRI measured at admission for the onset of all-cause death in patients with SCAD undergoing PCI; since this study was a secondary analysis of data from previous research and all data on subjects had been anonymized, Jiangxi Provincial People’s Hospital Ethics Committee had authorized this study and dispensed with repeatedly obtaining written informed consent from subjects (IRB: 2022-006). All procedures in the current study were following the Declaration of Helsinki [21].
Data collection and definitions
During the subjects’ hospitalization, the researchers collected extensive data on all subjects including clinical characteristics [sex, body mass index (BMI), weight, age, height, diastolic blood pressure (DBP), estimated glomerular filtration rate (eGFR), systolic blood pressure (SBP), estimated glomerular filtration rate (eGFR), smoking history, Multivessel PCI, history of previous stroke, history of diabetes], type of coronary lesions (calcified lesions, ostial lesion, bifurcation lesions, chronic coronary obstruction lesions), laboratory measurements [total cholesterol (TC), low-density lipoprotein (LDL-C), ALB, Glycohemoglobin (HbA1c), triglycerides (TG), high-density lipoprotein (HDL-C), C-reactive protein (CRP), hemoglobin (Hb)], echocardiography (left ventricular ejection fraction) and angiography data. Where ALB was measured using the bromocresol purple assay and the LABOSPECT 008 analyzer and anthropometric indicators were measured using standard methods. SCAD was diagnosed based on angiographic findings of ≥ 90% epicardial coronary stenosis or findings of ≥ 75% epicardial coronary stenosis with symptoms of exercise-induced chest pain or clinical evidence of myocardial ischemia induced by stress testing; PCI procedures and coronary angiography were performed in all subjects according to guidelines and standard protocols, and subjects were administered thienopyridines and aspirin prior to the procedure [19]. The primary follow-up endpoint event in our study was the occurrence of all-cause death, and the event was validated by reviewing the medical record data of treated patients.
Calculation of the GNRI
The marker used to evaluate nutritional conditions in this study was GNRI, which was calculated as GNRI = 1.489 × ALB (g/L) + 41.7 × actual weight (kg)/ideal weight (kg). Where ALB and actual weight were measured while the subjects were hospitalized, and ideal weight was calculated using the Lorenz formula, i.e., height (cm)-100- [height (cm)-150]/4 for men and height (cm)-100-([height (cm)-150]/2.5) for women. When the calculation result of the ideal weight < actual weight, the actual weight/ideal weight was considered as one; additionally, we defined GNRI ≤ 98 as the presence of malnutrition risk and GNRI > 98 as the absence of malnutrition risk according to the findings of the GNRI developer Bouillanne O et al. [13].
Statistical analysis
All statistical analyses for this study were completed on R Language 3.4.3 and Empower(R) 4.1 software and significance was set at two-tailed P < 0.05.
Information about subjects was described by grou** them according to their GNRI values at baseline and whether death occurred during follow-up, respectively. Measurement data with normal and nonnormal distributions were expressed by mean (standard deviation) and median (lower quartile, upper quartile), respectively, while count data were described using frequency (%). In the inter-group comparison of measurement data, two independent sample t-test was used for variables with normal distribution and the Kruskal-Wallis H test for nonnormal distribution; while the chi-square test was used for inter-group comparisons of count data.
The Kaplan-Meier curves were used to evaluate the cumulative incidence risk of all-cause death of subjects in different GNRI groups, and the log-rank test was used to compare between groups; in addition, we also determined whether the proportional risk hypothesis was valid by observing whether the Kaplan-Meier curves intersected between different groups [22]. Before building the Cox regression model, we also checked whether there was collinearity between GNRI and other covariates using multiple linear regression analysis, and covariates with a final variance inflation factor greater than 5 were defined as collinear variables and excluded from model adjustment [23]. In the Cox regression analysis, we included GNRI as a continuous variable and a categorical variable in the model and calculated the hazard ratio (HR) and 95% confidence interval (CI) after stepwise adjustment for other non-collinear variables [24]. First, an unadjusted Crude Model was established to initially assess the association of GNRI with all-cause death risk; then in Model 1, sex, age, height, and BMI were adjusted; Model 2 was adjusted for blood lipid and blood glucose parameters (TG, HbA1c, LDL-C, HDL-C) on the basis of Model 1; finally, Model 3 further adjusted for all remaining non-collinear variables based on Model 2. Additionally, to further explore the shape of the association, we also fitted a dose-response relationship curve based on the multivariable-adjusted Model 3 using a RCS regression model with 4 knots and then used the piecewise linear regression model to automatically calculate the possible best inflection point on the curve by a recursive method.
Based on Model 3 we also explored the possible effect of the types of subjects’ coronary lesions [calcified lesions, ostial lesions, bifurcation lesions, and chronic total obstruction (CTO) lesions] on the association of GNRI with all-cause death risk and checked whether the effect of various lesion types on the association was significant using the log-likelihood ratio test. Finally, we also plotted the ROC curve to explore the predictive performance of GNRI and BMI for all-cause death and recorded the area under the ROC curve (AUC) and the prediction threshold, and the predictive accuracy of both was compared using the Delong test.
Results
Clinical baseline characteristics
The average age of the 204 subjects in the current study was 72.6 years, with 62 (30.39%) women and 142 (69.61%) men. During 443.89 person-years (mean 2.18 years) of follow-up, eighteen deaths occurred with an incidence density of 40.55/1000 person-years. The subjects’ basic characteristics were shown in Tables 1 and 2. Table 1 shows comparative information on the baseline characteristics of subjects at risk of malnutrition (GNRI ≤ 98) compared to those at no risk of malnutrition (GNRI > 98). The results showed that subjects without risk of malnutrition were younger and had a higher prevalence of smokers and also had higher levels of height, weight, BMI, ALB, eGFR, TC, TG, LDL-C, SBP, DBP, Hb, LVEF and lower CRP levels than those with risk of malnutrition, but there were no significant differences in sex composition, proportion of complex PCI procedures, history of stroke, proportion of diabetic patients, HDL-C and HbA1c levels and types of coronary lesion. In Table 2, we further compared the clinical characteristics of subjects who died during the follow-up period with those who did not. Our analysis revealed that those in the dead group tended to exhibit characteristics of malnutrition, as they were generally older and had significantly lower height, LDL-C, weight, TG, GNRI, BMI, eGFR, TC, Hb, and ALB levels and had higher CRP levels and proportion of smokers, while there were also no significant differences in sex composition, proportion of complex PCI procedures, history of stroke, proportion of diabetic patients, HDL-C, HbA1c, SBP, DBP, LVEF levels, and types of coronary lesion. These results suggested clinical indicators that may be associated with GNRI and that the occurrence of death may be related to the risk of malnutrition.
Figure 1 shows the Kaplan-Meier survival curves of the GNRI ≤ 98 group and the GNRI > 98 group, and it can be seen that subjects in the group of GNRI ≤ 98 had significantly higher mortality than the GNRI > 98 group (log-rank P < 0.0001), and the curves between the two groups did not intersect indicating that the proportional hazard assumption was met in the current study.
Association between GNRI and all-cause death risk
Before establishing the multivariate-adjusted Cox regression models, the collinearity screening analysis screened out the collinear variables weight, ALB, and TC with GNRI, and they will not be brought into the model adjustment. Table 3 shows the results of the association analysis between GNRI and all-cause death risk showing that in the unadjusted Crude Model, GNRI was negatively associated with all-cause death risk, either as a continuous variable (HR 0.86, 95% CI 0.82, 0.91; P < 0.0001) or as a categorical variable (HR 0.03, 95% CI 0.00, 0.25; P = 0.001); and the negative correlation between GNRI and all-cause death risk was slightly attenuated after stepwise adjustment for multiple confounders in Models 1, 2, and 3. According to the results of Model 3, each 1 unit increase in GNRI was associated with an 14% reduced risk of all-cause death (HR 0.86, 95% CI 0.77, 0.95; P = 0.0041); in addition, patients without risk of malnutrition had a significantly lower all-cause death risk compared to those with risk of malnutrition (HR 0.04, 95% CI 0.00, 0.89; P = 0.0417).
Nonlinear association analysis between GNRI and all-cause death risk
Figure 2 shows the dose-response relationship curve of GNRI with all-cause death risk. We found that when GNRI was at a low level, the dose-response relationship curve was relatively flat and an increase in GNRI did not seem to significantly reduce all-cause death risk, whereas when GNRI reached to 95–100 interval, there was a clear inflection point on the curve and a significant reduction in GNRI-related all-cause death risk occurred after the inflection point. On this basis, we further calculated the best inflection point on the curve by piecewise linear regression analysis, and the results showed that the correlation of GNRI with all-cause death risk had the most significant change on both sides of the GNRI value equal to 98.28 (Log-likelihood ratio test P = 0.023) (Table 4). The HR for the association between GNRI and all-cause death risk was 0.87 (95% CI 0.76, 0.99; P = 0.0402) when the GNRI was less than 98.28, whereas the association was not significant at GNRI greater than 98.28, which may be attributable to the fact that there were no deaths among the subjects with a GNRI greater than 98.28 at admission in the current study. Therefore, the risk threshold on this dose-response relationship curve for all-cause death risk associated with GNRI may be located at 98.28, and GNRI equal to 98.28 may be the target value for nutritional interventions to reduce all-cause death risk in post-PCI SCAD patients.
Subgroup analysis
Considering that the types of coronary lesions in subjects may have an impact on the GNRI-related all-cause death risk, we also conducted stratified analyses based on whether the presence of calcified lesions, ostial lesions, bifurcation lesions, and CTO lesions in the subjects, and the results showed that these types of coronary lesions had no significant impact on the association between GNRI and all-cause death risk (All P for interaction > 0.05) (Table 5).
The predictive value of GNRI and BMI for future all-cause death event
In Fig. 3, we plotted ROC curves for assessing the predictive performance of baseline GNRI and BMI for future all-cause death events and found that GNRI measured on admission had excellent predictive accuracy for all-cause death events occurring in SCAD patients after PCI. When the prediction threshold of GNRI was 98.62, the AUC reached the highest 0.8844 (0.8184, 0.9505), the sensitivity and specificity at this time were 100% and 63.44%, respectively, and the Youden index was 0.6344; the extremely high sensitivity of this prediction threshold point may imply that all-cause death risk after PCI was significantly reduced when patients with new-onset SCAD have a GNRI above 98.62 during hospitalization. The AUC value for BMI was 0.7945, and after comparing with the Delong test, it was found that GNRI’s predictive accuracy for all-cause mortality after PCI in SCAD patients was significantly higher than that of BMI (P = 0.0232). Moreover, it is worth noting that the results of the ROC analysis were consistent with the results of the nonlinear analysis described above and that the optimal risk threshold point we selected in the dose-response curve was at GNRI equal to 98.28, which was also extremely close to the prediction threshold point of 98.62.
Discussion
The results of this cohort study suggested that the GNRI of elderly patients with newly diagnosed SCAD measured on admission was associated with all-cause death risk after PCI and that a high level of GNRI was a protective factor against the occurrence of all-cause death. In addition, GNRI also had very high predictive performance (AUC = 0.8844) for the occurrence of all-cause death events in SCAD patients after PCI, with an appropriate prediction threshold of 98.62. It is worth mentioning that in the RCS regression analysis and threshold effects analysis, we found that a GNRI value equal to 98.28 was the threshold point for the GNRI-related risk of all-cause death, a threshold point that was extremely close to the prediction threshold of 98.62 in the ROC analysis and was also extremely close to the threshold point recommended by Bouillanne O et al. for no risk of malnutrition (GNRI = 98). Therefore, to reduce all-cause death risk, controlling GNRI above 98.28 may be the nutritional intervention threshold for SCAD patients admitted for PCI.
CAD, one of the most common cardiovascular diseases worldwide, is usually caused by insufficient coronary artery blood supply [1, 25, 26]. With the global aging population, unhealthy lifestyles, and the global prevalence of metabolic diseases like obesity, diabetes, and hypertension, CAD has become a major cause of death in middle-aged and elderly populations in both developed and develo** countries [5, 27,28,29]. Recently, there has been great clinical progress in both the pharmacological treatment of CAD and invasive PCI treatment, especially since the successive introduction of drug-eluting stents and bioresorbable stents, the efficacy and safety of PCI treatment have been significantly improved [11, 30, 31]. Nevertheless, the clinical benefit of PCI therapy in SCAD patients seems to be limited to the relief of angina, reduction of the incidence of spontaneous myocardial infarction, and improvement of the survival quality of patients, without a significant impact on the long-term prognosis of SCAD patients, i.e., mortality [7, 32, 33], and therefore a large number of investigators are working to find clinical indicators that play an important role in the long-term prognosis of SCAD patients. Previous studies have shown that malnutrition is a common problem among hospitalized patients, and it is estimated that approximately 30-60% of hospitalized patients are at risk for malnutrition, with a higher prevalence in older hospitalized patients [14, 34]. The presence of malnutrition risk may further weaken the immune system, and increase the cardiac burden and risk of infection in elderly patients with cardiovascular disease thereby prolonging hospitalization, with negative and far-reaching effects on clinical outcomes in elderly patients with SCAD [35,36,37].
The GNRI is a simple and practical nutrition-related risk index, which evaluates nutritional conditions through three routinely measured parameters of height, weight, and ALB in hospitalized patients, and can be used for risk assessment and risk stratification of morbidity and mortality from diseases related to nutritional status in elderly patients [13]. In a previously published meta-analysis by Fan Y et al., they evaluated for the first time a total of 9277 CAD patients in eight cohort studies and analyzed the predictive value of GNRI for all-cause death or major adverse cardiac events in CAD patients, showing that GNRI was an independent predictor of all-cause death and major adverse cardiac events in patients with stable and acute CAD [38]. Moreover, the prognostic value of GNRI has been demonstrated in several studies; for example, Zhao Q et al. showed that lower GNRI was an important predictor of poor prognosis in patients with non-ST-segment elevation myocardial infarction treated with PCI [16], while Anzaki K et al. and Cheng L et al. considered that low GNRI was not only an independent risk factor for all-cause mortality and coronary artery calcification in CAD patients after PCI but also an independent predictor of long-term adverse cardiac events in CAD patients who have developed CTO lesions after PCI [10, 11]. Similar to Anzaki K et al. [11], our results showed that GNRI levels at admission in new-onset SCAD patients were significantly associated with all-cause death risk after PCI, even after adjustment of many clinical indicators like age, sex, BMI, and cardiac function parameters, and that better nutritional status was associated with a significantly lower risk of all-cause death (HR 0.86, 95% CI 0.77, 0.95; P = 0.0041); furthermore, by looking at Tables 1 and 2, the clinical characteristics of subjects at risk of malnutrition (GNRI ≤ 98) were very similar to the characteristics of subjects who had died during follow-up, both having higher age and CRP levels and having lower height, weight, GNRI, BMI, ALB, eGFR, TC, TG, LDL-C levels, further highlighting the relationship between nutritional status and clinical outcomes in SCAD patients after PCI. However, in contrast to the findings of Cheng L et al. [10], in the stratified analyses of this study, we found that the type of coronary artery lesions did not appear to have a significant effect on the association of GNRI with all-cause death risk (all P for interaction > 0.05), and no significant association was found between GNRI and all-cause death risk in the subgroup of subjects who developed CTO lesions, although this may be due to the small number of subjects (n = 12) who developed CTO lesions.
An innovative finding of this study was the discovery of a threshold point of nutritional intervention that may need to be reached when hospitalizing patients with newly diagnosed SCAD for elective PCI. On the one hand, in the ROC analysis of the current study, we found that GNRI had a very high predictive value for all-cause death events occurring in SCAD patients after PCI treatment and found the maximum AUC value of 0.8844 at a prediction threshold of 98.62 with 100% sensitivity and 63.44% specificity, and the very high sensitivity implying a very low false-negative rate [39]; this may mean that newly diagnosed SCAD patients with a GNRI above 98.62 on admission have a significantly lower risk of all-cause death after PCI. On the other hand, the results of the RCS regression analysis and piecewise linear regression analysis in this study further corroborated the reliability of this prediction threshold. We observed a significant inflection point in the interval 95 < GNRI < 100 on the dose-response curve of GNRI with all-cause death risk (Fig. 2), and further calculated the GNRI value of 98.28 for the inflection point by piecewise linear regression analysis (Log-likelihood ratio test P = 0.023). When GNRI was less than 98.28, increasing GNRI can significantly reduce all-cause death risk (HR 0.87, 95% CI 0.76, 0.99; P = 0.0402), whereas when GNRI was greater than 98.28, the association of GNRI with all-cause death risk was not statistically significant, but this result may be due to the fact that none of the subjects in the current study population with a GNRI greater than 98.28 on admission had died during the follow-up period. It is also worth noting that the risk threshold of 98.28 was also extremely close to the threshold value of GNRI = 98 recommended by Bouillanne O, the developer of GNRI, who defined GNRI > 98 as the threshold value for no malnutrition risk based on the threshold values of ALB and weight loss in the elderly [13]. In a word, after a comprehensive evaluation of the risk assessment and predictive performance of GNRI for the occurrence of all-cause death events in SCAD patients after PCI, we suggested that GNRI = 98.28 may be the most appropriate threshold point for nutritional intervention in elderly patients with newly diagnosed SCAD who are hospitalized for elective PCI. Clinicians should also assess the nutritional status of elderly SCAD patients by calculating GNRI while treating them with PCI, and inpatients with GNRI less than 98.28 should be given appropriate nutritional support to minimize the risk of poor prognosis after PCI [40].
The strength of this study lies in the use of rigorous statistical approaches that, after stepwise adjustment for a large number of clinical indicators, identified GNRI as an independent predictor of the occurrence of all-cause death events in newly diagnosed SCAD patients after PCI, and proposed for the first time a threshold point of nutritional intervention that may need to be achieved when hospitalizing elderly patients with newly diagnosed SCAD for elective PCI treatment. Certainly, there are several limitations: (1) The study population was relatively small and from a single center, and the follow-up period was relatively short; (2) The GNRI levels of the subjects in this study were assessed only once and were not repeatedly measured during the follow-up period; it would provide more useful information if it could be further evaluated whether an increase in GNRI during the follow-up period has an improving effect on the prognosis of SCAD patients; (3) The current study is a retrospective cohort study and there may be some residual confounding because some clinical indicators with important effects on all-cause death risk could not be collected [41]; furthermore, this study was unable to update information regarding the make and manufacturer of the machines used for coronary angiography and PCI. (4) The mean age of the subjects in the current study cohort was 72.59 years, so the results of the current study may be more appropriate for the elderly population, and further research is needed on the applicability to other age groups. (5) This study only compared the predictive performance of the basic nutritional assessment indicator, BMI, for all-cause mortality risk with GNRI. It lacks comparison data between GNRI and other nutritional assessment scores or models. Further refinement is needed in future studies. (6) All subjects in this study underwent PCI treatment. Although the PCI procedures for SCAD patients were relatively consistent, differences in surgical techniques or operator proficiency could still lead to varying treatment efficacy, potentially resulting in residual confounding.
Conclusion
The current study found that the nutritional status of SCAD patients, as assessed by GNRI at admission, was an independent predictor of all-cause death events after PCI. We also identified a GNRI threshold of 98.28, which may be an appropriate point for nutritional intervention in newly diagnosed elderly SCAD patients who are hospitalized for elective PCI. Clinicians can improve the long-term prognosis of SCAD patients by moderately increasing their protein and calorie intake if they fall below this nutritional threshold.
Availability of data and materials
The data used in this study have been uploaded to the "Dryad" database (https://doi.org/10.5061/dryad.fn6730j).
Abbreviations
- GNRI:
-
Geriatric Nutrition Risk Index
- PCI:
-
Percutaneous coronary intervention
- SCAD:
-
Stable coronary artery disease
- RCS:
-
Restricted cubic spline
- ROC:
-
Receiver operating characteristic curve
- ALB:
-
Albumin
- BMI:
-
Body mass index
- DBP:
-
Diastolic blood pressure
- eGFR:
-
Estimated glomerular filtration rate
- SBP:
-
Systolic blood pressure
- TC:
-
Total cholesterol
- LDL-C:
-
Low-density lipoprotein
- BbA1c:
-
Glycohemoglobin
- TG:
-
Triglycerides
- HDL-C:
-
High-density lipoprotein
- HR:
-
Hazard ratio
- CI:
-
95% confidence interval
- CTO:
-
Chronic total obstruction
References
Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation. 2005;111(25):3481–8. https://doi.org/10.1161/CIRCULATIONAHA.105.537878.
Fox KAA, Metra M, Morais J, Atar D. The myth of ‘stable’ coronary artery disease. Nat Rev Cardiol. 2020;17(1):9–21. https://doi.org/10.1038/s41569-019-0233-y.
Boudoulas KD, Triposciadis F, Geleris P, Boudoulas H. Coronary atherosclerosis: pathophysiologic basis for diagnosis and management. Prog Cardiovasc Dis. 2016;58(6):676–92. https://doi.org/10.1016/j.pcad.2016.04.003.
Malakar AK, Choudhury D, Halder B, Paul P, Uddin A, Chakraborty S. A review on coronary artery disease, its risk factors, and therapeutics. J Cell Physiol. 2019;234(10):16812–23. https://doi.org/10.1002/jcp.28350.
Madhavan MV, Gersh BJ, Alexander KP, Granger CB, Stone GW. Coronary artery disease in patients ≥ 80 years of age. J Am Coll Cardiol. 2018;71(18):2015–40. https://doi.org/10.1016/j.jacc.2017.12.068.
Rich MW, Chyun DA, Skolnick AH, Alexander KP, Forman DE, Kitzman DW, et al. Knowledge gaps in Cardiovascular Care of the older Adult Population: A Scientific Statement from the American Heart Association, American College of Cardiology, and American Geriatrics Society. Circulation. 2016;133(21):2103–22. https://doi.org/10.1161/CIR.0000000000000380.
Al-Lamee RK, Nowbar AN, Francis DP. Percutaneous coronary intervention for stable coronary artery disease. Heart. 2019;105(1):11–9. https://doi.org/10.1136/heartjnl-2017-312755.
Lamy A, Natarajan M, Yusuf S. Medical treatment, PCI, or CABG for coronary artery disease? BMJ. 2011;342:d966. https://doi.org/10.1136/bmj.d966.
Kang SH, Song HN, Moon JY, Kim SH, Sung JH, Kim IJ, et al. Prevalence and prognostic significance of malnutrition in patients with acute coronary syndrome treated with percutaneous coronary intervention. Med (Baltim). 2022;101(34):e30100. https://doi.org/10.1097/MD.0000000000030100.
Cheng L, Rong J, Zhuo X, Gao K, Meng Z, Wen X, et al. Prognostic value of malnutrition using geriatric nutritional risk index in patients with coronary chronic total occlusion after percutaneous coronary intervention. Clin Nutr. 2021;40(6):4171–9. https://doi.org/10.1016/j.clnu.2021.01.042.
Anzaki K, Kanda D, Ikeda Y, Takumi T, Tokushige A, Ohmure K, et al. Impact of malnutrition on prognosis and coronary artery calcification in patients with stable coronary artery disease. Curr Probl Cardiol. 2022;101185. https://doi.org/10.1016/j.cpcardiol.2022.101185.
Stenvinkel P, Heimbürger O, Lindholm B, Kaysen GA, Bergström J. Are there two types of malnutrition in chronic renal failure? Evidence for relationships between malnutrition, inflammation and atherosclerosis (MIA syndrome). Nephrol Dial Transpl. 2000;15(7):953–60. https://doi.org/10.1093/ndt/15.7.953.
Bouillanne O, Morineau G, Dupont C, Coulombel I, Vincent JP, Nicolis I, et al. Geriatric nutritional risk index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr. 2005;82(4):777–83. https://doi.org/10.1093/ajcn/82.4.777.
Li Y, Shen J, Hou X, Su Y, Jiao Y, Wang J, et al. Geriatric nutritional risk index predicts all-cause mortality in the oldest-old patients with acute coronary syndrome: a 10-year cohort study. Front Nutr. 2023;10:1129978. https://doi.org/10.3389/fnut.2023.1129978.
Katayama T, Hioki H, Kyono H, Watanabe Y, Yamamoto H, Kozuma K. Predictive value of the geriatric nutritional risk index in percutaneous coronary intervention with rotational atherectomy. Heart Vessels. 2020;35(7):887–93. https://doi.org/10.1007/s00380-020-01558-4.
Zhao Q, Zhang TY, Cheng YJ, Ma Y, Xu YK, Yang JQ, et al. Impacts of geriatric nutritional risk index on prognosis of patients with non-ST-segment elevation acute coronary syndrome: results from an observational cohort study in China. Nutr Metab Cardiovasc Dis. 2020;30(10):1685–96. https://doi.org/10.1016/j.numecd.2020.05.016.
Huang BT, Peng Y, Liu W, Zhang C, Chai H, Huang FY, et al. Nutritional State predicts all-cause death Independent of comorbidities in geriatric patients with coronary artery disease. J Nutr Health Aging. 2016;20(2):199–204. https://doi.org/10.1007/s12603-015-0572-2.
Wada H, Dohi T, Miyauchi K, Doi S, Naito R, Konishi H, et al. Prognostic impact of the Geriatric Nutritional Risk Index on Long-Term outcomes in patients who underwent percutaneous coronary intervention. Am J Cardiol. 2017;119(11):1740–5. https://doi.org/10.1016/j.amjcard.2017.02.051.
Suzuki S, Hashizume N, Kanzaki Y, Maruyama T, Kozuka A, Yahikozawa K. Prognostic significance of serum albumin in patients with stable coronary artery disease treated by percutaneous coronary intervention. PLoS ONE. 2019;14(7):e0219044. https://doi.org/10.1371/journal.pone.0219044.
Suzuki S, et al. Data from: prognostic significance of serum albumin in patients with stable coronary artery disease treated by percutaneous coronary intervention. Dataset: Dryad; 2019. https://doi.org/10.5061/dryad.fn6730j.
World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–4. https://doi.org/10.1001/jama.2013.281053.
Flynn R. Survival analysis. J Clin Nurs. 2012;21(19–20):2789–97. https://doi.org/10.1111/j.1365-2702.2011.04023.x.
Kim JH. Multicollinearity and misleading statistical results. Korean J Anesthesiol. 2019;72(6):558–69. https://doi.org/10.4097/kja.19087.
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The strengthening the reporting of Observational studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–7.
Ford TJ, Ong P, Sechtem U, Beltrame J, Camici PG, Crea F, et al. Assessment of Vascular Dysfunction in patients without obstructive coronary artery disease: why, how, and when. JACC Cardiovasc Interv. 2020;13(16):1847–64. https://doi.org/10.1016/j.jcin.2020.05.052.
Medina-Leyte DJ, Zepeda-García O, Domínguez-Pérez M, González-Garrido A, Villarreal-Molina T, Jacobo-Albavera L. Endothelial dysfunction, inflammation and coronary artery disease: potential biomarkers and promising therapeutical approaches. Int J Mol Sci. 2021;22(8):3850. https://doi.org/10.3390/ijms22083850.
Roth GA, Forouzanfar MH, Moran AE, Barber R, Nguyen G, Feigin VL, et al. Demographic and epidemiologic drivers of global cardiovascular mortality. N Engl J Med. 2015;372(14):1333–41. https://doi.org/10.1056/NEJMoa1406656.
Jørgensen T, Jacobsen RK, Toft U, Aadahl M, Glümer C, Pisinger C. Effect of screening and lifestyle counselling on incidence of ischaemic heart disease in general population: Inter99 randomised trial. BMJ. 2014;348:g3617. https://doi.org/10.1136/bmj.g3617.
Chen S, Shen Y, Liu YH, Dai Y, Wu ZM, Wang XQ, et al. Impact of glycemic control on the association of endothelial dysfunction and coronary artery disease in patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2021;20(1):64. https://doi.org/10.1186/s12933-021-01257-y.
Hoole SP, Bambrough P. Recent advances in percutaneous coronary intervention. Heart. 2020;106(18):1380–6. https://doi.org/10.1136/heartjnl-2019-315707.
Palmerini T, Benedetto U, Biondi-Zoccai G, Della Riva D, Bacchi-Reggiani L, Smits PC, et al. Long-term safety of drug-eluting and Bare-Metal stents: evidence from a Comprehensive Network Meta-Analysis. J Am Coll Cardiol. 2015;65(23):2496–507. https://doi.org/10.1016/j.jacc.2015.04.017.
Teoh Z, Al-Lamee RK, COURAGE, ORBITA. Percutaneous coronary intervention for stable coronary artery disease. Interv Cardiol Clin. 2020;9(4):469–82. https://doi.org/10.1016/j.iccl.2020.07.003.
Rothberg MB, Sivalingam SK, Ashraf J, Visintainer P, Joelson J, Kleppel R, et al. Patients’ and cardiologists’ perceptions of the benefits of percutaneous coronary intervention for stable coronary disease. Ann Intern Med. 2010;153(5):307–13. https://doi.org/10.7326/0003-4819-153-5-201009070-00005.
Tonet E, Campana R, Caglioni S, Gibiino F, Fiorio A, Chiaranda G, et al. Tools for the Assessment of the Malnutrition Status and possible interventions in Elderly with Cardiovascular diseases. J Clin Med. 2021;10(7):1508. https://doi.org/10.3390/jcm10071508.
Anker SD, Sharma R. The syndrome of cardiac cachexia. Int J Cardiol. 2002;85(1):51–66. https://doi.org/10.1016/s0167-5273(02)00233-4.
Norman K, Haß U, Pirlich M. Malnutrition in older adults-recent advances and remaining challenges. Nutrients. 2021;13(8):2764. https://doi.org/10.3390/nu13082764.
Komici K, Vitale DF, Mancini A, Bencivenga L, Conte M, Provenzano S, et al. Impact of Malnutrition on Long-Term Mortality in Elderly patients with Acute myocardial infarction. Nutrients. 2019;11(2):224. https://doi.org/10.3390/nu11020224.
Fan Y, He L, Zhou Y, Man C. Predictive value of Geriatric Nutritional Risk Index in patients with coronary artery disease: a Meta-analysis. Front Nutr. 2021;8:736884. https://doi.org/10.3389/fnut.2021.736884.
Gambino B. Reflections on accuracy. J Gambl Stud. 2006;22(4):393–404. https://doi.org/10.1007/s10899-006-9025-5.
Rozentryt P, von Haehling S, Lainscak M, Nowak JU, Kalantar-Zadeh K, Polonski L, et al. The effects of a high-caloric protein-rich oral nutritional supplement in patients with chronic heart failure and cachexia on quality of life, body composition, and inflammation markers: a randomized, double-blind pilot study. J Cachexia Sarcopenia Muscle. 2010;1(1):35–42. https://doi.org/10.1007/s13539-010-0008-0.
Fewell Z, Davey Smith G, Sterne JA. The impact of residual and unmeasured confounding in epidemiologic studies: a simulation study. Am J Epidemiol. 2007;166(6):646–55. https://doi.org/10.1093/aje/kwm165.
Acknowledgements
Thanks to Professor Suzuki S and his team for contributing available research data.
Funding
This work was supported by Natural Science Foundation of Jiangxi Province [No. 20232BAB216004 to YZ].
Author information
Authors and Affiliations
Contributions
Conceptualization, YZ and GT-S; Methodology, YZ, JJ-Q and MB-K; Validation, RJ-Y; Formal analysis, JJ-Q and MB-K; Investigation, JJ-Q, MB-K, RJ-Y, CW, XH, GB-X, GT-S and YZ; Data curation, YZ and GT-S; Writing–original draft, JJ-Q, MB-K and RJ-Y; Writing–review and editing, CW, XH, GB-X, GT-S and YZ; Visualization, CW, XH and GB-X; Supervision, GT-S and YZ; Project administration, YZ. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Since the current study was a secondary analysis of data from previous studies and all data on subjects had been anonymized, the Ethics Committee of Jiangxi Provincial People’s Hospital approved the current study and dispensed with repeatedly obtaining written informed consent from subjects (2022-006). All procedures in the current study were in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kuang, M., Qiu, J., Yang, R. et al. Prognostic value of geriatric nutritional risk index in patients with stable coronary artery disease undergoing percutaneous coronary intervention. BMC Cardiovasc Disord 24, 264 (2024). https://doi.org/10.1186/s12872-024-03940-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-024-03940-w