Abstract
Background
Primary cardiac schwannoma remains extremely rare and difficult to distinguish from other myocardial tumours. We report a case of cardiac schwannoma that occurred in the lateral wall of the right ventricle and grew in the myocardial walls. It is the third case of schwannoma that occurred in the free wall of the right ventricle. Moreover, we reviewed and summarised the literature for cases involving benign cardiac schwannomas.
Case presentation
We present a case of a 64-year-old woman who presented to our centre with syncope for 1–2 min. Echocardiogram and contrast-enhanced computed tomography subsequently revealed a 2.9 × 1.9 cm homogeneous mass originating from the anterior wall of the right ventricle. The patient underwent thoracotomy to resect the mass, which was pathologically verified as Schwann cell tumour.
Conclusions
This is a rare case added to the limited existing literature on cardiac schwannoma. Comprehensive analysis of various imaging examinations is helpful to determine the extent of the tumour. Complete surgical resection is recommended for similar cases involving cardiac schwannomas, especially when the patient has related symptoms. Patients generally have a good prognosis. The pathogenesis of cardiac schwannoma needs further research in order to prevent and manage this rare lesion.
Similar content being viewed by others
Background
Primary cardiac tumours are extremely rare tumours with a prevalence of 0.02%–0.056% [1], and cardiac Schwann cell tumours are even rarer. Schwannoma is a slow-growing tumour that arises from Schwann cells in the surrounding nerve sheath [2]. Primary cardiac schwannoma is believed to originate from the cardiac plexus or the cardiac branch of the vagus nerve [3]; but its pathogenesis remains unclear. We explored the pathogenesis of cardiac schwannomas that have not been explored in detail in previous literature. Cardiac schwannoma has a variety of clinical manifestations, ranging from asymptomatic findings on imaging studies to exertion, chest pain, tachypnoea and arrhythmia, which are related to tumour size and compression of adjacent structures (e.g. large vessels, cardiac chambers, mediastinal structure and coronary artery [4]). Preoperative diagnosis is difficult, but the identification of such tumours is of great value in the development of treatment strategies and prognostic assessment. In this report, we present a case of a cardiac schwannoma, which is the third case of schwannoma that occurred in the free wall of the right ventricle. In addition, we reviewed and summarised cardiac schwannomas, which have been reported in the English literature. This is the most detailed summary and discussion of cardiac schwannoma in the past 18 years (Table 1).
Case presentation
A 64-year-old woman was admitted to our institute with syncope for 1–2 min. She reported no shortness of breath, chest pain, dyspnoea or weight loss. Her medical history included lacunar infarction and ground glass nodule of the left upper lobe, which was suspected to be lung adenocarcinoma. On physical examination, the patient was afebrile and had a regular heart rate of 60 beats per minute, a blood pressure of 125/70 mmHg and a respiratory rate of 16 breaths per minute. The patient had no murmur. The results of laboratory investigations were unremarkable. Electrocardiogram revealed sinus bradycardia and a ventricular rate of 57 beats per minute.
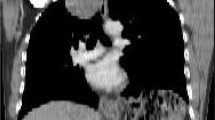
Echocardiogram revealed a mass with a size of 2.9 × 1.9 cm on the front wall of the right ventricle, which had a uniform internal echo and star-shaped blood flow signals (Fig. 1). Chest computed tomography (CT) scan demonstrated a 2.8 × 2.0 cm homogeneous mass originating from the anterior wall of the right ventricle, which has a relatively broad base. The boundary of the mass is clear and fixed. No obvious narrowing of the heart cavity was observed (Fig. 2A). The patient underwent a three-phase dynamic chest CT, which disclosed a myocardial tissue mass with slightly enhancement during the arterial phase (Fig. 2B) and a persistent moderate increase during the venous and delayed phases (Fig. 2C-D). The definite diagnosis was difficult. The patient was referred to thoracic surgery for thoracotomy and resection of the myocardial tumour under cardiopulmonary bypass (CPB). The defect of the right ventricle was repaired.
A An isodense mass on the anterior wall of the right ventricle with uniform internal density and clear boundary. No obvious narrowing of the heart cavity was notede, the CT value is approximately 29 HU. B A myocardial tissue mass with slightly enhancement during the arterial phase, the CT value is approximately 34 HU. C-D Lesions with a persistent enhancement during the venous and delayed phase, the CT value is approximately 38 HU (C) and 35HU (D)
The desquamated tissue was histopathologically examined and reported as a schwannoma. Microscopic sections revealed that Cells of Antoni A tissue have modest eosinophilic cytoplasm with on discernible cell borders and normochromic, elongated, tapered nuclei (Fig. 3A). Immunohistochemical studies showed that the tumour cells stained positively for S100 and SOX10 (Fig. 3B-C). Micrographs were acquired by using Nikon CI2 (Nikon) and NIS-Elements (Nikon) software. The resolution of each acquired images is 300 dots per inch.
A Cells of Antoni A tissue have modest eosinophilic cytoplasm with on discernible cell borders and normochromic, elongated,tapered nuclei. (Hematoxylin and eosin) B Tumour cells stained positively for S100 as determined by immunohistochemistry.(S100) C Tumour cells stained positively for SOX10 as determined by immunohistochemistry.(SOX10)
The patient recovered and was discharged on the 10th day after surgery without complications. The patient had a ground glass nodule resection, which was subsequently confirmed as microinvasive lung adenocarcinoma, and the pathologic TNM stage was T1N0M0. The patient recovered uneventfully and had no sign of recurrence at a follow-up duration of 5 years.
Discussion and conclusion
We searched the PubMed database until July 21 2022 using the keywords “Cardiac Schwannoma” and “Cardiac tumour and Schwannoma” to identify the relevant English medical literature. The search identified 332 results. After a careful analysis of the articles, approximately 24 articles met the inclusion criteria and were included. In addition, 3 patients dates from one of the abovementioned paper [5], which could not be retrieved from PubMed, were also added to this review. The study selection process is shown in Fig. 4. Two reviewers independently appraised all included studies using the Joanna Briggs Institute (JBI) checklist for case reports and case series.
Primary cardiac tumours are very rare, and benign cardiac schwannomas are even rarer. Our review of English literature showed that 27 cases of benign cardiac schwannomas including two cases of type II neurofibromatosis [12, 25] have been reported. The age range was 12–72 years old, the mean age was 50.7 years old, and the male-to-female ratio was about 1:2. These data were consistent with previous reports [5]. Primary cardiac schwannoma is believed to originate from the cardiac plexus or the cardiac branch of the vagus nerve; therefore, it is located primarily on the right side of the heart [26]. However, we found that the right atrium is the predominant site of cardiac schwannomas (12/28), and the incidences of left atrial, bilateral ventricular and aortic outflow tracts have no remarkable differences. This finding could be attributed to the distribution of the sinoatrial and atrioventricular nodes around the right atrium because the distribution of nerve fibres around these structures is remarkably higher than that in the surrounding working myocardium. The case that we reported occurred in the lateral wall of the right ventricle. It is the third case of schwannoma that occurred in the free wall of the right ventricle. This study provides an important supplement to explore the pathogenesis of the lesion and reflect the distribution of cardiac plexus.
Neurilemoma originates from the peripheral nerve sheath, and its pathogenesis remains unclear. No relevant literature has proposed hypotheses regarding its cause. The National Toxicology Program and Ramazzini Institute reported that radiofrequency electromagnetic field substantially increases glioma and schwannoma in the heart of rodents [27]. Stephen Factor et al. reported that a patient with cardiac neurilemoma who received a large total amount of radiotherapy or at least one course of radiotherapy directed to the lower thoracic vertebral region for the treatment of paravertebral mass may have peripherally involved the heart [6]. The relationship between human cardiac schwannoma and radiation needs further research. Additionally, Das Gupta et a1. studied 303 benign schwannomas and reported the interesting correlation of nerve sheath tumours with the past, concurrent or future development of a malignancy unrelated to peripheral nerves [28]. Through case review, we found that seven cases, including our case, were accompanied by other tumours, including six cases of malignancy and one unspecified case. The seven tumours included one autopsies case of ovarian cancer [6] and one autopsies cases of lung cancer [5], one case of renal cancer preceded cardiac schwannoma [3], one case of synchronous sigmoid colon cancer [21], one case of synchronous cancer of the left chest wall [4] and one case of synchronous cavernous mass of the bladder [14], one cases of synchronous lung adenocarcinoma (our case). The connection between schwannoma and other unrelated malignancy needs further experimental verification.
Primary cardiac schwannomas vary in size. The clinical symptoms are mostly caused by compression or obstruction, and some patients may have dyspnoea on exertion (5/28) [12, 17, 18, 23], chest pain (4/28) [3, 7, 8, 19], shortness of breath (4/28) [3, 7, 10, 24], palpitation (2/28) [15, 20], arrhythmia (2/28) [8, 10] and other discomfort. More than one third of patients (10/28) [4,5,6, 9, 14, 16, 21,22,23] had no related symptoms. Our case was hospitalised because of syncope, which is rarely reported in literature. The syncope may be caused by the sudden decrease or pause of cardiac output caused by the cardiac tumour.
Cardiac schwannoma can be detected by X-ray or echocardiogram, CT and magnetic resonance imaging (MRI), which can help to better determine the location and extent of the mass and the involvement of other structures [3]. Tumours are mostly heterogeneous masses with cystic changes, haemorrhages and calcifications. Uneven and mild enhancement may even occur. Some lesions have a broad base and shallow lobes, and most lesions have a clear boundary. The fibrous capsule is also one of the identification points of schwannomas from other tumours. Coronary angiography is required for patients at risk of coronary heart disease or with tumours that may involve the coronary artery [4]. When the exact origin of the tumour cannot be obtained by CT or MRI, 3D printing and model establishment can help to clearly identify the location of the tumour and its relationship with large blood vessels [20]. The nature of the tumour is difficult to identify through imaging.
Most patients with cardiac Schwann cell tumours undergo extensive radical tumour resection and cardiac reconstruction with autologous pericardium or artificial patch under CPB [5]. The degree of involvement and reconstruction of the atrioventricular valve, coronary artery, coronary sinus or pulmonary vein are also important [4, 5, 13]. Among the 28 patients, excepting for 2 autopsy patients, 2 patients whose survival/death was not mentioned in the literature, and 1 patient whose data was not available, the survival rate of the remaining 23 patients was 100% in the follow-up period, and the postoperative prognosis is good. Our operation was also successful, and no recurrence was observed after 5 years of follow-up.
In conclusion, this is a rare case added to the limited existing literature on cardiac schwannoma. Comprehensive analysis of various imaging examinations is helpful to determine the extent of the tumour. Complete surgical resection is recommended for similar cases involving cardiac schwannomas, especially when the patient has related symptoms. Patients generally have a good prognosis. The pathogenesis of cardiac schwannoma needs further research in order to prevent and manage this rare lesion.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files.
Abbreviations
- CT:
-
Computed tomography
- CPB:
-
Cardiopulmonary bypass
- MRI:
-
Magnetic resonance imaging
References
Butany J, Nair V, Naseemuddin A, Nair GM, Catton C, Yau T. Cardiac tumours: diagnosis and management. Lancet Oncol. 2005;6(4):219–28. https://doi.org/10.1016/S1470-2045(05)70093-0.
Zainab H, Kale AD, Hallikerimath S. Intraosseous schwannoma of the mandible. J Oral Maxillofac Pathol: JOMFP. 2012;16(2):294–6. https://doi.org/10.4103/0973-029X.99094.
Bizzarri F, Mondillo S, Tanganelli P, et al. A primary intracavitary right atrial neurilemoma. J Cardiovasc Surg (Torino). 2001;42(6):777–9.
La Francesca S, Gregoric ID, Cohn WE, Frazier OH. Successful resection of a primary left ventricular schwannoma. Ann Thorac Surg. 2007;83(5):1881–2. https://doi.org/10.1016/j.athoracsur.2006.12.012.
Yokoyama K, Yoshizaki T, Tasaki D. Left atrial schwannoma in schwannomatosis: a case report. Surg Case Rep. 2021;7(1):75 (Published 2021 Mar 23. doi:10.1186/s40792-021-01158-y).
Factor S, Turi G, Biempica L. Primary cardiac neurilemoma. Cancer. 1976;37(2):883–90. https://doi.org/10.1002/1097-0142(197602)37:2%3c883::aid-cncr2820370237%3e3.0.co;2-e.
Betancourt B, Defendini EA, Johnson C, et al. Severe right ventricular outflow tract obstruction caused by an intracavitary cardiac neurilemoma: succesful surgical removal and postoperative diagnosis. Chest. 1979;75(4):522–4. https://doi.org/10.1378/chest.75.4.522.
Forbes AD, Schmidt RA, Wood DE, Cochran RP, Munkenbeck F, Verrier ED. Schwannoma of the left atrium: diagnostic evaluation and surgical resection. Ann Thorac Surg. 1994;57(3):743–6. https://doi.org/10.1016/0003-4975(94)90581-9.
Hashimoto T, Eguchi S, Nakayama T, Ohzeki H, Hayashi J. Successful removal of massive cardiac neurilemoma with cardiopulmonary bypass. Ann Thorac Surg. 1998;66(2):553–5. https://doi.org/10.1016/s0003-4975(98)00473-1.
Sirlak M, Uymaz OK, Taşoz R, Erden E, Ozyurda U, Akalin H. Primary benign schwannoma of the heart. Cardiovasc Pathol. 2003;12(5):290–2. https://doi.org/10.1016/s1054-8807(03)00076-0.
Jassal DS, Légaré JF, Cummings B, et al. Primary cardiac ancient schwannoma. J Thorac Cardiovasc Surg. 2003;125(3):733–5. https://doi.org/10.1067/mtc.2003.26.
Nakamura K, Onitsuka T, Yano M, Yano Y. Surgical resection of right atrial neurilemoma extending to pulmonary vein. Eur J Cardiothorac Surg. 2003;24(5):840–2. https://doi.org/10.1016/s1010-7940(03)00494-9.
Rausche T, El-Mokthari NE, Krüger D, et al. Benign mediastinal schwannoma: cardiac considerations - case report and a short review of the literature. Clin Res Cardiol. 2006;95(8):422–4. https://doi.org/10.1007/s00392-006-0396-5.
Stolf NA, Santos GG, Sobral ML, Haddad VL. Primary schwannoma of the right atrium: successful surgical resection. Clinics (Sao Paulo). 2006;61(1):87–8. https://doi.org/10.1590/s1807-59322006000100016.
Sevimli S, Erkut B, Becit N, Aksakal E, Polat P. Primary benign schwannoma of the left ventricle coursing under the left anterior descending artery. Echocardiography. 2007;24(10):1093–5. https://doi.org/10.1111/j.1540-8175.2007.00529.x.
Early SA, McGuinness J, Galvin J, Kennedy M, Hurley J. Asymptomatic schwannoma of the heart. J Cardiothorac Surg. 2007;2:1. Published 2007 Jan 4. doi:https://doi.org/10.1186/1749-8090-2-1.
Anderson CD, Hashimi S, Brown T, Moyers J, Farivar RS. Primary benign interatrial schwannoma encountered during aortic valve replacement. J Card Surg. 2011;26(1):63–5. https://doi.org/10.1111/j.1540-8191.2010.01158.x.
Elstner K, Granger E, Wilson S, Kumaradevan N, Chew M, Harris C. Schwannoma of the pulmonary artery. Heart Lung Circ. 2013;22(3):231–3. https://doi.org/10.1016/j.hlc.2012.07.012.
Hwang SK, Jung SH. Schwannoma of the heart. Korean J Thorac Cardiovasc Surg. 2014;47(2):141–4. https://doi.org/10.5090/kjtcs.2014.47.2.141.
Son KH, Kim KW, Ahn CB, et al. Surgical Planning by 3D Printing for Primary Cardiac Schwannoma Resection. Yonsei Med J. 2015;56(6):1735–7. https://doi.org/10.3349/ymj.2015.56.6.1735.
Jung JC, Chang HW, Kim KH. An unusual presentation of schwannoma in the interatrial space. Korean J Thorac Cardiovasc Surg. 2015;48(1):95–7. https://doi.org/10.5090/kjtcs.2015.48.1.95.
Wang JG, Wang B, Hu Y, et al. Clinicopathologic features and outcomes of primary cardiac tumors: a 16-year-experience with 212 patients at a Chinese medical center. Cardiovasc Pathol. 2018;33:45–54. https://doi.org/10.1016/j.carpath.2018.01.003.
Huang Z. Successful resection of a huge schwannoma of the aortic root with 5-years follow-up. J Card Surg. 2020;35(8):2084–6. https://doi.org/10.1111/jocs.14797.
Wang SY, Liu JH, Yao S, Wang SX, Shao D. PET/CT and contrast-enhanced CT imaging findings in benign solitary schwannomas. Eur J Radiol. 2021;141:109820. https://doi.org/10.1016/j.ejrad.2021.109820.
Chen XD, Qian M, Tu WF, Liao QL, Zhou BC. Cardiac schwannoma: report of a case. Zhonghua Bing Li Xue Za Zhi. 2006;35(3):186–7.
Bottio T, Gerosa G. Clinical-pathologic conference in cardiac surgery: malignant schwannoma of the heart. J Thorac Cardiovasc Surg. 2005;130(1):202–5. https://doi.org/10.1016/j.jtcvs.2004.11.060.
Wall S, Wang ZM, Kendig T, Dobraca D, Lipsett M. Real-world cell phone radiofrequency electromagnetic field exposures. Environ Res. 2019;171:581–92. https://doi.org/10.1016/j.envres.2018.09.015.
Das Gupta TK, Brasfield RD, Strong EW, Hajdu SI. Benign solitary Schwannomas (neurilemomas). Cancer. 1969;24(2):355–66. https://doi.org/10.1002/1097-0142(196908)24:2%3c355::aid-cncr2820240218%3e3.0.co;2-2.
Acknowledgements
Not applicable.
Funding
This work was supported by National Natural Science Foundation of China [81571636]. The funding assisted in the collection of clinical data and publication fees.
Author information
Authors and Affiliations
Contributions
WF performed data analyses and wrote the manuscript. WF, LL, MH and CXX conducted the clinical diagnosis and data collection. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Medical Ethics Committee of Yantai Yuhuangding Hospital.
Consent for publication
Written informed consent was obtained from the patient for the publication of this report and any accompanying images. A copy of the written consent is available for review by the Editor at any time.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, F., Li, L., Ma, H. et al. A primary cardiac schwannoma of the right ventricle: a case report and literature review. BMC Cardiovasc Disord 22, 498 (2022). https://doi.org/10.1186/s12872-022-02941-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-022-02941-x